Grain Boundary Engineering To Maximize Sulfide Ionic Conductivity
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfide Solid Electrolyte Development Background and Objectives
Sulfide solid electrolytes have emerged as promising candidates for next-generation all-solid-state batteries due to their superior ionic conductivity compared to oxide-based counterparts. The development of these materials traces back to the 1980s when researchers first identified their potential for energy storage applications. Over the past four decades, significant advancements have been made in understanding and enhancing their fundamental properties, particularly their ionic conductivity mechanisms.
The evolution of sulfide solid electrolytes has been marked by several breakthrough discoveries, including the identification of thio-LISICON structures in the early 2000s and the more recent development of argyrodite-type materials. These milestones have progressively pushed ionic conductivity values from the range of 10^-5 S/cm to exceeding 10^-2 S/cm, approaching or even surpassing the conductivity of liquid electrolytes used in conventional lithium-ion batteries.
Despite these achievements, the technical objective remains clear: to develop sulfide solid electrolytes with consistently high ionic conductivity, improved electrochemical stability, and enhanced mechanical properties suitable for commercial battery applications. Particularly, understanding and engineering grain boundaries has become a critical focus area, as these interfaces often act as bottlenecks for ion transport, significantly reducing the overall conductivity of polycrystalline materials.
The primary goal of grain boundary engineering in sulfide electrolytes is to maximize ionic conductivity by minimizing resistance at these interfaces. This involves controlling the microstructure, composition, and crystallographic orientation of grains to create favorable pathways for ion migration. Additionally, reducing impurities and secondary phases at grain boundaries is essential for maintaining high conductivity across the entire material.
Current research objectives include developing scalable synthesis methods that can precisely control grain boundary characteristics, establishing comprehensive models to predict ion transport behavior at interfaces, and creating innovative doping strategies to modify grain boundary properties. These efforts aim to bridge the gap between laboratory demonstrations and practical implementation in commercial energy storage systems.
Furthermore, the development of sulfide solid electrolytes must address safety concerns related to their reactivity with moisture and air, as well as mechanical stability issues during battery cycling. The ultimate technical target is to create sulfide electrolytes that not only exhibit high ionic conductivity but also demonstrate long-term stability, compatibility with electrode materials, and feasibility for large-scale manufacturing processes.
As the field progresses, interdisciplinary approaches combining materials science, electrochemistry, and advanced characterization techniques will be crucial for overcoming the remaining challenges and realizing the full potential of sulfide solid electrolytes in next-generation energy storage technologies.
The evolution of sulfide solid electrolytes has been marked by several breakthrough discoveries, including the identification of thio-LISICON structures in the early 2000s and the more recent development of argyrodite-type materials. These milestones have progressively pushed ionic conductivity values from the range of 10^-5 S/cm to exceeding 10^-2 S/cm, approaching or even surpassing the conductivity of liquid electrolytes used in conventional lithium-ion batteries.
Despite these achievements, the technical objective remains clear: to develop sulfide solid electrolytes with consistently high ionic conductivity, improved electrochemical stability, and enhanced mechanical properties suitable for commercial battery applications. Particularly, understanding and engineering grain boundaries has become a critical focus area, as these interfaces often act as bottlenecks for ion transport, significantly reducing the overall conductivity of polycrystalline materials.
The primary goal of grain boundary engineering in sulfide electrolytes is to maximize ionic conductivity by minimizing resistance at these interfaces. This involves controlling the microstructure, composition, and crystallographic orientation of grains to create favorable pathways for ion migration. Additionally, reducing impurities and secondary phases at grain boundaries is essential for maintaining high conductivity across the entire material.
Current research objectives include developing scalable synthesis methods that can precisely control grain boundary characteristics, establishing comprehensive models to predict ion transport behavior at interfaces, and creating innovative doping strategies to modify grain boundary properties. These efforts aim to bridge the gap between laboratory demonstrations and practical implementation in commercial energy storage systems.
Furthermore, the development of sulfide solid electrolytes must address safety concerns related to their reactivity with moisture and air, as well as mechanical stability issues during battery cycling. The ultimate technical target is to create sulfide electrolytes that not only exhibit high ionic conductivity but also demonstrate long-term stability, compatibility with electrode materials, and feasibility for large-scale manufacturing processes.
As the field progresses, interdisciplinary approaches combining materials science, electrochemistry, and advanced characterization techniques will be crucial for overcoming the remaining challenges and realizing the full potential of sulfide solid electrolytes in next-generation energy storage technologies.
Market Analysis for High-Performance Solid-State Batteries
The solid-state battery market is experiencing unprecedented growth, driven by increasing demand for safer, higher energy density power solutions across multiple industries. Current market valuations place the global solid-state battery sector at approximately $500 million in 2023, with projections indicating a compound annual growth rate of 34.2% through 2030, potentially reaching a market value of $3.4 billion. This remarkable growth trajectory is primarily fueled by the automotive sector's aggressive transition toward electrification.
Within this expanding market, sulfide-based solid electrolytes represent a particularly promising segment due to their superior ionic conductivity compared to oxide and polymer alternatives. These materials demonstrate conductivity values approaching 10 mS/cm at room temperature, comparable to liquid electrolytes but without the associated safety risks. Market research indicates that sulfide electrolytes could capture up to 40% of the solid-state electrolyte market by 2028, representing a significant commercial opportunity.
The automotive industry remains the primary demand driver, with major manufacturers including Toyota, BMW, and Volkswagen investing heavily in sulfide-based solid-state battery technology. These companies recognize the potential for sulfide electrolytes with engineered grain boundaries to enable fast-charging capabilities and extended range in electric vehicles, addressing key consumer concerns that currently limit EV adoption.
Consumer electronics represents the second largest market segment, with manufacturers seeking higher energy density and improved safety profiles for next-generation devices. The wearable technology sector specifically shows strong demand potential, as miniaturized solid-state batteries with optimized grain boundary engineering could deliver both the form factor and performance requirements of advanced wearable devices.
Market analysis reveals significant regional variations in adoption patterns. Asia-Pacific currently dominates both production and consumption of advanced battery technologies, with Japan and South Korea leading research efforts in sulfide-based solid electrolytes. North America and Europe are rapidly expanding their research and manufacturing capabilities, driven by governmental initiatives to secure domestic battery supply chains.
Customer surveys indicate that manufacturers are willing to pay premium prices for solid electrolytes that demonstrate ionic conductivity improvements of 30% or greater through grain boundary engineering techniques. This price elasticity creates substantial market opportunities for companies that can successfully commercialize advanced sulfide electrolytes with optimized grain boundary structures.
The market landscape remains highly competitive, with both established battery manufacturers and specialized startups pursuing grain boundary engineering approaches. Recent venture capital investments in this specific technology area exceeded $800 million in 2022, highlighting strong investor confidence in the commercial potential of these advanced materials.
Within this expanding market, sulfide-based solid electrolytes represent a particularly promising segment due to their superior ionic conductivity compared to oxide and polymer alternatives. These materials demonstrate conductivity values approaching 10 mS/cm at room temperature, comparable to liquid electrolytes but without the associated safety risks. Market research indicates that sulfide electrolytes could capture up to 40% of the solid-state electrolyte market by 2028, representing a significant commercial opportunity.
The automotive industry remains the primary demand driver, with major manufacturers including Toyota, BMW, and Volkswagen investing heavily in sulfide-based solid-state battery technology. These companies recognize the potential for sulfide electrolytes with engineered grain boundaries to enable fast-charging capabilities and extended range in electric vehicles, addressing key consumer concerns that currently limit EV adoption.
Consumer electronics represents the second largest market segment, with manufacturers seeking higher energy density and improved safety profiles for next-generation devices. The wearable technology sector specifically shows strong demand potential, as miniaturized solid-state batteries with optimized grain boundary engineering could deliver both the form factor and performance requirements of advanced wearable devices.
Market analysis reveals significant regional variations in adoption patterns. Asia-Pacific currently dominates both production and consumption of advanced battery technologies, with Japan and South Korea leading research efforts in sulfide-based solid electrolytes. North America and Europe are rapidly expanding their research and manufacturing capabilities, driven by governmental initiatives to secure domestic battery supply chains.
Customer surveys indicate that manufacturers are willing to pay premium prices for solid electrolytes that demonstrate ionic conductivity improvements of 30% or greater through grain boundary engineering techniques. This price elasticity creates substantial market opportunities for companies that can successfully commercialize advanced sulfide electrolytes with optimized grain boundary structures.
The market landscape remains highly competitive, with both established battery manufacturers and specialized startups pursuing grain boundary engineering approaches. Recent venture capital investments in this specific technology area exceeded $800 million in 2022, highlighting strong investor confidence in the commercial potential of these advanced materials.
Current Challenges in Sulfide Ionic Conductivity Enhancement
Despite significant advancements in sulfide solid electrolytes for all-solid-state batteries, several critical challenges continue to impede the maximization of ionic conductivity through grain boundary engineering. The inherent chemical instability of sulfide materials presents a fundamental obstacle, as these compounds are highly sensitive to moisture and oxygen, often decomposing upon exposure to ambient conditions. This reactivity complicates both research methodologies and practical implementation, requiring specialized handling environments and manufacturing processes.
The mechanical properties of sulfide electrolytes pose another significant challenge. These materials typically exhibit brittle behavior and poor mechanical compliance, leading to contact loss and increased interfacial resistance during battery cycling. The volume changes that occur during lithium insertion and extraction further exacerbate these issues, creating microcracks and degrading grain boundary integrity over time.
Grain boundary resistance remains perhaps the most direct impediment to achieving optimal ionic conductivity. In sulfide solid electrolytes, grain boundaries often act as bottlenecks for ion transport, with conductivity at these interfaces typically orders of magnitude lower than bulk conductivity. This phenomenon stems from multiple factors including space charge effects, impurity segregation, and structural discontinuities between adjacent grains.
The complex phase behavior of many sulfide systems presents additional complications. Phase transitions during processing or operation can dramatically alter grain boundary properties, while secondary phases that precipitate at grain boundaries frequently possess poor ionic conductivity characteristics, further impeding ion transport across these critical interfaces.
Scalable manufacturing represents another substantial hurdle. Current laboratory techniques for grain boundary engineering, such as controlled sintering or dopant segregation, often prove difficult to scale to industrial production levels. The precise control required for optimal grain boundary properties is challenging to maintain in large-scale manufacturing environments.
Characterization limitations further complicate progress in this field. The nanoscale nature of grain boundaries makes direct observation and analysis challenging, while the high sensitivity of sulfide materials to electron beam damage restricts the application of many advanced microscopy techniques. This creates significant barriers to understanding the fundamental mechanisms governing ionic transport at grain boundaries.
Finally, computational modeling of grain boundary phenomena in sulfide electrolytes remains underdeveloped. The complex interplay of structural, chemical, and electronic factors at these interfaces exceeds the capabilities of many current simulation approaches, limiting the ability to predict and design optimal grain boundary configurations for enhanced ionic conductivity.
The mechanical properties of sulfide electrolytes pose another significant challenge. These materials typically exhibit brittle behavior and poor mechanical compliance, leading to contact loss and increased interfacial resistance during battery cycling. The volume changes that occur during lithium insertion and extraction further exacerbate these issues, creating microcracks and degrading grain boundary integrity over time.
Grain boundary resistance remains perhaps the most direct impediment to achieving optimal ionic conductivity. In sulfide solid electrolytes, grain boundaries often act as bottlenecks for ion transport, with conductivity at these interfaces typically orders of magnitude lower than bulk conductivity. This phenomenon stems from multiple factors including space charge effects, impurity segregation, and structural discontinuities between adjacent grains.
The complex phase behavior of many sulfide systems presents additional complications. Phase transitions during processing or operation can dramatically alter grain boundary properties, while secondary phases that precipitate at grain boundaries frequently possess poor ionic conductivity characteristics, further impeding ion transport across these critical interfaces.
Scalable manufacturing represents another substantial hurdle. Current laboratory techniques for grain boundary engineering, such as controlled sintering or dopant segregation, often prove difficult to scale to industrial production levels. The precise control required for optimal grain boundary properties is challenging to maintain in large-scale manufacturing environments.
Characterization limitations further complicate progress in this field. The nanoscale nature of grain boundaries makes direct observation and analysis challenging, while the high sensitivity of sulfide materials to electron beam damage restricts the application of many advanced microscopy techniques. This creates significant barriers to understanding the fundamental mechanisms governing ionic transport at grain boundaries.
Finally, computational modeling of grain boundary phenomena in sulfide electrolytes remains underdeveloped. The complex interplay of structural, chemical, and electronic factors at these interfaces exceeds the capabilities of many current simulation approaches, limiting the ability to predict and design optimal grain boundary configurations for enhanced ionic conductivity.
Current Approaches to Sulfide Grain Boundary Modification
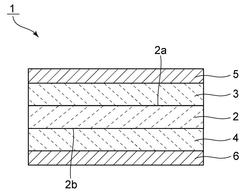
01 Composition of sulfide solid electrolytes
Sulfide solid electrolytes can be composed of various materials to enhance ionic conductivity. These compositions typically include lithium-containing sulfides such as Li2S combined with other elements like phosphorus, germanium, or silicon to form structures like Li3PS4, Li10GeP2S12, or Li6PS5Cl. The specific composition and crystal structure significantly influence the ionic conductivity properties, with certain atomic arrangements facilitating faster lithium ion movement through the electrolyte matrix.- Composition of sulfide solid electrolytes: Sulfide solid electrolytes can be composed of various materials to enhance ionic conductivity. These compositions typically include lithium sulfide (Li2S) as a base material combined with other sulfides such as phosphorus sulfide (P2S5), germanium sulfide (GeS2), or silicon sulfide (SiS2). The specific composition ratios significantly affect the ionic conductivity properties, with certain formulations achieving superior performance for lithium-ion transport in solid-state batteries.
- Doping strategies for improved conductivity: Doping sulfide solid electrolytes with specific elements or compounds can significantly enhance their ionic conductivity. Common dopants include halides (such as LiI, LiBr, LiCl), oxides, and other ionic compounds. These dopants can modify the crystal structure, create additional lithium vacancies, or form conductive interfaces that facilitate faster ion transport. Strategic doping has been shown to increase room temperature conductivity by orders of magnitude compared to undoped sulfide electrolytes.
- Synthesis methods affecting conductivity: The method used to synthesize sulfide solid electrolytes significantly impacts their ionic conductivity. Common synthesis approaches include mechanical milling, solution processing, and melt-quenching techniques. Each method produces materials with different crystallinity, grain boundary characteristics, and defect concentrations, all of which affect ion transport. Advanced synthesis methods that control particle size, crystallinity, and interfacial properties have been developed to optimize ionic conductivity while maintaining mechanical and chemical stability.
- Interface engineering for enhanced performance: Interface engineering is crucial for maximizing the ionic conductivity of sulfide solid electrolytes in practical applications. This involves designing and controlling the interfaces between the electrolyte and electrodes to minimize resistance to ion transport. Techniques include surface coatings, buffer layers, and gradient compositions that can reduce interfacial resistance. Properly engineered interfaces prevent unwanted side reactions while maintaining high ionic conductivity across the entire battery cell.
- Argyrodite-type sulfide electrolytes: Argyrodite-type sulfide electrolytes, particularly those with the formula Li6PS5X (where X is typically a halogen like Cl, Br, or I), have emerged as promising materials for solid-state batteries due to their exceptionally high ionic conductivity. These materials feature a unique crystal structure that provides fast lithium-ion transport pathways. Research has focused on optimizing the composition and structure of argyrodite sulfides to further enhance their conductivity while improving their stability against lithium metal anodes and high-voltage cathodes.
02 Doping strategies for improved conductivity
Doping sulfide solid electrolytes with specific elements can significantly enhance their ionic conductivity. Common dopants include halides (Cl, Br, I), metals (Al, Ga, In), and other elements that can modify the crystal structure or create additional lithium vacancies. These dopants can reduce energy barriers for ion migration, stabilize the conductive phases, and create favorable pathways for lithium ions, resulting in conductivity improvements of several orders of magnitude compared to undoped materials.Expand Specific Solutions03 Interface engineering and stability
Engineering the interfaces between sulfide solid electrolytes and electrodes is crucial for maintaining high ionic conductivity in practical applications. Strategies include creating buffer layers, surface modifications, and gradient compositions to minimize interfacial resistance. These approaches help prevent chemical reactions at interfaces that can form high-resistance layers, maintain structural integrity during cycling, and ensure consistent ionic conductivity throughout battery operation.Expand Specific Solutions04 Synthesis methods affecting conductivity
Various synthesis methods significantly impact the ionic conductivity of sulfide solid electrolytes. Techniques include mechanical milling, solution processing, and heat treatment under controlled atmospheres. The processing parameters such as milling time, annealing temperature, cooling rate, and pressure during synthesis can dramatically affect the crystallinity, grain boundaries, and defect concentration, all of which directly influence ionic conductivity. Optimized synthesis routes can produce materials with conductivities approaching those of liquid electrolytes.Expand Specific Solutions05 Composite and hybrid electrolyte systems
Combining sulfide solid electrolytes with other materials to create composite or hybrid systems can enhance overall ionic conductivity and electrochemical performance. These composites may incorporate polymers, oxides, or other sulfide materials to create synergistic effects. The resulting hybrid electrolytes often exhibit improved mechanical properties, better interfacial contacts, and enhanced ionic transport pathways, while potentially mitigating some of the challenges associated with pure sulfide electrolytes such as air sensitivity and mechanical brittleness.Expand Specific Solutions
Leading Research Groups and Companies in Solid Electrolytes
The sulfide ionic conductivity market is in a growth phase, characterized by increasing demand for solid-state batteries with enhanced safety and performance. The global market size for solid electrolytes is expanding rapidly, projected to reach significant scale as automotive and electronics industries seek alternatives to liquid electrolytes. Technologically, grain boundary engineering for sulfide conductors remains in early maturity stages, with key players advancing at different rates. FUJIFILM and Mitsubishi Materials lead in materials processing innovations, while academic institutions like MIT and Tianjin University contribute fundamental research breakthroughs. Companies including Samsung, Sharp, and Idemitsu Kosan are developing commercial applications, focusing on overcoming conductivity limitations at grain boundaries. Research collaborations between industry leaders and institutions like Shanghai Institute of Ceramics are accelerating technological maturity in this competitive landscape.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered advanced grain boundary engineering techniques for sulfide solid electrolytes, focusing on controlling the microstructure and composition at grain interfaces to enhance ionic conductivity. Their approach involves precise manipulation of grain boundary chemistry through dopant segregation strategies, where carefully selected elements are introduced to modify the space charge layers at interfaces. MIT researchers have demonstrated that controlling the crystallographic texture and orientation relationships between adjacent grains can create preferential pathways for lithium ion transport. Their work has shown that reducing grain boundary resistance can increase overall conductivity by up to 2-3 orders of magnitude in certain sulfide systems. Additionally, MIT has developed novel processing techniques including controlled cooling rates and pressure-assisted sintering to optimize grain boundary structures while maintaining the stability of the sulfide materials.
Strengths: Superior fundamental understanding of grain boundary physics; access to advanced characterization techniques allowing atomic-level analysis of interfaces; strong interdisciplinary collaboration between materials science, physics, and computational modeling teams. Weaknesses: Technologies may require sophisticated manufacturing processes that are challenging to scale commercially; some approaches may be limited to specific sulfide compositions.
Shanghai Institute of Ceramics, Chinese Academy of Sciences
Technical Solution: The Shanghai Institute of Ceramics has developed a comprehensive approach to grain boundary engineering in sulfide solid electrolytes, focusing on both compositional and structural modifications. Their research employs a dual-phase strategy where secondary phases are deliberately introduced at grain boundaries to create highly conductive pathways for ion transport. They've pioneered the use of liquid-phase sintering techniques that allow precise control over grain boundary formation while maintaining low processing temperatures compatible with sulfide stability. Their work has demonstrated that nanoscale amorphous intergranular films can significantly enhance ionic conductivity by providing fast diffusion channels that bypass the more resistive crystalline regions. The institute has also developed innovative hot-pressing protocols that simultaneously optimize grain size distribution and boundary characteristics, achieving room temperature conductivities exceeding 10 mS/cm in modified Li₆PS₅Cl systems. Their approach combines experimental techniques with advanced computational modeling to predict optimal dopant concentrations and distributions at grain interfaces.
Strengths: Exceptional capabilities in advanced ceramic processing techniques; strong integration of computational and experimental approaches; extensive experience with sulfide materials synthesis. Weaknesses: Some techniques require specialized equipment not widely available in industry; certain approaches may introduce stability issues at the engineered interfaces during long-term cycling.
Key Patents and Breakthroughs in Ionic Conductivity Enhancement
Method for manufacturing solid electrolyte layer, all-solid-state battery, and method for manufacturing same
PatentWO2025159151A1
Innovation
- A method involving laser irradiation of a powder mixture containing NASICON crystals and a carbon material to melt and solidify the material, forming a dense amorphous precursor, followed by heat treatment to recrystallize it at a lower temperature, thereby minimizing grain boundary formation and enhancing ionic conductivity.
Materials Processing and Scalability Considerations
The scalability of grain boundary engineering techniques for sulfide solid electrolytes represents a critical challenge in transitioning from laboratory-scale demonstrations to commercial production. Current processing methods for sulfide-based solid electrolytes typically involve mechanical ball milling, cold sintering, or hot pressing under inert atmospheres due to the air-sensitivity of these materials. These approaches, while effective at small scales, face significant hurdles when considered for mass production.
Cold sintering processes, which operate at relatively low temperatures (typically below 200°C), offer advantages for sulfide electrolytes by minimizing decomposition and side reactions. However, achieving uniform grain boundary modifications across large-format cells remains challenging. The introduction of dopants or secondary phases at grain boundaries must be precisely controlled to ensure homogeneous distribution throughout the material volume.
Mechanical processing techniques present additional complexities when scaled up. Ball milling, commonly used to reduce particle size and increase reactivity, becomes less efficient and more energy-intensive at larger scales. The extended processing times required for larger batches can lead to contamination issues and inconsistent grain boundary properties across the material.
Atmospheric control represents another significant manufacturing challenge. Most sulfide electrolytes are highly sensitive to moisture and oxygen, necessitating handling in controlled environments. The cost of maintaining inert atmospheres throughout large-scale production lines significantly impacts economic viability. Recent developments in dry room technology and specialized handling equipment have improved feasibility, but further innovations are needed to reduce these infrastructure requirements.
The selection of precursor materials also influences scalability. High-purity starting materials, often required for optimal ionic conductivity, can be expensive and limited in supply. Research into alternative synthesis routes using more abundant precursors, while maintaining control over grain boundary characteristics, could substantially improve commercial viability.
Post-processing treatments, such as controlled thermal annealing or chemical treatments to modify grain boundaries, must be adapted for continuous manufacturing processes. Batch-to-batch consistency becomes increasingly difficult to maintain as production volumes increase, potentially leading to variable electrochemical performance in final products.
Recent advances in additive manufacturing and solution-based processing offer promising alternatives for scalable production while maintaining precise control over microstructure. These emerging techniques may provide pathways to overcome current limitations in grain boundary engineering for sulfide solid electrolytes, enabling their widespread adoption in next-generation energy storage systems.
Cold sintering processes, which operate at relatively low temperatures (typically below 200°C), offer advantages for sulfide electrolytes by minimizing decomposition and side reactions. However, achieving uniform grain boundary modifications across large-format cells remains challenging. The introduction of dopants or secondary phases at grain boundaries must be precisely controlled to ensure homogeneous distribution throughout the material volume.
Mechanical processing techniques present additional complexities when scaled up. Ball milling, commonly used to reduce particle size and increase reactivity, becomes less efficient and more energy-intensive at larger scales. The extended processing times required for larger batches can lead to contamination issues and inconsistent grain boundary properties across the material.
Atmospheric control represents another significant manufacturing challenge. Most sulfide electrolytes are highly sensitive to moisture and oxygen, necessitating handling in controlled environments. The cost of maintaining inert atmospheres throughout large-scale production lines significantly impacts economic viability. Recent developments in dry room technology and specialized handling equipment have improved feasibility, but further innovations are needed to reduce these infrastructure requirements.
The selection of precursor materials also influences scalability. High-purity starting materials, often required for optimal ionic conductivity, can be expensive and limited in supply. Research into alternative synthesis routes using more abundant precursors, while maintaining control over grain boundary characteristics, could substantially improve commercial viability.
Post-processing treatments, such as controlled thermal annealing or chemical treatments to modify grain boundaries, must be adapted for continuous manufacturing processes. Batch-to-batch consistency becomes increasingly difficult to maintain as production volumes increase, potentially leading to variable electrochemical performance in final products.
Recent advances in additive manufacturing and solution-based processing offer promising alternatives for scalable production while maintaining precise control over microstructure. These emerging techniques may provide pathways to overcome current limitations in grain boundary engineering for sulfide solid electrolytes, enabling their widespread adoption in next-generation energy storage systems.
Interface Stability and Electrochemical Performance Metrics
The interface stability of sulfide solid electrolytes represents a critical factor in determining the overall performance and longevity of solid-state batteries. When evaluating grain boundary engineering approaches for maximizing sulfide ionic conductivity, the stability of interfaces between electrolyte grains and between the electrolyte and electrodes must be thoroughly assessed using standardized electrochemical performance metrics.
Electrochemical impedance spectroscopy (EIS) serves as a primary analytical tool for quantifying interfacial resistance contributions in sulfide systems. Well-engineered grain boundaries typically exhibit reduced impedance arcs in Nyquist plots, with characteristic frequencies that can be correlated to specific interfacial phenomena. The area-specific resistance (ASR) values derived from these measurements provide quantitative benchmarks for comparing different grain boundary engineering strategies, with state-of-the-art sulfide systems achieving ASR values below 10 Ω·cm² at room temperature.
Cycling stability represents another crucial metric, particularly for evaluating the long-term effectiveness of grain boundary modifications. Engineered interfaces must maintain their enhanced ionic conductivity over hundreds to thousands of charge-discharge cycles. Capacity retention measurements during extended cycling reveal the degradation kinetics at interfaces, with successful grain boundary engineering approaches demonstrating >80% capacity retention after 500 cycles at 1C rates.
Chemical stability against electrode materials must be evaluated through techniques such as X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (TOF-SIMS) to detect interfacial reaction products. The formation of space charge layers at grain boundaries and interfaces significantly impacts ionic transport, with the thickness and potential barrier height of these regions directly correlating with measured conductivity values.
Critical current density measurements provide insights into the mechanical stability of engineered interfaces under high current loads. Advanced grain boundary engineering approaches have demonstrated improvements in critical current density from typical values of 0.5-1 mA/cm² to over 3 mA/cm² through strategic dopant incorporation and microstructural control.
Temperature-dependent performance metrics reveal the activation energy for ion transport across engineered interfaces, with optimized grain boundaries showing reduced activation energies (typically below 0.3 eV) compared to unmodified systems. This parameter directly influences the practical operating temperature range for sulfide-based solid-state batteries with engineered grain boundaries.
Electrochemical impedance spectroscopy (EIS) serves as a primary analytical tool for quantifying interfacial resistance contributions in sulfide systems. Well-engineered grain boundaries typically exhibit reduced impedance arcs in Nyquist plots, with characteristic frequencies that can be correlated to specific interfacial phenomena. The area-specific resistance (ASR) values derived from these measurements provide quantitative benchmarks for comparing different grain boundary engineering strategies, with state-of-the-art sulfide systems achieving ASR values below 10 Ω·cm² at room temperature.
Cycling stability represents another crucial metric, particularly for evaluating the long-term effectiveness of grain boundary modifications. Engineered interfaces must maintain their enhanced ionic conductivity over hundreds to thousands of charge-discharge cycles. Capacity retention measurements during extended cycling reveal the degradation kinetics at interfaces, with successful grain boundary engineering approaches demonstrating >80% capacity retention after 500 cycles at 1C rates.
Chemical stability against electrode materials must be evaluated through techniques such as X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (TOF-SIMS) to detect interfacial reaction products. The formation of space charge layers at grain boundaries and interfaces significantly impacts ionic transport, with the thickness and potential barrier height of these regions directly correlating with measured conductivity values.
Critical current density measurements provide insights into the mechanical stability of engineered interfaces under high current loads. Advanced grain boundary engineering approaches have demonstrated improvements in critical current density from typical values of 0.5-1 mA/cm² to over 3 mA/cm² through strategic dopant incorporation and microstructural control.
Temperature-dependent performance metrics reveal the activation energy for ion transport across engineered interfaces, with optimized grain boundaries showing reduced activation energies (typically below 0.3 eV) compared to unmodified systems. This parameter directly influences the practical operating temperature range for sulfide-based solid-state batteries with engineered grain boundaries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!