Current Collector And Interlayer Choices For Sulfide-Based Pouch Cells
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfide-Based Battery Technology Background and Objectives
Sulfide-based solid-state batteries represent a significant advancement in energy storage technology, emerging as a promising alternative to conventional lithium-ion batteries with liquid electrolytes. The development of these batteries can be traced back to the early 2000s when researchers began exploring solid electrolytes to address safety concerns and energy density limitations of traditional batteries. Over the past decade, sulfide-based solid electrolytes have gained particular attention due to their high ionic conductivity, which approaches that of liquid electrolytes, while offering enhanced safety characteristics.
The technological evolution of sulfide-based batteries has been marked by several breakthrough discoveries, including the development of Li10GeP2S12 (LGPS) in 2011, which demonstrated unprecedented ionic conductivity of 12 mS/cm at room temperature. This milestone catalyzed intensive research into sulfide-based solid electrolytes, leading to the creation of various compositions with improved stability and conductivity properties.
Current research trends focus on addressing key challenges in sulfide-based battery technology, particularly the interface stability between the electrolyte and electrodes, mechanical integrity during cycling, and scalable manufacturing processes. The integration of sulfide electrolytes into pouch cell configurations represents a critical step toward commercialization, with specific attention to current collector and interlayer design to optimize performance and longevity.
The primary technical objectives for sulfide-based pouch cells include developing stable interfaces between the sulfide electrolyte and current collectors, minimizing interfacial resistance, preventing chemical reactions at the interfaces, and ensuring mechanical stability during battery operation. Additionally, researchers aim to design interlayers that can accommodate volume changes during cycling while maintaining excellent ionic conductivity.
From a broader perspective, sulfide-based solid-state batteries aim to achieve energy densities exceeding 400 Wh/kg and 1000 Wh/L, significantly surpassing current lithium-ion technologies. These batteries also target improved safety by eliminating flammable liquid electrolytes, extended cycle life of over 1000 cycles with minimal capacity degradation, and fast charging capabilities that enable 80% charge in less than 15 minutes.
The development trajectory suggests that sulfide-based batteries could potentially revolutionize multiple sectors, including electric vehicles, portable electronics, and grid storage systems. However, realizing these ambitious goals requires overcoming substantial technical hurdles, particularly in the design and optimization of current collectors and interlayers for pouch cell configurations, which represents the focal point of this technical investigation.
The technological evolution of sulfide-based batteries has been marked by several breakthrough discoveries, including the development of Li10GeP2S12 (LGPS) in 2011, which demonstrated unprecedented ionic conductivity of 12 mS/cm at room temperature. This milestone catalyzed intensive research into sulfide-based solid electrolytes, leading to the creation of various compositions with improved stability and conductivity properties.
Current research trends focus on addressing key challenges in sulfide-based battery technology, particularly the interface stability between the electrolyte and electrodes, mechanical integrity during cycling, and scalable manufacturing processes. The integration of sulfide electrolytes into pouch cell configurations represents a critical step toward commercialization, with specific attention to current collector and interlayer design to optimize performance and longevity.
The primary technical objectives for sulfide-based pouch cells include developing stable interfaces between the sulfide electrolyte and current collectors, minimizing interfacial resistance, preventing chemical reactions at the interfaces, and ensuring mechanical stability during battery operation. Additionally, researchers aim to design interlayers that can accommodate volume changes during cycling while maintaining excellent ionic conductivity.
From a broader perspective, sulfide-based solid-state batteries aim to achieve energy densities exceeding 400 Wh/kg and 1000 Wh/L, significantly surpassing current lithium-ion technologies. These batteries also target improved safety by eliminating flammable liquid electrolytes, extended cycle life of over 1000 cycles with minimal capacity degradation, and fast charging capabilities that enable 80% charge in less than 15 minutes.
The development trajectory suggests that sulfide-based batteries could potentially revolutionize multiple sectors, including electric vehicles, portable electronics, and grid storage systems. However, realizing these ambitious goals requires overcoming substantial technical hurdles, particularly in the design and optimization of current collectors and interlayers for pouch cell configurations, which represents the focal point of this technical investigation.
Market Analysis for Solid-State Battery Demand
The global solid-state battery market is experiencing unprecedented growth, driven by increasing demand for high-performance energy storage solutions across multiple sectors. Current projections indicate the market will reach approximately $6.5 billion by 2026, with a compound annual growth rate exceeding 30% between 2021 and 2026. This remarkable expansion is particularly relevant for sulfide-based solid-state batteries, which are emerging as a promising subset within this technology space.
Automotive applications represent the largest demand segment, accounting for over 45% of the projected market. Major automotive manufacturers have announced significant investments in solid-state battery technology, with several planning commercial vehicle launches featuring solid-state batteries between 2025 and 2030. The push toward electric vehicles with longer ranges, faster charging capabilities, and enhanced safety profiles is creating substantial pull for sulfide-based systems specifically.
Consumer electronics constitutes the second-largest market segment, representing approximately 30% of demand. Manufacturers are seeking higher energy density solutions that can enable slimmer device profiles while extending battery life. Sulfide-based solid-state batteries offer compelling advantages in this space due to their potential for higher energy density compared to conventional lithium-ion batteries.
Geographically, Asia-Pacific leads the market with Japan, South Korea, and China at the forefront of both research and commercialization efforts. North America and Europe follow closely, with significant research initiatives and strategic investments from both government and private sectors. The regional distribution of demand correlates strongly with centers of automotive and electronics manufacturing.
Market analysis reveals several key drivers accelerating demand for sulfide-based solid-state batteries. Safety concerns with conventional lithium-ion batteries remain paramount, particularly following high-profile thermal runaway incidents. The superior thermal stability of solid electrolytes addresses this critical market need. Additionally, the potential for higher energy density (potentially 2-3 times current lithium-ion batteries) aligns perfectly with consumer and industry demands for longer-lasting power sources.
Supply chain considerations are increasingly influencing market dynamics. The reduced reliance on critical materials like cobalt presents an attractive proposition for manufacturers concerned with supply security and ethical sourcing. However, challenges in scaling production of high-quality sulfide electrolytes currently limit market penetration, creating opportunities for companies that can solve manufacturing challenges related to current collectors and interlayers in pouch cell configurations.
Automotive applications represent the largest demand segment, accounting for over 45% of the projected market. Major automotive manufacturers have announced significant investments in solid-state battery technology, with several planning commercial vehicle launches featuring solid-state batteries between 2025 and 2030. The push toward electric vehicles with longer ranges, faster charging capabilities, and enhanced safety profiles is creating substantial pull for sulfide-based systems specifically.
Consumer electronics constitutes the second-largest market segment, representing approximately 30% of demand. Manufacturers are seeking higher energy density solutions that can enable slimmer device profiles while extending battery life. Sulfide-based solid-state batteries offer compelling advantages in this space due to their potential for higher energy density compared to conventional lithium-ion batteries.
Geographically, Asia-Pacific leads the market with Japan, South Korea, and China at the forefront of both research and commercialization efforts. North America and Europe follow closely, with significant research initiatives and strategic investments from both government and private sectors. The regional distribution of demand correlates strongly with centers of automotive and electronics manufacturing.
Market analysis reveals several key drivers accelerating demand for sulfide-based solid-state batteries. Safety concerns with conventional lithium-ion batteries remain paramount, particularly following high-profile thermal runaway incidents. The superior thermal stability of solid electrolytes addresses this critical market need. Additionally, the potential for higher energy density (potentially 2-3 times current lithium-ion batteries) aligns perfectly with consumer and industry demands for longer-lasting power sources.
Supply chain considerations are increasingly influencing market dynamics. The reduced reliance on critical materials like cobalt presents an attractive proposition for manufacturers concerned with supply security and ethical sourcing. However, challenges in scaling production of high-quality sulfide electrolytes currently limit market penetration, creating opportunities for companies that can solve manufacturing challenges related to current collectors and interlayers in pouch cell configurations.
Current Collector Technology Status and Challenges
The current collector technology for sulfide-based pouch cells faces significant challenges despite recent advancements. Traditional current collectors made of aluminum and copper, widely used in conventional lithium-ion batteries, exhibit compatibility issues with sulfide solid electrolytes. The high reactivity between aluminum and sulfide electrolytes leads to interfacial degradation, while copper experiences corrosion when exposed to sulfur-containing compounds, compromising long-term cell performance.
Global research efforts have focused on developing alternative current collector materials and protective coatings. Carbon-based current collectors, including carbon-coated aluminum foils and graphene-enhanced substrates, have shown promising results in laboratory settings. These materials offer reduced interfacial resistance and enhanced chemical stability. However, their mass production remains challenging due to cost constraints and scalability issues.
Nickel and nickel alloys represent another promising direction, demonstrating superior chemical stability with sulfide electrolytes compared to conventional materials. Research institutions in South Korea and Japan have pioneered the development of nickel-based current collectors with specialized surface treatments to enhance adhesion properties with sulfide electrolytes.
A major technical hurdle involves balancing electrical conductivity with chemical stability. While noble metals like gold and platinum exhibit excellent chemical compatibility with sulfide electrolytes, their prohibitive cost limits commercial viability. This has driven research toward composite structures and multi-layered designs that combine cost-effective base materials with thin protective layers.
The geographical distribution of current collector technology development shows concentration in East Asia, particularly Japan, South Korea, and China, where major battery manufacturers have established dedicated research facilities. European research institutions focus more on fundamental understanding of interfacial phenomena, while North American efforts emphasize innovative material solutions through startup ventures.
Manufacturing scalability presents another significant challenge. Laboratory-scale demonstrations of novel current collector technologies often fail to translate to mass production environments. Issues related to uniform coating deposition, adhesion strength, and process integration with existing manufacturing lines limit commercial adoption of promising technologies.
Recent advancements in atomic layer deposition and plasma-enhanced coating techniques have improved the feasibility of applying ultrathin protective layers to conventional current collectors, potentially offering a near-term solution that balances performance requirements with manufacturing practicality. However, these approaches still require optimization for cost-effectiveness in high-volume production scenarios.
Global research efforts have focused on developing alternative current collector materials and protective coatings. Carbon-based current collectors, including carbon-coated aluminum foils and graphene-enhanced substrates, have shown promising results in laboratory settings. These materials offer reduced interfacial resistance and enhanced chemical stability. However, their mass production remains challenging due to cost constraints and scalability issues.
Nickel and nickel alloys represent another promising direction, demonstrating superior chemical stability with sulfide electrolytes compared to conventional materials. Research institutions in South Korea and Japan have pioneered the development of nickel-based current collectors with specialized surface treatments to enhance adhesion properties with sulfide electrolytes.
A major technical hurdle involves balancing electrical conductivity with chemical stability. While noble metals like gold and platinum exhibit excellent chemical compatibility with sulfide electrolytes, their prohibitive cost limits commercial viability. This has driven research toward composite structures and multi-layered designs that combine cost-effective base materials with thin protective layers.
The geographical distribution of current collector technology development shows concentration in East Asia, particularly Japan, South Korea, and China, where major battery manufacturers have established dedicated research facilities. European research institutions focus more on fundamental understanding of interfacial phenomena, while North American efforts emphasize innovative material solutions through startup ventures.
Manufacturing scalability presents another significant challenge. Laboratory-scale demonstrations of novel current collector technologies often fail to translate to mass production environments. Issues related to uniform coating deposition, adhesion strength, and process integration with existing manufacturing lines limit commercial adoption of promising technologies.
Recent advancements in atomic layer deposition and plasma-enhanced coating techniques have improved the feasibility of applying ultrathin protective layers to conventional current collectors, potentially offering a near-term solution that balances performance requirements with manufacturing practicality. However, these approaches still require optimization for cost-effectiveness in high-volume production scenarios.
Current Interlayer Solutions for Sulfide-Based Pouch Cells
01 Current collector materials for sulfide-based pouch cells
Various materials can be used as current collectors in sulfide-based pouch cells to enhance performance and stability. These materials include aluminum, copper, nickel, and their alloys, which offer different advantages in terms of conductivity, corrosion resistance, and compatibility with sulfide electrolytes. The selection of appropriate current collector materials is crucial for preventing unwanted reactions at the electrode-electrolyte interface and ensuring long-term cell performance.- Current collector materials for sulfide-based pouch cells: Various materials can be used as current collectors in sulfide-based pouch cells to enhance performance and stability. These materials include aluminum, copper, nickel, and carbon-coated metals that offer good electrical conductivity while minimizing reactions with sulfide electrolytes. The selection of appropriate current collector materials is crucial for preventing degradation at the electrode-electrolyte interface and improving the overall cycle life of the battery.
- Protective interlayers for sulfide electrolyte interfaces: Protective interlayers are employed between the electrode materials and sulfide solid electrolytes to prevent unwanted side reactions and improve interface stability. These interlayers can be composed of oxide materials, polymers, or composite structures that act as buffer zones while maintaining ionic conductivity. The implementation of these protective layers significantly reduces interfacial resistance and enhances the electrochemical performance of sulfide-based pouch cells.
- Manufacturing techniques for sulfide-based pouch cell assemblies: Specialized manufacturing techniques are essential for the successful assembly of sulfide-based pouch cells. These include dry-room processing, pressure-assisted sintering, and novel lamination methods that prevent moisture contamination and ensure good contact between cell components. Advanced manufacturing approaches help to create uniform interfaces between the current collectors, active materials, and electrolytes, which is critical for achieving optimal cell performance.
- Interface engineering for reduced resistance in sulfide systems: Interface engineering strategies are employed to reduce contact resistance between sulfide electrolytes and current collectors. These approaches include surface modification of current collectors, application of conductive coatings, and creation of gradient interfaces that facilitate ion transport. By minimizing interfacial resistance, these engineering solutions improve power density and rate capability of sulfide-based pouch cells.
- Novel electrode architectures for sulfide-based battery systems: Innovative electrode architectures are being developed specifically for sulfide-based battery systems. These designs incorporate 3D structures, porous frameworks, and composite electrodes that maximize active material utilization while accommodating volume changes during cycling. The advanced architectures enable better integration with current collectors and interlayers, resulting in improved energy density and cycle stability of sulfide-based pouch cells.
02 Interlayer designs for sulfide solid electrolyte batteries
Interlayers play a critical role in sulfide-based pouch cells by mitigating interfacial resistance and preventing chemical reactions between the electrodes and sulfide electrolytes. These interlayers can be composed of various materials including oxides, polymers, or composite structures that act as buffer zones. Properly designed interlayers can significantly improve ionic conductivity, mechanical stability, and cycle life of sulfide-based batteries while reducing degradation mechanisms.Expand Specific Solutions03 Surface modification techniques for current collectors
Surface modification of current collectors in sulfide-based pouch cells can significantly improve electrode-electrolyte interface stability. Techniques include coating with protective layers, surface roughening to enhance adhesion, and chemical treatments to create favorable surface properties. These modifications help prevent side reactions, reduce interfacial resistance, and improve the overall electrochemical performance of the battery by creating more stable interfaces between the current collector and active materials.Expand Specific Solutions04 Composite interlayers for interface stabilization
Composite interlayers combining multiple functional materials can effectively address the challenges at electrode-electrolyte interfaces in sulfide-based pouch cells. These composite structures may incorporate inorganic particles dispersed in polymer matrices, gradient compositions, or multilayer architectures. Such designs can simultaneously provide mechanical support, facilitate ion transport, block electron transfer, and prevent chemical cross-reactions, resulting in improved cycling stability and rate capability of the battery.Expand Specific Solutions05 Manufacturing processes for pouch cell assembly with sulfide electrolytes
Specialized manufacturing processes are required for assembling sulfide-based pouch cells due to the moisture sensitivity and mechanical properties of sulfide electrolytes. These processes include dry room assembly, hot pressing techniques for improved interfacial contact, and novel sealing methods to ensure cell integrity. Proper alignment and stacking of current collectors, electrodes, and interlayers during assembly are critical for achieving uniform performance and preventing short circuits in the finished pouch cells.Expand Specific Solutions
Leading Companies in Sulfide-Based Battery Development
The sulfide-based pouch cell market is currently in an early growth phase, characterized by increasing R&D investments as the industry transitions toward solid-state battery technologies. The market size is projected to expand significantly as automotive manufacturers like GM, Toyota, Nissan, and Renault accelerate electrification strategies. Technical maturity varies across players, with established battery manufacturers such as LG Energy Solution, CATL, and Sunwoda leading in current collector and interlayer development. Research-focused entities like Toyota, Johnson Matthey, and university collaborations are advancing innovative materials solutions. Automotive OEMs are strategically partnering with technology specialists, while companies like Sakuu are exploring novel manufacturing approaches. The competitive landscape reflects a balance between traditional battery manufacturers optimizing existing technologies and research-oriented organizations pursuing breakthrough solutions.
GM Global Technology Operations LLC
Technical Solution: GM has developed a comprehensive current collector and interlayer system specifically engineered for sulfide-based solid-state batteries in pouch format. Their solution features aluminum current collectors with a proprietary surface treatment that enhances adhesion while preventing corrosion from the reactive sulfide components. The company's multi-functional interlayer design incorporates a gradient structure with carbon-rich components near the current collector transitioning to sulfide-compatible materials at the electrolyte interface. This approach effectively manages the chemical and electrochemical stability issues at the critical interfaces while maintaining excellent electronic conductivity. GM's technology utilizes specialized polymer binders in the interlayer that accommodate volume changes during cycling while maintaining intimate contact between components. Their manufacturing process includes precise control of pressure distribution during cell assembly to ensure uniform contact across the large-format pouch cells, addressing one of the key challenges in solid-state battery production. The company has also implemented edge protection technologies that prevent moisture ingress and electrolyte degradation at the cell periphery, which is particularly important for sulfide-based systems known for their moisture sensitivity.
Strengths: Excellent scalability for automotive-scale production; superior pressure distribution management for large-format cells; effective moisture protection system for the highly sensitive sulfide components. Weaknesses: Relatively complex manufacturing process requiring specialized equipment; potential challenges with long-term cycling at elevated temperatures; higher initial costs compared to conventional lithium-ion technology.
Toyota Motor Corp.
Technical Solution: Toyota has developed an innovative current collector and interlayer system for sulfide-based solid-state batteries that addresses the key challenges of chemical compatibility and interfacial resistance. Their approach utilizes a carbon-coated aluminum current collector with precisely controlled surface roughness to optimize contact with the interlayer materials. Toyota's proprietary interlayer consists of a gradient structure with varying compositions that transition from highly electronically conductive materials near the current collector to more ionically conductive and sulfide-compatible materials at the electrolyte interface. This design minimizes interfacial resistance while preventing direct contact between the reactive sulfide electrolyte and the metal current collector. The company has implemented specialized coating techniques that ensure uniform coverage and strong adhesion between layers, critical for maintaining performance during thermal and mechanical stresses experienced in automotive applications. Toyota's solution also incorporates specific additives in the interlayer that act as sacrificial agents to scavenge trace moisture, protecting the moisture-sensitive sulfide electrolyte from degradation during cell assembly and operation.
Strengths: Excellent thermal stability suitable for automotive temperature ranges; superior mechanical integrity during cycling; effective protection against moisture-induced degradation of sulfide electrolytes. Weaknesses: Complex manufacturing process requiring precise control of multiple material interfaces; potentially higher cost compared to conventional lithium-ion battery components; challenges in achieving consistent quality at scale.
Key Innovations in Current Collector-Electrolyte Interfaces
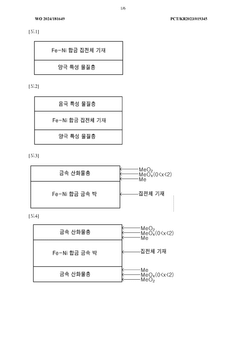
Current collector having anodic, cathodic, or both anodic and cathodic properties and secondary battery comprising same
PatentWO2024181649A1
Innovation
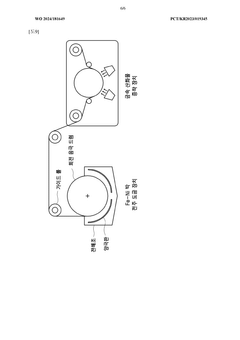
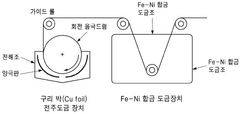
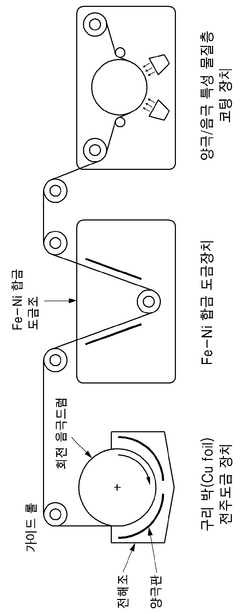
- A current collector made from an Fe-Ni alloy with a metal oxide layer, manufactured through electroplating, offering excellent corrosion resistance and high strength, allowing for the use as both negative and positive electrode collectors, and reducing the amount of active material needed.
Fe-ni alloy current collector having low resistance multilayer structure and secondary battery comprising same
PatentWO2025105762A1
Innovation
- A low-resistance multilayer Fe-Ni alloy current collector is developed, featuring a copper foil with Fe-Ni alloy layers on both sides, which provides excellent corrosion resistance and bipolar characteristics, enabling both negative and positive electrode functionality.
Safety and Stability Considerations for Sulfide Electrolytes
Safety considerations for sulfide-based solid electrolytes are paramount due to their inherent reactivity with moisture and air. When exposed to humidity, these materials undergo hydrolysis reactions that generate toxic hydrogen sulfide (H2S) gas, posing significant health hazards in manufacturing environments and potential risks during cell operation if packaging integrity is compromised. This reactivity necessitates stringent handling protocols, including dry room or glove box processing with moisture levels maintained below 100 ppm.
The chemical stability of sulfide electrolytes presents another critical challenge, particularly at the electrolyte-electrode interfaces. Many sulfide systems exhibit narrow electrochemical stability windows (typically 1.7-2.5V), leading to decomposition reactions at both high and low potentials. At the cathode interface, oxidative decomposition forms insulating layers that increase interfacial resistance, while reductive decomposition at the anode interface can form mixed ionic-electronic conducting layers that compromise long-term cycling performance.
Thermal stability concerns also affect sulfide electrolyte implementation in pouch cell configurations. Most sulfide systems demonstrate phase transitions or decomposition at elevated temperatures (typically above 200-250°C), which can trigger thermal runaway events. The thermal expansion coefficient mismatch between sulfide electrolytes and electrode materials introduces additional mechanical stress during temperature fluctuations, potentially creating micro-cracks that serve as failure initiation points.
Mechanical stability represents another significant consideration for pouch cell designs. Sulfide electrolytes exhibit relatively low fracture toughness compared to polymer or liquid electrolytes, making them susceptible to cracking during cell assembly and operation. The volume changes associated with electrode materials during cycling (particularly silicon or sulfur electrodes) can induce mechanical stress on the electrolyte layer, leading to progressive degradation of ionic pathways and capacity fade.
Interface engineering strategies have emerged as essential approaches to mitigate these safety and stability concerns. Protective coatings on current collectors, such as Al2O3 or LiNbO3 layers, can prevent direct contact between reactive sulfide electrolytes and metal surfaces. Buffer layers composed of more stable solid electrolytes or artificial SEI formulations help isolate the sulfide electrolyte from extreme potential regions, extending the effective electrochemical stability window and improving cycling performance.
The chemical stability of sulfide electrolytes presents another critical challenge, particularly at the electrolyte-electrode interfaces. Many sulfide systems exhibit narrow electrochemical stability windows (typically 1.7-2.5V), leading to decomposition reactions at both high and low potentials. At the cathode interface, oxidative decomposition forms insulating layers that increase interfacial resistance, while reductive decomposition at the anode interface can form mixed ionic-electronic conducting layers that compromise long-term cycling performance.
Thermal stability concerns also affect sulfide electrolyte implementation in pouch cell configurations. Most sulfide systems demonstrate phase transitions or decomposition at elevated temperatures (typically above 200-250°C), which can trigger thermal runaway events. The thermal expansion coefficient mismatch between sulfide electrolytes and electrode materials introduces additional mechanical stress during temperature fluctuations, potentially creating micro-cracks that serve as failure initiation points.
Mechanical stability represents another significant consideration for pouch cell designs. Sulfide electrolytes exhibit relatively low fracture toughness compared to polymer or liquid electrolytes, making them susceptible to cracking during cell assembly and operation. The volume changes associated with electrode materials during cycling (particularly silicon or sulfur electrodes) can induce mechanical stress on the electrolyte layer, leading to progressive degradation of ionic pathways and capacity fade.
Interface engineering strategies have emerged as essential approaches to mitigate these safety and stability concerns. Protective coatings on current collectors, such as Al2O3 or LiNbO3 layers, can prevent direct contact between reactive sulfide electrolytes and metal surfaces. Buffer layers composed of more stable solid electrolytes or artificial SEI formulations help isolate the sulfide electrolyte from extreme potential regions, extending the effective electrochemical stability window and improving cycling performance.
Manufacturing Scalability of Sulfide-Based Pouch Cells
The scalability of manufacturing processes for sulfide-based pouch cells represents a critical challenge in their commercial viability. Current production methods for these cells remain predominantly laboratory-scale, with significant barriers to industrial-scale manufacturing that must be addressed to enable mass market adoption.
Material handling presents the foremost challenge, as sulfide solid electrolytes are highly sensitive to moisture and air. Manufacturing facilities require specialized dry rooms with humidity levels below 100 ppm or inert atmosphere gloveboxes, substantially increasing production costs and complexity compared to conventional lithium-ion battery manufacturing.
The interface formation between current collectors and sulfide electrolytes demands precise control during scale-up. Traditional aluminum and copper current collectors may form resistive interfaces with sulfide electrolytes, necessitating specialized coatings or interlayers that must be applied uniformly across large-area electrodes—a process that becomes increasingly difficult at industrial scales.
Equipment compatibility poses another significant hurdle. Conventional battery manufacturing equipment requires substantial modification to accommodate the unique processing requirements of sulfide electrolytes. The pressure-sensitive nature of these materials demands carefully calibrated calendering and stacking processes that differ markedly from liquid electrolyte systems.
Throughput optimization remains underdeveloped, with current laboratory processes requiring extended mixing, pressing, and annealing times that are incompatible with high-volume production targets. Industry standards suggest that cycle times must be reduced by at least 70% to achieve economically viable production rates.
Quality control methodologies for sulfide-based cells are still evolving. Non-destructive testing techniques capable of detecting internal short circuits, electrolyte homogeneity, and interface quality at production speeds have yet to be fully developed, creating a significant gap in manufacturing readiness.
Cost considerations further complicate scalability. Current production costs for sulfide electrolytes exceed $1000/kg, compared to approximately $15/kg for liquid electrolytes. While economies of scale will reduce this disparity, significant process innovations and supply chain development are required to approach cost parity.
Recent pilot-scale demonstrations by companies like Toyota and Solid Power have shown promising results, achieving production rates of hundreds of cells per month. However, true gigafactory-scale production, requiring millions of cells annually, remains several years from realization without breakthrough advances in manufacturing technology.
Material handling presents the foremost challenge, as sulfide solid electrolytes are highly sensitive to moisture and air. Manufacturing facilities require specialized dry rooms with humidity levels below 100 ppm or inert atmosphere gloveboxes, substantially increasing production costs and complexity compared to conventional lithium-ion battery manufacturing.
The interface formation between current collectors and sulfide electrolytes demands precise control during scale-up. Traditional aluminum and copper current collectors may form resistive interfaces with sulfide electrolytes, necessitating specialized coatings or interlayers that must be applied uniformly across large-area electrodes—a process that becomes increasingly difficult at industrial scales.
Equipment compatibility poses another significant hurdle. Conventional battery manufacturing equipment requires substantial modification to accommodate the unique processing requirements of sulfide electrolytes. The pressure-sensitive nature of these materials demands carefully calibrated calendering and stacking processes that differ markedly from liquid electrolyte systems.
Throughput optimization remains underdeveloped, with current laboratory processes requiring extended mixing, pressing, and annealing times that are incompatible with high-volume production targets. Industry standards suggest that cycle times must be reduced by at least 70% to achieve economically viable production rates.
Quality control methodologies for sulfide-based cells are still evolving. Non-destructive testing techniques capable of detecting internal short circuits, electrolyte homogeneity, and interface quality at production speeds have yet to be fully developed, creating a significant gap in manufacturing readiness.
Cost considerations further complicate scalability. Current production costs for sulfide electrolytes exceed $1000/kg, compared to approximately $15/kg for liquid electrolytes. While economies of scale will reduce this disparity, significant process innovations and supply chain development are required to approach cost parity.
Recent pilot-scale demonstrations by companies like Toyota and Solid Power have shown promising results, achieving production rates of hundreds of cells per month. However, true gigafactory-scale production, requiring millions of cells annually, remains several years from realization without breakthrough advances in manufacturing technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!