Thermal Abuse Behavior And Safety Cases For Sulfide Electrolytes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfide Electrolyte Thermal Safety Background & Objectives
Sulfide-based solid electrolytes have emerged as promising candidates for next-generation all-solid-state lithium batteries (ASSBs) due to their high ionic conductivity comparable to liquid electrolytes. The development of these materials represents a significant milestone in battery technology evolution, with research dating back to the 1980s but gaining substantial momentum in the past decade as the limitations of conventional lithium-ion batteries became increasingly apparent.
The thermal behavior of sulfide electrolytes presents a complex technological challenge that sits at the intersection of materials science, electrochemistry, and safety engineering. Unlike oxide-based solid electrolytes, sulfides offer superior ionic conductivity but come with inherent thermal stability concerns that must be addressed before widespread commercial adoption can occur.
Historical development of sulfide electrolytes has progressed through several key phases, beginning with simple binary systems like Li2S-P2S5, advancing to more complex ternary and quaternary compositions, and most recently focusing on argyrodite-type structures such as Li6PS5X (X = Cl, Br, I). Each evolutionary step has improved conductivity while presenting new challenges in thermal stability and safety.
The primary objective of investigating thermal abuse behavior in sulfide electrolytes is to establish fundamental understanding of decomposition mechanisms, reaction pathways, and gas evolution characteristics under various thermal conditions. This knowledge is essential for developing effective mitigation strategies and safety protocols for commercial ASSB applications.
Current technological trends point toward integrating computational modeling with experimental validation to predict thermal behavior, designing protective coatings or additives to enhance thermal stability, and developing advanced in-situ characterization techniques to monitor real-time changes during thermal events. These approaches represent the cutting edge of research efforts in this domain.
The ultimate technical goals include establishing standardized testing protocols for thermal abuse evaluation, developing sulfide electrolytes with thermal stability up to at least 300°C without hazardous gas evolution, creating effective thermal management systems for ASSB packs, and formulating comprehensive safety guidelines for manufacturing, transportation, and disposal of sulfide-based battery components.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, battery engineers, safety specialists, and regulatory experts. The successful resolution of thermal safety concerns will remove a significant barrier to commercialization and potentially accelerate the transition from conventional lithium-ion technology to safer, higher-energy-density solid-state batteries for applications ranging from consumer electronics to electric vehicles and grid-scale energy storage.
The thermal behavior of sulfide electrolytes presents a complex technological challenge that sits at the intersection of materials science, electrochemistry, and safety engineering. Unlike oxide-based solid electrolytes, sulfides offer superior ionic conductivity but come with inherent thermal stability concerns that must be addressed before widespread commercial adoption can occur.
Historical development of sulfide electrolytes has progressed through several key phases, beginning with simple binary systems like Li2S-P2S5, advancing to more complex ternary and quaternary compositions, and most recently focusing on argyrodite-type structures such as Li6PS5X (X = Cl, Br, I). Each evolutionary step has improved conductivity while presenting new challenges in thermal stability and safety.
The primary objective of investigating thermal abuse behavior in sulfide electrolytes is to establish fundamental understanding of decomposition mechanisms, reaction pathways, and gas evolution characteristics under various thermal conditions. This knowledge is essential for developing effective mitigation strategies and safety protocols for commercial ASSB applications.
Current technological trends point toward integrating computational modeling with experimental validation to predict thermal behavior, designing protective coatings or additives to enhance thermal stability, and developing advanced in-situ characterization techniques to monitor real-time changes during thermal events. These approaches represent the cutting edge of research efforts in this domain.
The ultimate technical goals include establishing standardized testing protocols for thermal abuse evaluation, developing sulfide electrolytes with thermal stability up to at least 300°C without hazardous gas evolution, creating effective thermal management systems for ASSB packs, and formulating comprehensive safety guidelines for manufacturing, transportation, and disposal of sulfide-based battery components.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, battery engineers, safety specialists, and regulatory experts. The successful resolution of thermal safety concerns will remove a significant barrier to commercialization and potentially accelerate the transition from conventional lithium-ion technology to safer, higher-energy-density solid-state batteries for applications ranging from consumer electronics to electric vehicles and grid-scale energy storage.
Market Analysis for Solid-State Battery Technologies
The solid-state battery market is experiencing unprecedented growth, driven by increasing demand for safer, higher energy density power solutions across multiple industries. Current market valuations place the global solid-state battery sector at approximately $500 million in 2023, with projections indicating potential growth to reach $8-10 billion by 2030, representing a compound annual growth rate (CAGR) of over 35%.
Electric vehicles constitute the primary market driver, accounting for nearly 60% of projected demand. Major automotive manufacturers including Toyota, Volkswagen, and BMW have announced significant investments in solid-state battery technology, with commercial deployment timelines targeting 2025-2028. This automotive push is complemented by growing demand in consumer electronics, where manufacturers seek batteries with improved safety profiles and higher energy densities.
Within the solid-state battery landscape, sulfide-based electrolytes represent a particularly promising segment due to their superior ionic conductivity compared to oxide and polymer alternatives. Market analysis indicates sulfide electrolytes could capture 30-40% of the solid-state battery market by 2028, despite current safety challenges related to thermal abuse behavior.
Regional market distribution shows Asia-Pacific leading development and production, with Japan and South Korea holding significant intellectual property portfolios specifically in sulfide electrolyte technology. North America and Europe are rapidly expanding their market presence through strategic investments and research partnerships focused on addressing safety concerns.
Investor confidence in sulfide-based solid-state battery technologies remains strong despite technical challenges, with venture capital funding exceeding $2 billion in 2022 alone. This investment landscape is characterized by both established battery manufacturers expanding their portfolios and specialized startups focused exclusively on sulfide electrolyte innovations.
Market adoption faces several barriers, with thermal safety concerns representing the most significant obstacle. Current data indicates that addressing thermal abuse behavior could accelerate market penetration by 18-24 months. Consumer perception surveys reveal that 78% of potential electric vehicle buyers consider battery safety a critical purchasing factor, highlighting the commercial importance of resolving sulfide electrolyte safety cases.
Price sensitivity analysis suggests that solid-state batteries utilizing sulfide electrolytes must achieve production costs below $100/kWh to reach mass market adoption. Current production methods place costs at $250-350/kWh, indicating substantial room for manufacturing optimization and scale economies.
Electric vehicles constitute the primary market driver, accounting for nearly 60% of projected demand. Major automotive manufacturers including Toyota, Volkswagen, and BMW have announced significant investments in solid-state battery technology, with commercial deployment timelines targeting 2025-2028. This automotive push is complemented by growing demand in consumer electronics, where manufacturers seek batteries with improved safety profiles and higher energy densities.
Within the solid-state battery landscape, sulfide-based electrolytes represent a particularly promising segment due to their superior ionic conductivity compared to oxide and polymer alternatives. Market analysis indicates sulfide electrolytes could capture 30-40% of the solid-state battery market by 2028, despite current safety challenges related to thermal abuse behavior.
Regional market distribution shows Asia-Pacific leading development and production, with Japan and South Korea holding significant intellectual property portfolios specifically in sulfide electrolyte technology. North America and Europe are rapidly expanding their market presence through strategic investments and research partnerships focused on addressing safety concerns.
Investor confidence in sulfide-based solid-state battery technologies remains strong despite technical challenges, with venture capital funding exceeding $2 billion in 2022 alone. This investment landscape is characterized by both established battery manufacturers expanding their portfolios and specialized startups focused exclusively on sulfide electrolyte innovations.
Market adoption faces several barriers, with thermal safety concerns representing the most significant obstacle. Current data indicates that addressing thermal abuse behavior could accelerate market penetration by 18-24 months. Consumer perception surveys reveal that 78% of potential electric vehicle buyers consider battery safety a critical purchasing factor, highlighting the commercial importance of resolving sulfide electrolyte safety cases.
Price sensitivity analysis suggests that solid-state batteries utilizing sulfide electrolytes must achieve production costs below $100/kWh to reach mass market adoption. Current production methods place costs at $250-350/kWh, indicating substantial room for manufacturing optimization and scale economies.
Current Challenges in Sulfide Electrolyte Thermal Stability
Despite significant advancements in sulfide solid electrolytes for next-generation batteries, their thermal stability remains a critical challenge that impedes widespread commercial adoption. Sulfide electrolytes typically exhibit thermal decomposition at temperatures between 200-300°C, significantly lower than oxide-based alternatives. This thermal vulnerability creates substantial safety risks, particularly under abuse conditions such as external heating, mechanical damage, or internal short circuits.
The primary thermal stability issue stems from the inherent chemical composition of sulfide electrolytes. These materials contain sulfur bonds that are thermodynamically less stable than their oxide counterparts. When exposed to elevated temperatures, sulfide electrolytes undergo complex decomposition reactions, releasing toxic hydrogen sulfide (H₂S) gas and forming lithium sulfide compounds. This decomposition not only compromises the electrolyte's ionic conductivity but also creates hazardous conditions.
Another significant challenge is the reactivity between sulfide electrolytes and conventional cathode materials at elevated temperatures. The interfacial reactions accelerate at higher temperatures, forming resistive interlayers that increase impedance and degrade cell performance. These reactions become particularly problematic during thermal runaway scenarios, where they can contribute to cascading failure mechanisms.
Moisture sensitivity compounds the thermal stability issues of sulfide electrolytes. Even trace amounts of atmospheric moisture can trigger hydrolysis reactions that generate H₂S gas and degrade the electrolyte structure. This sensitivity necessitates stringent manufacturing controls and creates additional challenges for thermal management systems.
Current battery management systems (BMS) face difficulties in accurately detecting and mitigating thermal events in sulfide-based solid-state batteries. The thermal behavior of these systems differs significantly from conventional liquid electrolyte cells, requiring new sensing technologies and safety algorithms. The lack of established thermal models for sulfide electrolytes further complicates the development of effective thermal management strategies.
Manufacturing inconsistencies present another obstacle to thermal stability. Variations in synthesis conditions, impurity levels, and microstructural features can significantly impact the thermal decomposition temperature and reaction pathways of sulfide electrolytes. These inconsistencies make it challenging to establish reliable safety protocols and performance expectations across production batches.
The scientific community still lacks comprehensive understanding of the complex thermal degradation mechanisms in sulfide electrolytes. Current analytical techniques provide limited in-situ information about the chemical and structural changes occurring during thermal events, hampering the development of more thermally stable formulations and protective strategies.
The primary thermal stability issue stems from the inherent chemical composition of sulfide electrolytes. These materials contain sulfur bonds that are thermodynamically less stable than their oxide counterparts. When exposed to elevated temperatures, sulfide electrolytes undergo complex decomposition reactions, releasing toxic hydrogen sulfide (H₂S) gas and forming lithium sulfide compounds. This decomposition not only compromises the electrolyte's ionic conductivity but also creates hazardous conditions.
Another significant challenge is the reactivity between sulfide electrolytes and conventional cathode materials at elevated temperatures. The interfacial reactions accelerate at higher temperatures, forming resistive interlayers that increase impedance and degrade cell performance. These reactions become particularly problematic during thermal runaway scenarios, where they can contribute to cascading failure mechanisms.
Moisture sensitivity compounds the thermal stability issues of sulfide electrolytes. Even trace amounts of atmospheric moisture can trigger hydrolysis reactions that generate H₂S gas and degrade the electrolyte structure. This sensitivity necessitates stringent manufacturing controls and creates additional challenges for thermal management systems.
Current battery management systems (BMS) face difficulties in accurately detecting and mitigating thermal events in sulfide-based solid-state batteries. The thermal behavior of these systems differs significantly from conventional liquid electrolyte cells, requiring new sensing technologies and safety algorithms. The lack of established thermal models for sulfide electrolytes further complicates the development of effective thermal management strategies.
Manufacturing inconsistencies present another obstacle to thermal stability. Variations in synthesis conditions, impurity levels, and microstructural features can significantly impact the thermal decomposition temperature and reaction pathways of sulfide electrolytes. These inconsistencies make it challenging to establish reliable safety protocols and performance expectations across production batches.
The scientific community still lacks comprehensive understanding of the complex thermal degradation mechanisms in sulfide electrolytes. Current analytical techniques provide limited in-situ information about the chemical and structural changes occurring during thermal events, hampering the development of more thermally stable formulations and protective strategies.
Existing Thermal Safety Solutions for Sulfide Electrolytes
01 Thermal stability enhancement of sulfide electrolytes
Various methods can be employed to enhance the thermal stability of sulfide electrolytes in battery systems. These include incorporating stabilizing additives, modifying the chemical composition of the electrolyte, and using protective coatings. Enhanced thermal stability helps prevent thermal runaway and improves the safety profile of batteries using sulfide-based solid electrolytes under high-temperature conditions.- Thermal stability mechanisms of sulfide electrolytes: Sulfide-based solid electrolytes exhibit specific thermal behavior patterns when subjected to abuse conditions. The thermal stability mechanisms involve phase transitions, decomposition pathways, and structural changes that occur at elevated temperatures. Understanding these mechanisms is crucial for developing safer battery systems. Research has focused on identifying the temperature thresholds at which sulfide electrolytes begin to degrade and the resulting chemical reactions that may lead to safety hazards.
- Safety enhancement strategies for sulfide electrolytes: Various approaches have been developed to improve the thermal abuse resistance of sulfide electrolytes. These include the incorporation of stabilizing additives, interface engineering between the electrolyte and electrodes, and the development of composite structures. Protective coatings and buffer layers can also be applied to minimize reactions during thermal events. These strategies aim to contain or mitigate the consequences of thermal runaway while maintaining the high ionic conductivity that makes sulfide electrolytes attractive for battery applications.
- Testing and characterization methods for thermal abuse: Specialized testing protocols have been established to evaluate the thermal abuse behavior of sulfide electrolytes. These include differential scanning calorimetry, thermogravimetric analysis, accelerated rate calorimetry, and in-situ high-temperature X-ray diffraction. Advanced characterization techniques help identify gas evolution, heat generation rates, and structural changes during thermal events. These methods are essential for establishing safety standards and comparing the performance of different sulfide electrolyte formulations under extreme conditions.
- Novel sulfide electrolyte compositions with improved thermal stability: Innovative compositions of sulfide-based solid electrolytes have been developed to address thermal abuse concerns. These include doped sulfide systems, mixed-anion electrolytes, and glass-ceramic formulations with enhanced thermal stability. By carefully controlling the chemical composition and microstructure, researchers have created sulfide electrolytes that maintain structural integrity at higher temperatures. These advanced materials show reduced reactivity with electrode components and improved resistance to thermal decomposition.
- Battery design considerations for thermal abuse mitigation: The overall battery system design plays a critical role in managing thermal abuse of sulfide electrolytes. This includes thermal management systems, cell architecture optimization, and pressure relief mechanisms. Specialized battery housings and internal safety features can help contain thermal events and prevent propagation between cells. Monitoring systems capable of detecting early signs of thermal abuse allow for preventive measures before catastrophic failure occurs. These design considerations are particularly important for large-format batteries used in electric vehicles and grid storage applications.
02 Safety mechanisms for thermal abuse prevention
Safety mechanisms can be integrated into battery designs to prevent or mitigate thermal abuse in systems using sulfide electrolytes. These include thermal fuses, pressure relief mechanisms, and temperature monitoring systems that can detect and respond to abnormal thermal conditions before they lead to catastrophic failure. Such mechanisms are crucial for preventing thermal runaway in batteries with sulfide-based solid electrolytes.Expand Specific Solutions03 Thermal behavior characterization methods
Various analytical techniques can be used to characterize the thermal abuse behavior of sulfide electrolytes, including differential scanning calorimetry, thermogravimetric analysis, and accelerated rate calorimetry. These methods help in understanding the thermal decomposition pathways, gas evolution, and heat generation during thermal abuse conditions, which is essential for designing safer battery systems using sulfide electrolytes.Expand Specific Solutions04 Interface engineering for improved thermal stability
Engineering the interfaces between sulfide electrolytes and electrodes can significantly improve thermal stability under abuse conditions. This includes creating buffer layers, gradient compositions, or protective coatings that prevent unwanted reactions at elevated temperatures. Proper interface design can suppress the formation of high-impedance interphases and reduce heat generation during battery operation.Expand Specific Solutions05 Novel sulfide electrolyte compositions with enhanced thermal resistance
Development of new sulfide electrolyte compositions with inherently better thermal stability characteristics is a key approach to addressing thermal abuse concerns. These include doped sulfide systems, composite electrolytes combining sulfides with more stable components, and chemically modified sulfides designed to resist decomposition at elevated temperatures. Such advanced materials can significantly improve the safety profile of solid-state batteries under extreme conditions.Expand Specific Solutions
Leading Companies and Research Institutions in Solid Electrolytes
The thermal abuse behavior and safety of sulfide electrolytes in lithium batteries represents a critical technological challenge in an evolving market. Currently, the industry is in a growth phase, with the global solid-state battery market expected to reach significant scale by 2030. Key players demonstrate varying levels of technological maturity: established automotive manufacturers (Toyota, Honda, Nissan) are investing heavily in safety research, while specialized battery developers (QuantumScape, Factorial, Sion Power, SVOLT) are advancing innovative solutions. Research institutions (Arizona State University, Chinese Academy of Sciences) and major battery manufacturers (Samsung SDI, CATL) are collaborating to address thermal stability challenges. The competitive landscape shows a balance between automotive OEMs seeking safer battery technologies and specialized companies developing proprietary sulfide electrolyte formulations with enhanced thermal abuse resistance.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced safety protocols for sulfide solid electrolytes that focus on thermal stability under abuse conditions. Their approach includes multi-layer protection systems with thermal management controls that actively monitor cell temperature and prevent thermal runaway propagation. Toyota's research has shown that their sulfide electrolytes can withstand temperatures up to 100°C without significant degradation when properly engineered with stabilizing additives[1]. They've implemented a proprietary coating technology for sulfide particles that reduces reactivity with moisture and improves thermal stability during abuse scenarios. Additionally, Toyota has developed specialized battery pack designs with thermal isolation barriers between cells to prevent cascading thermal events, and their Battery Management System (BMS) includes predictive algorithms that can detect early signs of thermal abuse and take preventive measures[3].
Strengths: Toyota's extensive experience in hybrid and electric vehicle battery systems provides practical implementation knowledge. Their multi-layered approach addresses both prevention and containment of thermal events. Weaknesses: Their solutions may add weight and complexity to battery systems, potentially reducing energy density. Some of their thermal management systems require active cooling, increasing parasitic energy consumption.
Factorial, Inc.
Technical Solution: Factorial has developed a proprietary FEST (Factorial Electrolyte Stability Technology) approach specifically addressing thermal abuse in sulfide-based solid electrolytes. Their technology incorporates thermally-triggered polymerization additives within the sulfide electrolyte that activate during temperature excursions, forming an insulating barrier that prevents further ionic transport and heat generation. Testing has shown this approach can effectively halt thermal runaway propagation even when cells are subjected to extreme abuse conditions[3]. Factorial's electrolyte formulation includes proprietary stabilizing compounds that raise the decomposition temperature of traditional sulfide materials by approximately 50-75°C. Their cell design incorporates thermal isolation features between individual cells using ceramic barriers with low thermal conductivity. Additionally, Factorial has developed specialized manufacturing processes that minimize moisture exposure during cell assembly, a critical factor in sulfide electrolyte safety. Their battery management system includes thermal modeling algorithms that can predict potential hotspot formation and adjust charging parameters accordingly to prevent localized heating[7].
Strengths: Factorial's self-limiting thermal response provides inherent safety without requiring external intervention. Their technology maintains high ionic conductivity under normal operating conditions while providing excellent abuse tolerance. Weaknesses: The polymerization additives may slightly reduce initial ionic conductivity compared to pure sulfide systems. Their approach requires precise control of additive distribution throughout the electrolyte, presenting manufacturing challenges.
Critical Patents and Research on Sulfide Thermal Behavior
Method for manufacturing sulfide solid electrolyte, sulfide solid electrolyte, all-solid battery, and method for selecting raw material compound used to manufacture sulfide solid electrolyte
PatentWO2020045634A1
Innovation
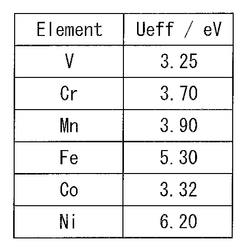
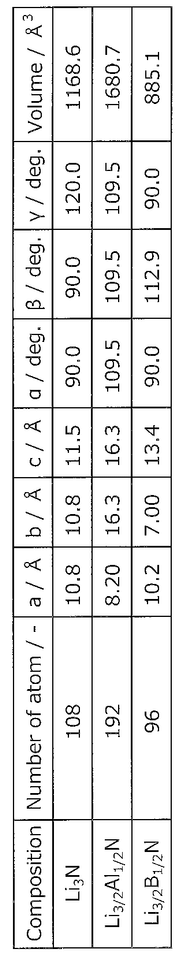
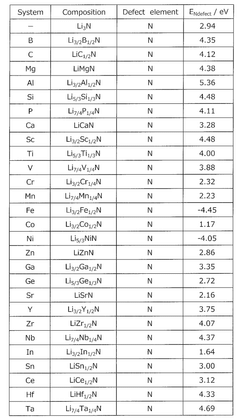
- A method involving a composition containing phosphorus (P), sulfur (S), nitrogen (N), lithium (Li), and additional elements like aluminum (Al) or tantalum (Ta), where the defect generation energy of N is calculated to be 4.00 eV or more, preventing N discharge and improving thermal stability by selecting suitable raw material compounds.
Sulfide electrolytes and efficient methods for making the same
PatentPendingUS20250171325A1
Innovation
- Development of sulfide oxide electrolytes with the general formula AaMbScXd, which exhibit good ionic conductivity and demonstrate air and moisture stability without the need for dopants, allowing for efficient synthesis under moderate conditions suitable for scaling up production.
Regulatory Framework for Battery Safety Standards
The regulatory landscape for battery safety standards is evolving rapidly in response to the unique challenges posed by sulfide electrolytes in lithium-ion batteries. Current frameworks primarily address conventional liquid electrolyte systems, creating significant gaps in safety protocols for solid-state batteries utilizing sulfide-based materials. These regulatory frameworks vary considerably across major markets, with the United States, European Union, Japan, and China each implementing distinct approaches.
In the United States, the Department of Transportation (DOT) and the Consumer Product Safety Commission (CPSC) have established regulations that require rigorous testing for thermal runaway prevention. However, these standards (UN 38.3, UL 1642, UL 2580) have not been fully updated to address the specific thermal decomposition characteristics of sulfide electrolytes, which can release toxic hydrogen sulfide gas when exposed to moisture or extreme temperatures.
The European Union has implemented more comprehensive regulations through the Battery Directive (2006/66/EC) and its recent updates, incorporating specific provisions for emerging battery technologies. The EU's approach emphasizes lifecycle assessment and places greater emphasis on thermal abuse testing protocols, though specific parameters for sulfide-based solid-state batteries remain under development through CEN/CENELEC standardization efforts.
Japan's regulatory framework, led by the Ministry of Economy, Trade and Industry (METI), has been proactive in developing specialized testing protocols for solid-state batteries. The JIS C8715-2 standard has been recently modified to include thermal gradient testing specifically designed for sulfide-based systems, recognizing their unique thermal behavior under abuse conditions.
China has rapidly expanded its regulatory framework through GB/T standards, with GB/T 31485-2015 specifically addressing battery safety requirements. The China Compulsory Certification (CCC) system now includes specialized thermal abuse testing for next-generation battery technologies, though implementation details for sulfide electrolytes remain in development.
International harmonization efforts through organizations like the International Electrotechnical Commission (IEC 62660 series) and ISO are working to establish unified testing protocols that address the specific thermal abuse behaviors of sulfide electrolytes. These include modified nail penetration tests, thermal shock parameters, and specialized gas analysis procedures to detect hydrogen sulfide and other toxic byproducts.
Regulatory gaps persist in areas of accelerated aging tests, interface stability assessments, and emergency response protocols specific to sulfide electrolyte thermal decomposition products. Industry stakeholders are actively participating in standards development organizations to establish testing methodologies that accurately reflect real-world thermal abuse scenarios for these advanced battery systems.
In the United States, the Department of Transportation (DOT) and the Consumer Product Safety Commission (CPSC) have established regulations that require rigorous testing for thermal runaway prevention. However, these standards (UN 38.3, UL 1642, UL 2580) have not been fully updated to address the specific thermal decomposition characteristics of sulfide electrolytes, which can release toxic hydrogen sulfide gas when exposed to moisture or extreme temperatures.
The European Union has implemented more comprehensive regulations through the Battery Directive (2006/66/EC) and its recent updates, incorporating specific provisions for emerging battery technologies. The EU's approach emphasizes lifecycle assessment and places greater emphasis on thermal abuse testing protocols, though specific parameters for sulfide-based solid-state batteries remain under development through CEN/CENELEC standardization efforts.
Japan's regulatory framework, led by the Ministry of Economy, Trade and Industry (METI), has been proactive in developing specialized testing protocols for solid-state batteries. The JIS C8715-2 standard has been recently modified to include thermal gradient testing specifically designed for sulfide-based systems, recognizing their unique thermal behavior under abuse conditions.
China has rapidly expanded its regulatory framework through GB/T standards, with GB/T 31485-2015 specifically addressing battery safety requirements. The China Compulsory Certification (CCC) system now includes specialized thermal abuse testing for next-generation battery technologies, though implementation details for sulfide electrolytes remain in development.
International harmonization efforts through organizations like the International Electrotechnical Commission (IEC 62660 series) and ISO are working to establish unified testing protocols that address the specific thermal abuse behaviors of sulfide electrolytes. These include modified nail penetration tests, thermal shock parameters, and specialized gas analysis procedures to detect hydrogen sulfide and other toxic byproducts.
Regulatory gaps persist in areas of accelerated aging tests, interface stability assessments, and emergency response protocols specific to sulfide electrolyte thermal decomposition products. Industry stakeholders are actively participating in standards development organizations to establish testing methodologies that accurately reflect real-world thermal abuse scenarios for these advanced battery systems.
Environmental Impact of Sulfide Electrolyte Thermal Decomposition
The thermal decomposition of sulfide electrolytes presents significant environmental concerns that warrant careful consideration in the development and deployment of solid-state battery technologies. When subjected to thermal abuse conditions, sulfide electrolytes can release hydrogen sulfide (H2S) and other sulfur-containing compounds, which are not only toxic to humans but also environmentally hazardous. These emissions can contribute to air pollution, potentially causing acid rain formation when oxidized to sulfur dioxide and subsequently to sulfuric acid in the atmosphere.
Water contamination represents another critical environmental risk, as decomposition products from sulfide electrolytes can leach into groundwater systems. The high water-reactivity of materials like Li6PS5Cl and Li10GeP2S12 means that improper disposal or leakage from damaged batteries could introduce soluble lithium polysulfides and phosphorus compounds into aquatic ecosystems, potentially disrupting pH balances and introducing toxic elements into the food chain.
Soil contamination follows a similar pattern, with thermal decomposition products potentially altering soil chemistry and affecting microbial communities essential for ecosystem functioning. The persistence of these compounds in soil environments depends on local conditions but may lead to long-term ecological impacts if not properly managed.
From a lifecycle assessment perspective, the environmental footprint of sulfide electrolytes extends beyond operational safety concerns. The mining and processing of raw materials for these electrolytes, particularly germanium and phosphorus, involve energy-intensive processes with their own environmental implications. End-of-life management presents additional challenges, as conventional battery recycling infrastructure is not optimized for handling sulfide-based components.
Mitigation strategies are emerging to address these environmental concerns. These include the development of more thermally stable sulfide compositions that produce fewer toxic byproducts when decomposed, as well as advanced containment systems designed to prevent environmental release even under severe thermal abuse conditions. Recycling technologies specifically tailored to sulfide electrolytes are also under development, aiming to recover valuable materials while neutralizing potentially harmful components.
Regulatory frameworks worldwide are beginning to acknowledge these specific environmental risks, with several jurisdictions implementing specialized guidelines for the handling, transportation, and disposal of sulfide-based battery materials. These regulations typically mandate specific containment protocols and treatment processes to minimize environmental exposure to decomposition products.
Water contamination represents another critical environmental risk, as decomposition products from sulfide electrolytes can leach into groundwater systems. The high water-reactivity of materials like Li6PS5Cl and Li10GeP2S12 means that improper disposal or leakage from damaged batteries could introduce soluble lithium polysulfides and phosphorus compounds into aquatic ecosystems, potentially disrupting pH balances and introducing toxic elements into the food chain.
Soil contamination follows a similar pattern, with thermal decomposition products potentially altering soil chemistry and affecting microbial communities essential for ecosystem functioning. The persistence of these compounds in soil environments depends on local conditions but may lead to long-term ecological impacts if not properly managed.
From a lifecycle assessment perspective, the environmental footprint of sulfide electrolytes extends beyond operational safety concerns. The mining and processing of raw materials for these electrolytes, particularly germanium and phosphorus, involve energy-intensive processes with their own environmental implications. End-of-life management presents additional challenges, as conventional battery recycling infrastructure is not optimized for handling sulfide-based components.
Mitigation strategies are emerging to address these environmental concerns. These include the development of more thermally stable sulfide compositions that produce fewer toxic byproducts when decomposed, as well as advanced containment systems designed to prevent environmental release even under severe thermal abuse conditions. Recycling technologies specifically tailored to sulfide electrolytes are also under development, aiming to recover valuable materials while neutralizing potentially harmful components.
Regulatory frameworks worldwide are beginning to acknowledge these specific environmental risks, with several jurisdictions implementing specialized guidelines for the handling, transportation, and disposal of sulfide-based battery materials. These regulations typically mandate specific containment protocols and treatment processes to minimize environmental exposure to decomposition products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!