Comparison of Ammonium Hydroxide and Sodium Hydroxide in Alkaline Cleaning
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkaline Cleaning Evolution
Alkaline cleaning has undergone significant evolution since its inception in the early 20th century. Initially, the process primarily relied on sodium hydroxide (NaOH) as the main cleaning agent due to its strong alkaline properties and effectiveness in removing organic contaminants. However, as industrial needs diversified and environmental concerns grew, the field of alkaline cleaning began to explore alternative solutions.
The 1950s and 1960s saw the introduction of ammonium hydroxide (NH4OH) as a potential alternative to sodium hydroxide in certain applications. This shift was driven by the need for gentler cleaning agents that could effectively clean sensitive materials without causing damage. Ammonium hydroxide offered a milder alkaline solution while still maintaining good cleaning properties, particularly for removing light oils and greases.
Throughout the 1970s and 1980s, research focused on optimizing the formulations of alkaline cleaners. This period saw the development of complex blends that incorporated both sodium hydroxide and ammonium hydroxide, along with surfactants and chelating agents. These formulations aimed to combine the strengths of both alkaline compounds while mitigating their individual weaknesses.
The 1990s marked a turning point in alkaline cleaning evolution with the rise of environmental awareness. This led to increased scrutiny of sodium hydroxide due to its potential environmental impact and handling hazards. Consequently, there was a renewed interest in ammonium hydroxide and other less caustic alternatives. Research during this time focused on developing eco-friendly formulations that maintained cleaning efficacy while reducing environmental footprint.
In the early 2000s, advancements in nanotechnology began to influence alkaline cleaning. Researchers explored the use of nanoparticles in conjunction with alkaline solutions to enhance cleaning performance. This approach showed promise in improving the efficiency of both sodium hydroxide and ammonium hydroxide-based cleaners, particularly in removing stubborn contaminants at lower concentrations.
The past decade has seen a shift towards precision cleaning in various industries, driving further innovation in alkaline cleaning technologies. This has led to the development of highly specialized alkaline cleaning solutions tailored for specific applications, such as semiconductor manufacturing or medical device cleaning. In these contexts, the choice between ammonium hydroxide and sodium hydroxide is often determined by the specific material being cleaned and the level of cleanliness required.
Today, the field of alkaline cleaning continues to evolve, with ongoing research into new formulations and applications. The comparison between ammonium hydroxide and sodium hydroxide remains relevant, with each compound finding its niche in different industrial applications based on their unique properties and the specific cleaning requirements of various sectors.
The 1950s and 1960s saw the introduction of ammonium hydroxide (NH4OH) as a potential alternative to sodium hydroxide in certain applications. This shift was driven by the need for gentler cleaning agents that could effectively clean sensitive materials without causing damage. Ammonium hydroxide offered a milder alkaline solution while still maintaining good cleaning properties, particularly for removing light oils and greases.
Throughout the 1970s and 1980s, research focused on optimizing the formulations of alkaline cleaners. This period saw the development of complex blends that incorporated both sodium hydroxide and ammonium hydroxide, along with surfactants and chelating agents. These formulations aimed to combine the strengths of both alkaline compounds while mitigating their individual weaknesses.
The 1990s marked a turning point in alkaline cleaning evolution with the rise of environmental awareness. This led to increased scrutiny of sodium hydroxide due to its potential environmental impact and handling hazards. Consequently, there was a renewed interest in ammonium hydroxide and other less caustic alternatives. Research during this time focused on developing eco-friendly formulations that maintained cleaning efficacy while reducing environmental footprint.
In the early 2000s, advancements in nanotechnology began to influence alkaline cleaning. Researchers explored the use of nanoparticles in conjunction with alkaline solutions to enhance cleaning performance. This approach showed promise in improving the efficiency of both sodium hydroxide and ammonium hydroxide-based cleaners, particularly in removing stubborn contaminants at lower concentrations.
The past decade has seen a shift towards precision cleaning in various industries, driving further innovation in alkaline cleaning technologies. This has led to the development of highly specialized alkaline cleaning solutions tailored for specific applications, such as semiconductor manufacturing or medical device cleaning. In these contexts, the choice between ammonium hydroxide and sodium hydroxide is often determined by the specific material being cleaned and the level of cleanliness required.
Today, the field of alkaline cleaning continues to evolve, with ongoing research into new formulations and applications. The comparison between ammonium hydroxide and sodium hydroxide remains relevant, with each compound finding its niche in different industrial applications based on their unique properties and the specific cleaning requirements of various sectors.
Market Demand Analysis
The market demand for alkaline cleaning solutions, particularly those utilizing ammonium hydroxide and sodium hydroxide, has been steadily growing across various industries. This growth is primarily driven by the increasing need for effective and efficient cleaning processes in manufacturing, electronics, healthcare, and other sectors.
In the industrial cleaning market, there is a rising demand for environmentally friendly and cost-effective solutions. Ammonium hydroxide and sodium hydroxide both offer advantages in this regard, as they are relatively inexpensive and can be used in lower concentrations compared to some other cleaning agents. This aligns with the global trend towards sustainable and eco-friendly industrial practices.
The electronics industry, in particular, has shown a significant increase in demand for alkaline cleaning solutions. As electronic components become more complex and miniaturized, the need for precision cleaning has intensified. Both ammonium hydroxide and sodium hydroxide are widely used in this sector for removing flux residues, oxides, and other contaminants from printed circuit boards and semiconductor wafers.
In the healthcare and pharmaceutical industries, there is a growing emphasis on stringent cleaning and disinfection protocols. Alkaline cleaning solutions play a crucial role in maintaining hygiene standards in medical facilities and in the production of pharmaceuticals. The market demand in this sector is expected to continue its upward trajectory due to increased focus on infection control and regulatory compliance.
The automotive industry is another significant consumer of alkaline cleaning solutions. With the trend towards lightweight materials and complex surface treatments in vehicle manufacturing, the demand for effective cleaning agents has increased. Both ammonium hydroxide and sodium hydroxide are used in various stages of automotive production, from parts cleaning to surface preparation for painting.
Market analysis indicates that the Asia-Pacific region is experiencing the fastest growth in demand for alkaline cleaning solutions. This is attributed to the rapid industrialization in countries like China and India, coupled with the expansion of electronics manufacturing in the region. North America and Europe continue to be significant markets, driven by technological advancements and stringent environmental regulations.
The global market for alkaline cleaning chemicals is projected to grow at a compound annual growth rate (CAGR) of several percentage points over the next five years. This growth is fueled by the expansion of end-use industries and the continuous development of more efficient and environmentally friendly cleaning formulations.
In the industrial cleaning market, there is a rising demand for environmentally friendly and cost-effective solutions. Ammonium hydroxide and sodium hydroxide both offer advantages in this regard, as they are relatively inexpensive and can be used in lower concentrations compared to some other cleaning agents. This aligns with the global trend towards sustainable and eco-friendly industrial practices.
The electronics industry, in particular, has shown a significant increase in demand for alkaline cleaning solutions. As electronic components become more complex and miniaturized, the need for precision cleaning has intensified. Both ammonium hydroxide and sodium hydroxide are widely used in this sector for removing flux residues, oxides, and other contaminants from printed circuit boards and semiconductor wafers.
In the healthcare and pharmaceutical industries, there is a growing emphasis on stringent cleaning and disinfection protocols. Alkaline cleaning solutions play a crucial role in maintaining hygiene standards in medical facilities and in the production of pharmaceuticals. The market demand in this sector is expected to continue its upward trajectory due to increased focus on infection control and regulatory compliance.
The automotive industry is another significant consumer of alkaline cleaning solutions. With the trend towards lightweight materials and complex surface treatments in vehicle manufacturing, the demand for effective cleaning agents has increased. Both ammonium hydroxide and sodium hydroxide are used in various stages of automotive production, from parts cleaning to surface preparation for painting.
Market analysis indicates that the Asia-Pacific region is experiencing the fastest growth in demand for alkaline cleaning solutions. This is attributed to the rapid industrialization in countries like China and India, coupled with the expansion of electronics manufacturing in the region. North America and Europe continue to be significant markets, driven by technological advancements and stringent environmental regulations.
The global market for alkaline cleaning chemicals is projected to grow at a compound annual growth rate (CAGR) of several percentage points over the next five years. This growth is fueled by the expansion of end-use industries and the continuous development of more efficient and environmentally friendly cleaning formulations.
Current Challenges
The alkaline cleaning industry faces several significant challenges when comparing the use of ammonium hydroxide and sodium hydroxide. One of the primary issues is the environmental impact of these chemicals. While both are effective cleaning agents, they pose different risks to ecosystems when released into the environment. Ammonium hydroxide can contribute to eutrophication in water bodies, while sodium hydroxide can cause severe pH changes, affecting aquatic life.
Safety concerns for workers and end-users present another major challenge. Sodium hydroxide is highly corrosive and can cause severe burns upon contact with skin or eyes. Ammonium hydroxide, while less corrosive, produces strong fumes that can irritate the respiratory system. Balancing cleaning efficacy with worker safety remains a constant struggle for manufacturers and industrial users.
The cost-effectiveness of these alkaline cleaning solutions is also a significant consideration. Sodium hydroxide is generally less expensive and more widely available, making it a preferred choice for large-scale industrial applications. However, ammonium hydroxide may be more suitable for certain specialized cleaning tasks, particularly where residue-free cleaning is crucial. This cost-benefit analysis becomes more complex when factoring in the potential long-term effects on equipment and surfaces being cleaned.
Regulatory compliance presents an ongoing challenge for the industry. Different regions and countries have varying regulations regarding the use and disposal of these alkaline cleaning agents. Companies must navigate a complex landscape of environmental and safety regulations, which can impact their choice between ammonium hydroxide and sodium hydroxide.
Another technical challenge lies in the formulation of cleaning products. Achieving the right balance of cleaning power, safety, and material compatibility often requires extensive research and development. The interaction of these alkaline agents with other components in cleaning formulations can affect their performance and stability, necessitating careful consideration in product design.
The disposal of waste from alkaline cleaning processes poses additional challenges. Both ammonium hydroxide and sodium hydroxide solutions require specialized treatment before disposal to neutralize their high pH levels and remove potentially harmful byproducts. This adds complexity and cost to the overall cleaning process, particularly for industries that generate large volumes of alkaline waste.
Lastly, there is an ongoing challenge in educating users about the proper handling and application of these alkaline cleaning agents. Misuse can lead to ineffective cleaning, damage to surfaces, or safety hazards. Developing clear guidelines and training programs for various industry sectors remains a critical need in the field of alkaline cleaning.
Safety concerns for workers and end-users present another major challenge. Sodium hydroxide is highly corrosive and can cause severe burns upon contact with skin or eyes. Ammonium hydroxide, while less corrosive, produces strong fumes that can irritate the respiratory system. Balancing cleaning efficacy with worker safety remains a constant struggle for manufacturers and industrial users.
The cost-effectiveness of these alkaline cleaning solutions is also a significant consideration. Sodium hydroxide is generally less expensive and more widely available, making it a preferred choice for large-scale industrial applications. However, ammonium hydroxide may be more suitable for certain specialized cleaning tasks, particularly where residue-free cleaning is crucial. This cost-benefit analysis becomes more complex when factoring in the potential long-term effects on equipment and surfaces being cleaned.
Regulatory compliance presents an ongoing challenge for the industry. Different regions and countries have varying regulations regarding the use and disposal of these alkaline cleaning agents. Companies must navigate a complex landscape of environmental and safety regulations, which can impact their choice between ammonium hydroxide and sodium hydroxide.
Another technical challenge lies in the formulation of cleaning products. Achieving the right balance of cleaning power, safety, and material compatibility often requires extensive research and development. The interaction of these alkaline agents with other components in cleaning formulations can affect their performance and stability, necessitating careful consideration in product design.
The disposal of waste from alkaline cleaning processes poses additional challenges. Both ammonium hydroxide and sodium hydroxide solutions require specialized treatment before disposal to neutralize their high pH levels and remove potentially harmful byproducts. This adds complexity and cost to the overall cleaning process, particularly for industries that generate large volumes of alkaline waste.
Lastly, there is an ongoing challenge in educating users about the proper handling and application of these alkaline cleaning agents. Misuse can lead to ineffective cleaning, damage to surfaces, or safety hazards. Developing clear guidelines and training programs for various industry sectors remains a critical need in the field of alkaline cleaning.
Existing Cleaning Solutions
01 Comparative cleaning effectiveness of ammonium hydroxide and sodium hydroxide
Studies have been conducted to compare the cleaning effectiveness of ammonium hydroxide and sodium hydroxide in various applications. Both compounds are strong bases and can effectively remove organic contaminants, grease, and oils. However, their effectiveness may vary depending on the specific cleaning task and surface material.- Comparison of cleaning effectiveness: Both ammonium hydroxide and sodium hydroxide are effective cleaning agents, but their effectiveness can vary depending on the specific application. Ammonium hydroxide is generally milder and less corrosive than sodium hydroxide, making it suitable for more delicate surfaces. Sodium hydroxide, being a stronger base, is often more effective for heavy-duty cleaning tasks and removing stubborn stains or grease.
- Industrial cleaning applications: In industrial settings, both ammonium hydroxide and sodium hydroxide are used for various cleaning purposes. Sodium hydroxide is commonly employed in metal cleaning, degreasing, and removing scale from industrial equipment. Ammonium hydroxide finds applications in cleaning electronic components, glass surfaces, and as a component in specialized cleaning formulations for industrial use.
- Environmental and safety considerations: When comparing the cleaning effectiveness of ammonium hydroxide and sodium hydroxide, it's important to consider their environmental impact and safety profiles. Ammonium hydroxide is generally considered less harmful to the environment and safer to handle, while sodium hydroxide requires more stringent safety precautions due to its corrosive nature. The choice between the two often depends on balancing cleaning efficacy with safety and environmental concerns.
- Formulation with other cleaning agents: To enhance cleaning effectiveness, both ammonium hydroxide and sodium hydroxide are often formulated with other cleaning agents. These formulations may include surfactants, chelating agents, or other additives to improve their cleaning power, stability, or specific properties. The combination of these alkaline substances with other components can result in synergistic effects, leading to more effective cleaning solutions for various applications.
- Specialized cleaning applications: Ammonium hydroxide and sodium hydroxide have specific advantages in certain specialized cleaning applications. For instance, ammonium hydroxide is particularly effective in cleaning glass surfaces without leaving streaks, while sodium hydroxide excels in removing organic materials and is often used in cleaning processes for food processing equipment. The choice between the two depends on the specific requirements of the cleaning task at hand.
02 Use of ammonium hydroxide and sodium hydroxide in industrial cleaning processes
Ammonium hydroxide and sodium hydroxide are widely used in industrial cleaning processes due to their strong alkaline properties. They are effective in removing stubborn stains, scale deposits, and organic residues from various surfaces and equipment. These compounds are often used in the cleaning of manufacturing equipment, pipelines, and storage tanks.Expand Specific Solutions03 Environmental and safety considerations in using ammonium hydroxide and sodium hydroxide for cleaning
When using ammonium hydroxide and sodium hydroxide for cleaning purposes, it is important to consider their environmental impact and safety implications. Both compounds are corrosive and can be harmful if not handled properly. Proper dilution, personal protective equipment, and disposal methods are crucial to ensure safe and environmentally friendly cleaning practices.Expand Specific Solutions04 Formulation of cleaning products containing ammonium hydroxide or sodium hydroxide
Cleaning products containing ammonium hydroxide or sodium hydroxide are formulated to optimize their cleaning effectiveness while minimizing potential risks. These formulations may include additional ingredients such as surfactants, chelating agents, and pH buffers to enhance cleaning performance and stability. The choice between ammonium hydroxide and sodium hydroxide in product formulations depends on factors such as target soil types and surface compatibility.Expand Specific Solutions05 Application-specific cleaning effectiveness of ammonium hydroxide and sodium hydroxide
The cleaning effectiveness of ammonium hydroxide and sodium hydroxide can vary depending on the specific application. For example, in semiconductor manufacturing, these compounds may be used for wafer cleaning and photoresist removal. In the food industry, they are employed for equipment sanitation and CIP (Clean-in-Place) systems. The choice between the two compounds is often based on their performance in removing specific contaminants and their compatibility with the materials being cleaned.Expand Specific Solutions
Key Industry Players
The competitive landscape for alkaline cleaning using ammonium hydroxide and sodium hydroxide is characterized by a mature industry with established players. The market size is substantial, driven by widespread applications in industrial and consumer cleaning products. Technological maturity is high, with companies like Ecolab, Henkel, and S.C. Johnson & Son leading innovation. These firms, along with others such as The Clorox Co. and L'Oréal, have extensive experience in formulating and manufacturing cleaning solutions. The competition is intense, with companies focusing on product differentiation, efficiency, and environmental sustainability to gain market share in this well-developed sector.
Ecolab USA, Inc.
Technical Solution: Ecolab has developed advanced alkaline cleaning solutions that utilize both ammonium hydroxide and sodium hydroxide. Their proprietary formulations optimize the balance between these two alkaline agents to achieve superior cleaning performance across various industries. Ecolab's approach involves using ammonium hydroxide for its excellent grease-cutting properties and low residue, while incorporating sodium hydroxide for its strong alkalinity and ability to saponify fats[1]. The company has also implemented automated dosing systems that can adjust the ratio of these alkaline agents based on specific cleaning requirements, water hardness, and soil types[3].
Strengths: Customizable solutions, broad industry applications, advanced dosing technology. Weaknesses: Higher cost compared to single-agent cleaners, potential environmental concerns with ammonia emissions.
The Clorox Co.
Technical Solution: Clorox has focused on developing consumer-grade cleaning products that primarily utilize sodium hydroxide as the main alkaline agent. Their research has shown that for household applications, sodium hydroxide provides a more stable and safer alternative to ammonium hydroxide. Clorox has engineered formulations that combine sodium hydroxide with surfactants and chelating agents to enhance its cleaning power while minimizing potential surface damage[2]. The company has also invested in eco-friendly packaging and concentrated formulas to reduce environmental impact[4].
Strengths: Strong consumer trust, widely available products, safer for household use. Weaknesses: Limited industrial applications, potentially less effective on certain types of soils compared to ammonium hydroxide-based cleaners.
Core Chemical Innovations
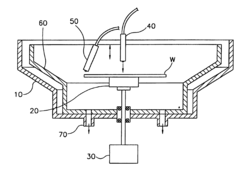
Novel synthetic caustic composition
PatentActiveUS20210122971A1
Innovation
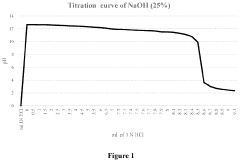
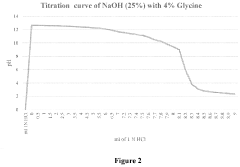
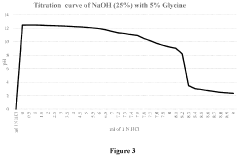
- A novel caustic composition comprising a caustic component, such as potassium hydroxide or sodium hydroxide, combined with glycine as an additive to provide an extended and linear buffering effect, reducing dermal damage and lowering the freeze point, along with water, to enhance safety and environmental responsibility.
Cleaning solution and method of cleaning anti-reflective coating composition using the same
PatentInactiveUS6777379B2
Innovation
- A cleaning solution comprising 5-30% ammonium hydroxide salt, 23-70% organic solvent, and 10-50% water is used to effectively remove both cured and non-cured organic ARC compositions from equipment, utilizing the swelling and solvency power of the solution to facilitate efficient cleaning.
Environmental Impact
The environmental impact of alkaline cleaning agents, particularly ammonium hydroxide and sodium hydroxide, is a critical consideration in industrial and domestic applications. Both compounds have distinct environmental footprints that warrant careful examination.
Ammonium hydroxide, when released into the environment, can contribute to eutrophication in aquatic ecosystems. Its high nitrogen content can lead to excessive algal growth, potentially disrupting the balance of aquatic life. However, it is relatively volatile and tends to dissipate quickly in the atmosphere, reducing its long-term environmental persistence.
Sodium hydroxide, on the other hand, does not directly contribute to eutrophication but can have severe localized effects on aquatic ecosystems due to its high alkalinity. When discharged in high concentrations, it can dramatically alter the pH of water bodies, potentially harming or killing aquatic organisms. Unlike ammonium hydroxide, sodium hydroxide is not volatile and persists in the environment until neutralized.
Both compounds can have significant impacts on soil chemistry when released in large quantities. Ammonium hydroxide can temporarily increase soil nitrogen levels, potentially benefiting plant growth in the short term but risking soil acidification over time. Sodium hydroxide can increase soil pH, potentially altering soil structure and affecting plant nutrient availability.
In terms of production and transportation, sodium hydroxide generally has a lower carbon footprint. It is typically produced through the chloralkali process, which has been optimized for energy efficiency over many years. Ammonium hydroxide production, often involving the Haber-Bosch process for ammonia synthesis, tends to be more energy-intensive and thus has a higher associated carbon footprint.
Waste management is another crucial aspect of environmental impact. Sodium hydroxide can be neutralized relatively easily and safely, often with acidic waste streams, making it manageable in industrial settings. Ammonium hydroxide disposal requires more careful handling to prevent ammonia gas release, which can contribute to air pollution and pose health risks.
Water consumption in the production and use of these compounds also differs. Sodium hydroxide production typically requires less water than ammonium hydroxide, which can be a significant factor in water-stressed regions. However, in cleaning applications, the water usage for both compounds can vary depending on the specific process and concentration used.
Considering the broader lifecycle impact, the production of raw materials for both compounds should be evaluated. Sodium hydroxide relies on salt (NaCl) as a primary raw material, which is abundant and relatively easy to extract. Ammonium hydroxide production depends on natural gas for hydrogen production, linking it to fossil fuel extraction and its associated environmental impacts.
Ammonium hydroxide, when released into the environment, can contribute to eutrophication in aquatic ecosystems. Its high nitrogen content can lead to excessive algal growth, potentially disrupting the balance of aquatic life. However, it is relatively volatile and tends to dissipate quickly in the atmosphere, reducing its long-term environmental persistence.
Sodium hydroxide, on the other hand, does not directly contribute to eutrophication but can have severe localized effects on aquatic ecosystems due to its high alkalinity. When discharged in high concentrations, it can dramatically alter the pH of water bodies, potentially harming or killing aquatic organisms. Unlike ammonium hydroxide, sodium hydroxide is not volatile and persists in the environment until neutralized.
Both compounds can have significant impacts on soil chemistry when released in large quantities. Ammonium hydroxide can temporarily increase soil nitrogen levels, potentially benefiting plant growth in the short term but risking soil acidification over time. Sodium hydroxide can increase soil pH, potentially altering soil structure and affecting plant nutrient availability.
In terms of production and transportation, sodium hydroxide generally has a lower carbon footprint. It is typically produced through the chloralkali process, which has been optimized for energy efficiency over many years. Ammonium hydroxide production, often involving the Haber-Bosch process for ammonia synthesis, tends to be more energy-intensive and thus has a higher associated carbon footprint.
Waste management is another crucial aspect of environmental impact. Sodium hydroxide can be neutralized relatively easily and safely, often with acidic waste streams, making it manageable in industrial settings. Ammonium hydroxide disposal requires more careful handling to prevent ammonia gas release, which can contribute to air pollution and pose health risks.
Water consumption in the production and use of these compounds also differs. Sodium hydroxide production typically requires less water than ammonium hydroxide, which can be a significant factor in water-stressed regions. However, in cleaning applications, the water usage for both compounds can vary depending on the specific process and concentration used.
Considering the broader lifecycle impact, the production of raw materials for both compounds should be evaluated. Sodium hydroxide relies on salt (NaCl) as a primary raw material, which is abundant and relatively easy to extract. Ammonium hydroxide production depends on natural gas for hydrogen production, linking it to fossil fuel extraction and its associated environmental impacts.
Safety Regulations
Safety regulations play a crucial role in the use of ammonium hydroxide and sodium hydroxide for alkaline cleaning processes. Both chemicals are highly corrosive and pose significant risks to human health and the environment if not handled properly. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have established strict guidelines for their use in industrial settings.
For ammonium hydroxide, safety regulations typically require proper ventilation systems to prevent the accumulation of ammonia vapors, which can cause respiratory irritation and, in high concentrations, be potentially fatal. Personal protective equipment (PPE) such as chemical-resistant gloves, goggles, and respiratory protection is mandatory when handling this substance. Storage requirements include keeping ammonium hydroxide in tightly sealed containers in well-ventilated areas, away from heat sources and incompatible materials.
Sodium hydroxide, commonly known as lye or caustic soda, is subject to equally stringent safety regulations. Due to its highly corrosive nature, regulations mandate the use of specialized containment systems and transfer equipment to prevent spills and splashes. Workers must be provided with comprehensive training on proper handling techniques and emergency procedures. Eye wash stations and safety showers must be readily accessible in areas where sodium hydroxide is used or stored.
Both chemicals are subject to transportation regulations set by agencies such as the Department of Transportation (DOT) in the US and the International Maritime Dangerous Goods (IMDG) Code for international shipments. These regulations specify requirements for packaging, labeling, and documentation to ensure safe transport and handling throughout the supply chain.
Environmental regulations also govern the disposal of ammonium hydroxide and sodium hydroxide. Many jurisdictions classify these substances as hazardous waste when disposed of in certain concentrations. Companies must adhere to specific waste management protocols, including proper neutralization before disposal and compliance with local wastewater treatment regulations.
Safety data sheets (SDS) for both chemicals must be readily available and regularly updated in accordance with the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). These documents provide critical information on hazards, handling precautions, and emergency response procedures.
Regulatory compliance also extends to workplace monitoring and exposure limits. OSHA has established Permissible Exposure Limits (PELs) for both ammonia and sodium hydroxide, which employers must ensure are not exceeded through regular air quality monitoring and implementation of engineering controls.
In the context of alkaline cleaning processes, regulations often require the implementation of closed-loop systems or other containment measures to minimize worker exposure and environmental release. Regular safety audits and risk assessments are typically mandated to ensure ongoing compliance with evolving safety standards and best practices in the industry.
For ammonium hydroxide, safety regulations typically require proper ventilation systems to prevent the accumulation of ammonia vapors, which can cause respiratory irritation and, in high concentrations, be potentially fatal. Personal protective equipment (PPE) such as chemical-resistant gloves, goggles, and respiratory protection is mandatory when handling this substance. Storage requirements include keeping ammonium hydroxide in tightly sealed containers in well-ventilated areas, away from heat sources and incompatible materials.
Sodium hydroxide, commonly known as lye or caustic soda, is subject to equally stringent safety regulations. Due to its highly corrosive nature, regulations mandate the use of specialized containment systems and transfer equipment to prevent spills and splashes. Workers must be provided with comprehensive training on proper handling techniques and emergency procedures. Eye wash stations and safety showers must be readily accessible in areas where sodium hydroxide is used or stored.
Both chemicals are subject to transportation regulations set by agencies such as the Department of Transportation (DOT) in the US and the International Maritime Dangerous Goods (IMDG) Code for international shipments. These regulations specify requirements for packaging, labeling, and documentation to ensure safe transport and handling throughout the supply chain.
Environmental regulations also govern the disposal of ammonium hydroxide and sodium hydroxide. Many jurisdictions classify these substances as hazardous waste when disposed of in certain concentrations. Companies must adhere to specific waste management protocols, including proper neutralization before disposal and compliance with local wastewater treatment regulations.
Safety data sheets (SDS) for both chemicals must be readily available and regularly updated in accordance with the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). These documents provide critical information on hazards, handling precautions, and emergency response procedures.
Regulatory compliance also extends to workplace monitoring and exposure limits. OSHA has established Permissible Exposure Limits (PELs) for both ammonia and sodium hydroxide, which employers must ensure are not exceeded through regular air quality monitoring and implementation of engineering controls.
In the context of alkaline cleaning processes, regulations often require the implementation of closed-loop systems or other containment measures to minimize worker exposure and environmental release. Regular safety audits and risk assessments are typically mandated to ensure ongoing compliance with evolving safety standards and best practices in the industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!