Electrochemical Stability Windows And Redox Side Reactions In Sulfides
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfide Electrolytes Background and Research Objectives
Sulfide-based solid electrolytes have emerged as promising candidates for next-generation all-solid-state batteries (ASSBs) due to their high ionic conductivity, which can reach levels comparable to or even exceeding those of liquid electrolytes. The development of these materials represents a significant milestone in the evolution of energy storage technology, potentially addressing critical limitations of conventional lithium-ion batteries such as safety concerns, limited energy density, and restricted operational temperature ranges.
The history of sulfide electrolytes dates back to the 1980s when researchers first identified their potential for fast ion conduction. However, it wasn't until the early 2000s that significant breakthroughs in synthesis methods and compositional optimization led to dramatic improvements in their ionic conductivity. The discovery of superionic conductors like Li10GeP2S12 (LGPS) in 2011 marked a turning point, demonstrating room-temperature conductivities exceeding 10 mS/cm.
Despite these advances, the practical implementation of sulfide electrolytes faces substantial challenges, particularly regarding their electrochemical stability windows and susceptibility to redox side reactions. These materials often exhibit narrow electrochemical stability windows, typically ranging from 1.7 to 2.5 V vs. Li/Li+, which is insufficient for high-voltage cathode materials. When operating outside this stability window, sulfide electrolytes undergo decomposition reactions, forming interphases that can be either beneficial or detrimental to battery performance.
The redox side reactions in sulfide electrolytes primarily involve the reduction of sulfur species at the anode interface and oxidation at the cathode interface. These reactions can lead to increased interfacial resistance, capacity fading, and compromised cycle life. Understanding and controlling these reactions represents one of the most critical challenges in the development of practical sulfide-based ASSBs.
The primary objectives of this research are to comprehensively investigate the electrochemical stability windows of various sulfide electrolyte systems, identify the mechanisms and products of redox side reactions, and develop strategies to mitigate their negative impacts. This includes exploring compositional modifications, protective coatings, and interfacial engineering approaches to expand the effective electrochemical stability window.
Additionally, this research aims to establish standardized testing protocols for evaluating the electrochemical stability of sulfide electrolytes, as current methodologies often yield inconsistent results across different research groups. By developing more reliable assessment techniques, we can better compare different electrolyte systems and accelerate the optimization process.
The ultimate goal is to enable the development of sulfide electrolytes with enhanced electrochemical stability that can be successfully integrated with high-voltage cathodes and lithium metal anodes, thereby unlocking the full potential of ASSBs for next-generation energy storage applications in electric vehicles, grid storage, and portable electronics.
The history of sulfide electrolytes dates back to the 1980s when researchers first identified their potential for fast ion conduction. However, it wasn't until the early 2000s that significant breakthroughs in synthesis methods and compositional optimization led to dramatic improvements in their ionic conductivity. The discovery of superionic conductors like Li10GeP2S12 (LGPS) in 2011 marked a turning point, demonstrating room-temperature conductivities exceeding 10 mS/cm.
Despite these advances, the practical implementation of sulfide electrolytes faces substantial challenges, particularly regarding their electrochemical stability windows and susceptibility to redox side reactions. These materials often exhibit narrow electrochemical stability windows, typically ranging from 1.7 to 2.5 V vs. Li/Li+, which is insufficient for high-voltage cathode materials. When operating outside this stability window, sulfide electrolytes undergo decomposition reactions, forming interphases that can be either beneficial or detrimental to battery performance.
The redox side reactions in sulfide electrolytes primarily involve the reduction of sulfur species at the anode interface and oxidation at the cathode interface. These reactions can lead to increased interfacial resistance, capacity fading, and compromised cycle life. Understanding and controlling these reactions represents one of the most critical challenges in the development of practical sulfide-based ASSBs.
The primary objectives of this research are to comprehensively investigate the electrochemical stability windows of various sulfide electrolyte systems, identify the mechanisms and products of redox side reactions, and develop strategies to mitigate their negative impacts. This includes exploring compositional modifications, protective coatings, and interfacial engineering approaches to expand the effective electrochemical stability window.
Additionally, this research aims to establish standardized testing protocols for evaluating the electrochemical stability of sulfide electrolytes, as current methodologies often yield inconsistent results across different research groups. By developing more reliable assessment techniques, we can better compare different electrolyte systems and accelerate the optimization process.
The ultimate goal is to enable the development of sulfide electrolytes with enhanced electrochemical stability that can be successfully integrated with high-voltage cathodes and lithium metal anodes, thereby unlocking the full potential of ASSBs for next-generation energy storage applications in electric vehicles, grid storage, and portable electronics.
Market Analysis for Solid-State Battery Technologies
The solid-state battery market is experiencing unprecedented growth, driven by increasing demand for safer, higher energy density power solutions across multiple industries. Current market valuations place the global solid-state battery sector at approximately $500 million in 2023, with projections indicating potential expansion to $8-10 billion by 2030, representing a compound annual growth rate exceeding 35%.
Sulfide-based solid electrolytes have emerged as particularly promising materials due to their high ionic conductivity comparable to liquid electrolytes. Market research indicates that among various solid electrolyte categories, sulfides currently represent about 25% of research focus but only 15% of commercial development efforts, highlighting a significant opportunity gap.
The automotive sector remains the primary driver for solid-state battery adoption, with major manufacturers including Toyota, Volkswagen, and BMW investing heavily in sulfide-based technologies. These investments are motivated by the potential for faster charging capabilities and higher energy densities that sulfide electrolytes theoretically enable, despite challenges with electrochemical stability windows.
Consumer electronics represents the second largest market segment, with companies seeking longer-lasting, safer battery solutions. This sector values the reduced risk of thermal runaway that solid-state technologies offer, particularly as device manufacturers face increasing regulatory scrutiny regarding battery safety.
Market analysis reveals a significant barrier to widespread sulfide electrolyte adoption: the narrow electrochemical stability windows and redox side reactions that occur at electrode interfaces. These technical challenges directly impact product longevity and performance, creating hesitancy among potential adopters and investors.
Regional market distribution shows Asia-Pacific leading development and production, with Japan and South Korea holding approximately 60% of sulfide-based solid-state battery patents. North America follows with strong research initiatives but fewer commercialization efforts, while Europe demonstrates balanced investment across both research and commercialization phases.
Customer willingness-to-pay metrics indicate premium pricing potential of 30-40% over conventional lithium-ion batteries, provided that cycle life and energy density improvements can be demonstrated. However, this premium erodes significantly if electrochemical stability issues remain unresolved, highlighting the direct market impact of the technical challenges associated with sulfide electrolytes.
Market forecasts suggest that breakthroughs in managing electrochemical stability windows and mitigating redox side reactions could accelerate market penetration by 3-5 years compared to current adoption timelines, underscoring the economic importance of addressing these specific technical challenges.
Sulfide-based solid electrolytes have emerged as particularly promising materials due to their high ionic conductivity comparable to liquid electrolytes. Market research indicates that among various solid electrolyte categories, sulfides currently represent about 25% of research focus but only 15% of commercial development efforts, highlighting a significant opportunity gap.
The automotive sector remains the primary driver for solid-state battery adoption, with major manufacturers including Toyota, Volkswagen, and BMW investing heavily in sulfide-based technologies. These investments are motivated by the potential for faster charging capabilities and higher energy densities that sulfide electrolytes theoretically enable, despite challenges with electrochemical stability windows.
Consumer electronics represents the second largest market segment, with companies seeking longer-lasting, safer battery solutions. This sector values the reduced risk of thermal runaway that solid-state technologies offer, particularly as device manufacturers face increasing regulatory scrutiny regarding battery safety.
Market analysis reveals a significant barrier to widespread sulfide electrolyte adoption: the narrow electrochemical stability windows and redox side reactions that occur at electrode interfaces. These technical challenges directly impact product longevity and performance, creating hesitancy among potential adopters and investors.
Regional market distribution shows Asia-Pacific leading development and production, with Japan and South Korea holding approximately 60% of sulfide-based solid-state battery patents. North America follows with strong research initiatives but fewer commercialization efforts, while Europe demonstrates balanced investment across both research and commercialization phases.
Customer willingness-to-pay metrics indicate premium pricing potential of 30-40% over conventional lithium-ion batteries, provided that cycle life and energy density improvements can be demonstrated. However, this premium erodes significantly if electrochemical stability issues remain unresolved, highlighting the direct market impact of the technical challenges associated with sulfide electrolytes.
Market forecasts suggest that breakthroughs in managing electrochemical stability windows and mitigating redox side reactions could accelerate market penetration by 3-5 years compared to current adoption timelines, underscoring the economic importance of addressing these specific technical challenges.
Current Challenges in Sulfide Electrochemical Stability
Sulfide-based solid electrolytes have garnered significant attention in the development of all-solid-state batteries due to their high ionic conductivity. However, their practical implementation faces substantial challenges related to electrochemical stability. The narrow electrochemical stability windows of most sulfide electrolytes (typically 1-3V) severely limit their compatibility with high-voltage cathode materials and lithium metal anodes, creating a fundamental barrier to achieving high energy density batteries.
When operating outside their stability windows, sulfide electrolytes undergo detrimental redox reactions. At high potentials (>2.5V vs. Li/Li+), oxidative decomposition occurs, forming insulating phases like Li2S, P2S5, and elemental sulfur. These decomposition products increase interfacial resistance and impede lithium-ion transport. Conversely, at low potentials (<1.7V vs. Li/Li+), reductive decomposition leads to the formation of Li2S and lithium phosphides, which can cause capacity fade and safety concerns.
The reactivity of sulfides with moisture presents another critical challenge. Even trace amounts of atmospheric moisture can trigger the formation of toxic H2S gas and degrade the electrolyte structure. This high sensitivity necessitates stringent manufacturing conditions and complicates practical handling and integration into commercial battery systems.
Interface stability issues between sulfide electrolytes and electrode materials represent a significant technical hurdle. Chemical and electrochemical reactions at these interfaces form interphases with poor ionic conductivity, increasing cell impedance and accelerating capacity fade. The volume changes during cycling further exacerbate these interfacial problems, creating mechanical stress that can lead to contact loss and cell failure.
Current mitigation strategies, such as protective coatings and buffer layers, offer only partial solutions and often introduce additional complexity and cost to the manufacturing process. The development of artificial solid electrolyte interphases (SEIs) shows promise but faces challenges in achieving uniform coverage and long-term stability under realistic operating conditions.
The fundamental understanding of degradation mechanisms in sulfide electrolytes remains incomplete. Real-time characterization of interfacial reactions under operating conditions is technically challenging, limiting the development of effective stabilization strategies. Advanced in-situ and operando analytical techniques are needed to elucidate the complex redox processes occurring at electrolyte-electrode interfaces.
Computational modeling efforts to predict stability windows and reaction pathways are hampered by the complexity of the multi-component systems and the dynamic nature of the interfaces. Bridging the gap between theoretical predictions and experimental observations represents a significant scientific challenge that must be addressed to enable rational design of more stable sulfide electrolytes.
When operating outside their stability windows, sulfide electrolytes undergo detrimental redox reactions. At high potentials (>2.5V vs. Li/Li+), oxidative decomposition occurs, forming insulating phases like Li2S, P2S5, and elemental sulfur. These decomposition products increase interfacial resistance and impede lithium-ion transport. Conversely, at low potentials (<1.7V vs. Li/Li+), reductive decomposition leads to the formation of Li2S and lithium phosphides, which can cause capacity fade and safety concerns.
The reactivity of sulfides with moisture presents another critical challenge. Even trace amounts of atmospheric moisture can trigger the formation of toxic H2S gas and degrade the electrolyte structure. This high sensitivity necessitates stringent manufacturing conditions and complicates practical handling and integration into commercial battery systems.
Interface stability issues between sulfide electrolytes and electrode materials represent a significant technical hurdle. Chemical and electrochemical reactions at these interfaces form interphases with poor ionic conductivity, increasing cell impedance and accelerating capacity fade. The volume changes during cycling further exacerbate these interfacial problems, creating mechanical stress that can lead to contact loss and cell failure.
Current mitigation strategies, such as protective coatings and buffer layers, offer only partial solutions and often introduce additional complexity and cost to the manufacturing process. The development of artificial solid electrolyte interphases (SEIs) shows promise but faces challenges in achieving uniform coverage and long-term stability under realistic operating conditions.
The fundamental understanding of degradation mechanisms in sulfide electrolytes remains incomplete. Real-time characterization of interfacial reactions under operating conditions is technically challenging, limiting the development of effective stabilization strategies. Advanced in-situ and operando analytical techniques are needed to elucidate the complex redox processes occurring at electrolyte-electrode interfaces.
Computational modeling efforts to predict stability windows and reaction pathways are hampered by the complexity of the multi-component systems and the dynamic nature of the interfaces. Bridging the gap between theoretical predictions and experimental observations represents a significant scientific challenge that must be addressed to enable rational design of more stable sulfide electrolytes.
State-of-the-Art Solutions for Redox Stability
01 Sulfide-based solid electrolytes for batteries
Sulfide-based solid electrolytes are used in batteries due to their high ionic conductivity and electrochemical stability. These materials provide wide electrochemical stability windows, making them suitable for high-voltage applications. The stability window can be enhanced through compositional modifications and surface treatments, allowing for improved battery performance and safety. These electrolytes typically contain lithium sulfide combined with other elements to optimize their electrochemical properties.- Sulfide-based solid electrolytes for batteries: Sulfide-based solid electrolytes are used in all-solid-state batteries due to their high ionic conductivity and wide electrochemical stability windows. These materials provide improved safety and energy density compared to conventional liquid electrolytes. The electrochemical stability window of sulfide electrolytes can be enhanced through compositional modifications and protective coatings, allowing them to operate effectively at various voltage ranges without decomposition.
- Metal sulfide electrodes for energy storage: Metal sulfides serve as electrode materials in various electrochemical energy storage devices. These materials offer high theoretical capacity and favorable redox properties. The electrochemical stability window of metal sulfide electrodes can be optimized through nanostructuring, carbon compositing, and surface modifications, which help prevent dissolution and structural degradation during cycling, thereby extending the operational voltage range.
- Polymer-sulfide composite electrolytes: Polymer-sulfide composite electrolytes combine the flexibility of polymers with the high ionic conductivity of sulfide materials. These composites can achieve wider electrochemical stability windows than pure sulfide electrolytes. The polymer matrix helps suppress interfacial reactions between the sulfide components and electrodes, enabling stable operation across broader voltage ranges while maintaining good mechanical properties.
- Interface engineering for sulfide stability: Interface engineering techniques are employed to expand the effective electrochemical stability window of sulfide materials. These approaches include the application of buffer layers, artificial solid electrolyte interphases, and gradient compositions at electrode-electrolyte interfaces. Such modifications help prevent direct contact between sulfides and reactive electrode materials, mitigating decomposition reactions and extending the operational voltage range.
- Characterization methods for sulfide stability windows: Various analytical techniques are used to determine the electrochemical stability windows of sulfide materials. These include cyclic voltammetry, impedance spectroscopy, in-situ X-ray diffraction, and computational modeling. Advanced characterization methods help identify decomposition mechanisms and stability limits, guiding the development of sulfide materials with expanded electrochemical windows for specific applications.
02 Metal sulfide electrodes for energy storage
Metal sulfides are utilized as electrode materials in various energy storage devices due to their favorable electrochemical stability windows. These materials can undergo reversible redox reactions within specific voltage ranges without decomposition. The electrochemical stability window of metal sulfide electrodes can be tailored by controlling their composition, morphology, and crystallinity. This enables their application in batteries, supercapacitors, and other electrochemical systems with enhanced cycling stability.Expand Specific Solutions03 Polymer-sulfide composite electrolytes
Polymer-sulfide composite electrolytes combine the flexibility of polymers with the high ionic conductivity of sulfides to create materials with improved electrochemical stability windows. These composites can withstand wider voltage ranges without degradation compared to pure sulfide electrolytes. The polymer matrix helps to stabilize the sulfide components and prevent unwanted side reactions at electrode interfaces. This approach enables the development of safer and more durable electrochemical devices.Expand Specific Solutions04 Sulfide interface engineering for extended stability
Engineering the interfaces between sulfide materials and electrodes is crucial for extending electrochemical stability windows. Protective coatings, buffer layers, and surface modifications can prevent direct contact between reactive components, thereby suppressing unwanted reactions that would narrow the stability window. Various techniques such as atomic layer deposition, solution processing, and solid-state reactions are employed to create these engineered interfaces, resulting in improved electrochemical performance and longevity.Expand Specific Solutions05 Electrochemical characterization of sulfide stability
Methods for accurately determining the electrochemical stability windows of sulfide materials are essential for their application in energy storage and conversion devices. Techniques such as cyclic voltammetry, linear sweep voltammetry, and electrochemical impedance spectroscopy are commonly used to measure the voltage range within which sulfides remain stable. Advanced in-situ and operando characterization approaches provide deeper insights into degradation mechanisms and stability limits, enabling the rational design of sulfide materials with optimized electrochemical properties.Expand Specific Solutions
Leading Organizations in Sulfide Electrolyte Research
The electrochemical stability window (ESW) and redox side reactions in sulfides represent a critical technological challenge in the energy storage sector, currently in its growth phase with an estimated market size exceeding $5 billion. The technology is approaching maturity but faces stability limitations. Leading players include LG Energy Solution and Samsung SDI, who are advancing sulfide solid electrolytes for next-generation batteries, while research institutions like Dalian Institute of Chemical Physics and Harvard College focus on fundamental electrochemical mechanisms. Companies such as Toyota and Rivian are exploring sulfide applications in electric vehicles, while materials specialists like Soulbrain and Air Products & Chemicals develop enhanced sulfide compositions with improved stability windows to minimize detrimental redox reactions that currently limit commercial viability.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced sulfide-based solid electrolytes with enhanced electrochemical stability windows (ESW) through surface modification techniques. Their approach involves coating sulfide particles with lithium niobium oxide (LNO) layers that create a protective interface between the electrolyte and electrodes. This coating significantly widens the ESW from typical 1.7-2.1V to over 3V, enabling compatibility with high-voltage cathode materials. LG Chem's proprietary "gradient interface engineering" introduces concentration gradients of elements like Al, Ta, and Nb at electrolyte-electrode interfaces, which effectively suppresses redox side reactions by creating kinetic barriers against lithium dendrite formation and preventing decomposition products from propagating through the electrolyte bulk. Their sulfide systems incorporate halogen doping (particularly chlorine and bromine) to enhance ionic conductivity while simultaneously improving oxidative stability.
Strengths: Superior ionic conductivity (>5 mS/cm at room temperature) compared to oxide systems while maintaining improved stability; scalable manufacturing process compatible with existing battery production lines. Weaknesses: Still requires protective coatings for high-voltage operation; some interface engineering solutions increase overall cell resistance; potential long-term degradation from accumulated decomposition products at interfaces.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute has developed an innovative approach to sulfide electrolyte stability through their "dual-phase composite electrolyte" technology. Their research focuses on creating engineered heterojunctions between different sulfide phases (typically Li3PS4 and Li7P3S11) that exhibit complementary electrochemical stability properties. By precisely controlling the spatial distribution and interfacial characteristics of these phases, they've created electrolyte systems with expanded effective stability windows. Their proprietary "gradient concentration profile" technique introduces controlled amounts of oxide components (such as Li2O, Li3PO4) that gradually increase in concentration toward electrode interfaces, creating a natural barrier against redox reactions. The institute has also pioneered the use of lithium thiophosphate glass-ceramic electrolytes with nano-sized Li3PS4 crystals embedded in an amorphous matrix, which demonstrates superior resistance to lithium dendrite penetration while maintaining high ionic conductivity. Their recent work incorporates graphene oxide nanosheets as protective layers at sulfide-cathode interfaces, which effectively block electron transfer while allowing lithium ion transport, significantly reducing interfacial side reactions.
Strengths: Innovative heterojunction approach provides both high conductivity and improved stability; demonstrated effectiveness against lithium dendrite formation; relatively simple manufacturing compared to complex coating processes. Weaknesses: Potential long-term phase separation issues in composite systems; some approaches reduce overall ionic conductivity; challenges in achieving uniform distribution of protective components throughout the electrolyte.
Critical Patents in Sulfide Electrochemical Windows
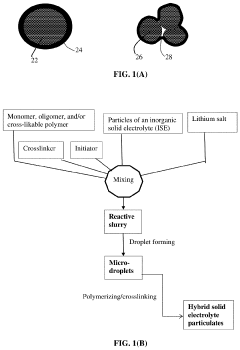
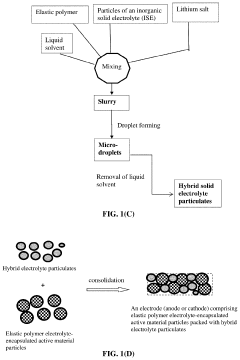
Elastomer/Inorganic Hybrid Solid-State Electrolytes, Lithium Batteries Containing Same, and Production Processes
PatentPendingUS20230238575A1
Innovation
- A hybrid solid electrolyte system comprising inorganic solid electrolyte particles encapsulated in an elastic polymer shell, which provides lithium-ion conductivity from 10^-6 to 5×10^-2 S/cm, minimizes electrolyte volume, and forms a contiguous phase with electrode active materials, while being compatible with existing battery production facilities.
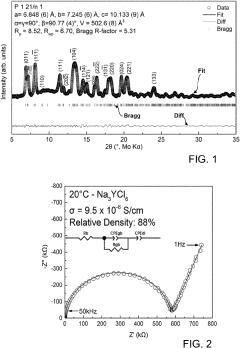
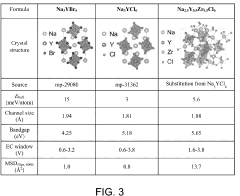
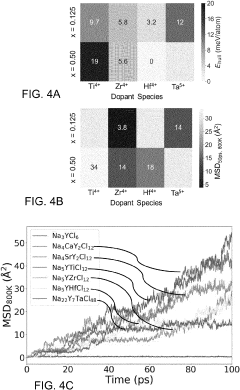
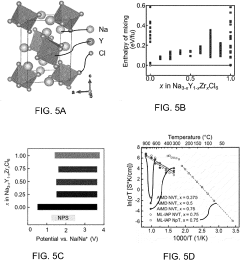
Chlorine-Based Sodium Solid Electrolyte
PatentPendingUS20230115956A1
Innovation
- Development of chloride-based sodium-ion conductors, specifically Na3-xY1-xZrxCl6 compounds, which enhance sodium diffusion and provide superior chemical and electrochemical stability with high voltage cathodes, preventing unwanted reactions and degradation, and are used in a cathode composite without protective coatings.
Safety and Environmental Implications of Sulfide Materials
The safety and environmental implications of sulfide-based materials present significant considerations for their widespread adoption in energy storage technologies. Sulfide solid electrolytes, while promising for next-generation batteries, pose unique hazards that require careful management throughout their lifecycle.
When exposed to moisture or humid air, many sulfide materials undergo hydrolysis reactions that generate toxic hydrogen sulfide (H2S) gas. This reaction not only compromises the electrochemical performance of the materials but also creates immediate safety risks in manufacturing, handling, and disposal environments. The threshold limit value for H2S exposure is extremely low at 10 ppm, highlighting the severity of this concern.
Manufacturing processes for sulfide materials typically require stringent moisture-free environments, necessitating specialized equipment and protocols that increase production costs and complexity. These requirements extend to battery assembly operations, where any moisture ingress can trigger degradation reactions and compromise cell integrity.
Environmental considerations extend beyond operational safety to end-of-life management. The potential leaching of sulfur compounds from improperly disposed materials could lead to soil and water contamination. Current recycling infrastructure is inadequately prepared for the specific challenges posed by sulfide-containing batteries, creating a significant gap in the circular economy approach necessary for sustainable battery technologies.
Toxicological studies on various sulfide materials remain limited, particularly regarding long-term exposure effects and environmental persistence. This knowledge gap complicates risk assessment and regulatory compliance, potentially slowing commercial deployment of these technologies.
The electrochemical instability windows of sulfides also contribute to safety concerns through possible formation of polysulfides during cycling. These compounds can participate in shuttle reactions that not only reduce battery efficiency but may also create additional safety hazards through pressure buildup and thermal events.
Addressing these challenges requires multidisciplinary approaches, including development of protective coatings to prevent hydrolysis, improved encapsulation technologies, and dedicated recycling processes. Some researchers are exploring chemical modifications to reduce reactivity while maintaining ionic conductivity properties.
Regulatory frameworks worldwide are beginning to acknowledge these unique challenges, with evolving standards for handling, transportation, and disposal of sulfide-containing energy storage systems. Industry-academic collaborations are increasingly focused on developing comprehensive safety protocols and environmental impact assessments specific to sulfide materials.
When exposed to moisture or humid air, many sulfide materials undergo hydrolysis reactions that generate toxic hydrogen sulfide (H2S) gas. This reaction not only compromises the electrochemical performance of the materials but also creates immediate safety risks in manufacturing, handling, and disposal environments. The threshold limit value for H2S exposure is extremely low at 10 ppm, highlighting the severity of this concern.
Manufacturing processes for sulfide materials typically require stringent moisture-free environments, necessitating specialized equipment and protocols that increase production costs and complexity. These requirements extend to battery assembly operations, where any moisture ingress can trigger degradation reactions and compromise cell integrity.
Environmental considerations extend beyond operational safety to end-of-life management. The potential leaching of sulfur compounds from improperly disposed materials could lead to soil and water contamination. Current recycling infrastructure is inadequately prepared for the specific challenges posed by sulfide-containing batteries, creating a significant gap in the circular economy approach necessary for sustainable battery technologies.
Toxicological studies on various sulfide materials remain limited, particularly regarding long-term exposure effects and environmental persistence. This knowledge gap complicates risk assessment and regulatory compliance, potentially slowing commercial deployment of these technologies.
The electrochemical instability windows of sulfides also contribute to safety concerns through possible formation of polysulfides during cycling. These compounds can participate in shuttle reactions that not only reduce battery efficiency but may also create additional safety hazards through pressure buildup and thermal events.
Addressing these challenges requires multidisciplinary approaches, including development of protective coatings to prevent hydrolysis, improved encapsulation technologies, and dedicated recycling processes. Some researchers are exploring chemical modifications to reduce reactivity while maintaining ionic conductivity properties.
Regulatory frameworks worldwide are beginning to acknowledge these unique challenges, with evolving standards for handling, transportation, and disposal of sulfide-containing energy storage systems. Industry-academic collaborations are increasingly focused on developing comprehensive safety protocols and environmental impact assessments specific to sulfide materials.
Manufacturing Scalability of Sulfide-Based Batteries
The scalability of manufacturing processes for sulfide-based batteries represents a critical challenge in transitioning from laboratory-scale research to commercial production. Current manufacturing methods for sulfide solid electrolytes often involve complex processes such as mechanical ball milling, solution processing, or high-temperature synthesis, which present significant barriers to large-scale implementation.
The electrochemical stability windows and redox side reactions in sulfides directly impact manufacturing scalability. Sulfide electrolytes typically exhibit narrow electrochemical stability windows (approximately 1-3V), making them susceptible to decomposition during battery operation. This instability necessitates additional manufacturing steps to incorporate protective coatings or buffer layers, increasing production complexity and cost.
Material handling presents another major challenge in scaling production. Sulfide electrolytes are highly sensitive to moisture and oxygen, requiring strictly controlled dry-room or inert atmosphere conditions throughout the manufacturing process. This environmental control adds substantial infrastructure costs and operational complexity to production facilities, limiting the number of potential manufacturers capable of producing these materials at scale.
The reactivity of sulfides with conventional electrode materials creates additional manufacturing hurdles. Interface engineering becomes essential to prevent undesired side reactions, often requiring specialized coating processes or the development of compatible composite materials. These additional processing steps further complicate the manufacturing workflow and increase production costs.
Raw material supply chains for high-purity sulfur compounds and other precursors needed for sulfide electrolyte synthesis remain underdeveloped compared to those for conventional battery materials. This supply limitation creates potential bottlenecks in scaling production to commercial levels and introduces volatility in material costs.
Equipment compatibility issues also emerge when scaling sulfide-based battery production. Conventional battery manufacturing equipment may require significant modifications to accommodate the unique processing requirements of sulfide materials, particularly regarding air sensitivity and mechanical properties. These modifications represent substantial capital investments for manufacturers entering this space.
Despite these challenges, recent advances in processing technologies show promise for improving manufacturing scalability. Developments in dry coating methods, solvent-free processing, and rapid synthesis techniques are gradually addressing key production bottlenecks. Additionally, innovative approaches to interface stabilization are reducing the complexity of cell assembly processes, potentially enabling more streamlined manufacturing workflows for sulfide-based battery systems.
The electrochemical stability windows and redox side reactions in sulfides directly impact manufacturing scalability. Sulfide electrolytes typically exhibit narrow electrochemical stability windows (approximately 1-3V), making them susceptible to decomposition during battery operation. This instability necessitates additional manufacturing steps to incorporate protective coatings or buffer layers, increasing production complexity and cost.
Material handling presents another major challenge in scaling production. Sulfide electrolytes are highly sensitive to moisture and oxygen, requiring strictly controlled dry-room or inert atmosphere conditions throughout the manufacturing process. This environmental control adds substantial infrastructure costs and operational complexity to production facilities, limiting the number of potential manufacturers capable of producing these materials at scale.
The reactivity of sulfides with conventional electrode materials creates additional manufacturing hurdles. Interface engineering becomes essential to prevent undesired side reactions, often requiring specialized coating processes or the development of compatible composite materials. These additional processing steps further complicate the manufacturing workflow and increase production costs.
Raw material supply chains for high-purity sulfur compounds and other precursors needed for sulfide electrolyte synthesis remain underdeveloped compared to those for conventional battery materials. This supply limitation creates potential bottlenecks in scaling production to commercial levels and introduces volatility in material costs.
Equipment compatibility issues also emerge when scaling sulfide-based battery production. Conventional battery manufacturing equipment may require significant modifications to accommodate the unique processing requirements of sulfide materials, particularly regarding air sensitivity and mechanical properties. These modifications represent substantial capital investments for manufacturers entering this space.
Despite these challenges, recent advances in processing technologies show promise for improving manufacturing scalability. Developments in dry coating methods, solvent-free processing, and rapid synthesis techniques are gradually addressing key production bottlenecks. Additionally, innovative approaches to interface stabilization are reducing the complexity of cell assembly processes, potentially enabling more streamlined manufacturing workflows for sulfide-based battery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!