Lithium Metal Nucleation And Void Mitigation With Sulfide SSEs
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Metal Battery Evolution and Research Objectives
The evolution of lithium-ion batteries has been a cornerstone of modern portable electronics and electric vehicles since their commercial introduction by Sony in 1991. However, conventional lithium-ion batteries with graphite anodes have approached their theoretical energy density limits, creating an urgent need for next-generation energy storage solutions. Lithium metal batteries (LMBs) have emerged as promising candidates due to their significantly higher theoretical capacity (3860 mAh/g) compared to graphite anodes (372 mAh/g), potentially enabling energy densities exceeding 500 Wh/kg at the cell level.
The historical development of lithium metal batteries dates back to the 1970s, but their commercialization was hindered by safety concerns related to dendrite formation and poor cycling stability. These challenges led to the pivot toward intercalation-based lithium-ion batteries. Recent advances in materials science and electrolyte engineering have reignited interest in lithium metal anodes, particularly when paired with solid-state electrolytes that could potentially address the historical limitations.
Sulfide solid-state electrolytes (SSEs) have gained significant attention due to their high ionic conductivity (often exceeding 10^-3 S/cm at room temperature), favorable mechanical properties, and potential to suppress lithium dendrite growth. Materials such as Li10GeP2S12 (LGPS), Li6PS5Cl, and Li3PS4 represent breakthrough developments in this field, offering conductivities comparable to or exceeding those of liquid electrolytes while potentially providing enhanced safety and electrochemical stability.
The current research landscape focuses on understanding the fundamental mechanisms of lithium metal nucleation and growth at the interface with sulfide SSEs. Despite their promising properties, challenges remain in controlling lithium deposition morphology and preventing void formation during cycling, which leads to increased interfacial resistance and eventual cell failure. These issues are particularly critical for practical applications requiring long cycle life and high reliability.
Our research objectives center on elucidating the physicochemical processes governing lithium nucleation behavior at sulfide SSE interfaces and developing strategies to mitigate void formation during lithium plating/stripping cycles. Specifically, we aim to: (1) investigate the correlation between SSE surface chemistry and lithium nucleation patterns; (2) understand the role of mechanical pressure and interfacial engineering in promoting uniform lithium deposition; (3) develop novel sulfide SSE compositions with optimized interfaces for lithium metal anodes; and (4) establish design principles for practical lithium metal batteries utilizing sulfide electrolytes.
Through this research, we anticipate contributing to the fundamental understanding of solid-state electrochemistry while advancing practical solutions for next-generation energy storage systems that could revolutionize electric transportation, portable electronics, and grid-scale applications.
The historical development of lithium metal batteries dates back to the 1970s, but their commercialization was hindered by safety concerns related to dendrite formation and poor cycling stability. These challenges led to the pivot toward intercalation-based lithium-ion batteries. Recent advances in materials science and electrolyte engineering have reignited interest in lithium metal anodes, particularly when paired with solid-state electrolytes that could potentially address the historical limitations.
Sulfide solid-state electrolytes (SSEs) have gained significant attention due to their high ionic conductivity (often exceeding 10^-3 S/cm at room temperature), favorable mechanical properties, and potential to suppress lithium dendrite growth. Materials such as Li10GeP2S12 (LGPS), Li6PS5Cl, and Li3PS4 represent breakthrough developments in this field, offering conductivities comparable to or exceeding those of liquid electrolytes while potentially providing enhanced safety and electrochemical stability.
The current research landscape focuses on understanding the fundamental mechanisms of lithium metal nucleation and growth at the interface with sulfide SSEs. Despite their promising properties, challenges remain in controlling lithium deposition morphology and preventing void formation during cycling, which leads to increased interfacial resistance and eventual cell failure. These issues are particularly critical for practical applications requiring long cycle life and high reliability.
Our research objectives center on elucidating the physicochemical processes governing lithium nucleation behavior at sulfide SSE interfaces and developing strategies to mitigate void formation during lithium plating/stripping cycles. Specifically, we aim to: (1) investigate the correlation between SSE surface chemistry and lithium nucleation patterns; (2) understand the role of mechanical pressure and interfacial engineering in promoting uniform lithium deposition; (3) develop novel sulfide SSE compositions with optimized interfaces for lithium metal anodes; and (4) establish design principles for practical lithium metal batteries utilizing sulfide electrolytes.
Through this research, we anticipate contributing to the fundamental understanding of solid-state electrochemistry while advancing practical solutions for next-generation energy storage systems that could revolutionize electric transportation, portable electronics, and grid-scale applications.
Market Analysis for Solid-State Battery Technologies
The solid-state battery market is experiencing unprecedented growth, driven by increasing demand for safer, higher energy density power solutions across multiple industries. Current market valuations place the global solid-state battery sector at approximately $500 million in 2023, with projections indicating expansion to $8-10 billion by 2030, representing a compound annual growth rate exceeding 35%.
Automotive applications constitute the primary market driver, with major manufacturers including Toyota, Volkswagen, and BMW making substantial investments in solid-state technology. These companies view sulfide-based solid-state electrolytes as critical to achieving the 500+ mile range targets necessary for mainstream electric vehicle adoption while addressing safety concerns associated with conventional lithium-ion batteries.
Consumer electronics represents the second largest market segment, with manufacturers seeking higher energy density and improved safety profiles for next-generation devices. The premium smartphone and wearable technology sectors have demonstrated willingness to adopt higher-cost battery solutions that deliver tangible performance improvements.
Regionally, Asia-Pacific dominates the solid-state battery landscape, with Japan and South Korea leading research initiatives specifically focused on sulfide-based electrolytes. North America and Europe are rapidly expanding their research capabilities, with significant government funding supporting development programs targeting lithium metal anode technologies.
Market analysis reveals that sulfide-based solid electrolytes hold particular commercial promise due to their superior ionic conductivity compared to oxide and polymer alternatives. Companies specializing in sulfide electrolytes, including Solid Power and QuantumScape, have secured substantial investment based on their potential to address lithium metal nucleation and void formation challenges.
Investor confidence in sulfide-based solid-state technology remains strong despite technical hurdles, with venture capital funding exceeding $2 billion in 2022 alone. This investment surge reflects recognition that solving the lithium metal nucleation and void formation challenges represents a critical inflection point for commercialization.
Market forecasts indicate that initial commercial applications of sulfide-based solid-state batteries will emerge in premium consumer electronics by 2025, followed by limited automotive deployment by 2027. Mass market penetration is anticipated by 2030, contingent upon successful resolution of manufacturing scalability challenges and continued progress in addressing lithium metal interface stability issues.
Automotive applications constitute the primary market driver, with major manufacturers including Toyota, Volkswagen, and BMW making substantial investments in solid-state technology. These companies view sulfide-based solid-state electrolytes as critical to achieving the 500+ mile range targets necessary for mainstream electric vehicle adoption while addressing safety concerns associated with conventional lithium-ion batteries.
Consumer electronics represents the second largest market segment, with manufacturers seeking higher energy density and improved safety profiles for next-generation devices. The premium smartphone and wearable technology sectors have demonstrated willingness to adopt higher-cost battery solutions that deliver tangible performance improvements.
Regionally, Asia-Pacific dominates the solid-state battery landscape, with Japan and South Korea leading research initiatives specifically focused on sulfide-based electrolytes. North America and Europe are rapidly expanding their research capabilities, with significant government funding supporting development programs targeting lithium metal anode technologies.
Market analysis reveals that sulfide-based solid electrolytes hold particular commercial promise due to their superior ionic conductivity compared to oxide and polymer alternatives. Companies specializing in sulfide electrolytes, including Solid Power and QuantumScape, have secured substantial investment based on their potential to address lithium metal nucleation and void formation challenges.
Investor confidence in sulfide-based solid-state technology remains strong despite technical hurdles, with venture capital funding exceeding $2 billion in 2022 alone. This investment surge reflects recognition that solving the lithium metal nucleation and void formation challenges represents a critical inflection point for commercialization.
Market forecasts indicate that initial commercial applications of sulfide-based solid-state batteries will emerge in premium consumer electronics by 2025, followed by limited automotive deployment by 2027. Mass market penetration is anticipated by 2030, contingent upon successful resolution of manufacturing scalability challenges and continued progress in addressing lithium metal interface stability issues.
Current Challenges in Sulfide SSE Implementation
Despite the promising potential of sulfide solid-state electrolytes (SSEs) in next-generation lithium metal batteries, several critical challenges impede their widespread implementation. The most significant issue is the high interfacial resistance between lithium metal and sulfide SSEs, which stems from both chemical and mechanical incompatibilities. Chemically, most sulfide SSEs react with lithium metal to form an interphase layer composed of Li2S, Li3P, and other decomposition products, increasing resistance and impeding ion transport.
Mechanically, the nucleation and growth of lithium during cycling creates significant volume changes that the rigid sulfide electrolytes cannot accommodate. This leads to contact loss, void formation, and increased local current densities that accelerate dendrite growth. The problem is particularly pronounced during lithium stripping, where voids form at the interface and persist through subsequent cycles, progressively degrading cell performance.
The brittle nature of sulfide SSEs (with elastic moduli typically between 18-25 GPa) makes them vulnerable to fracture under the stress induced by lithium deposition. These microcracks become pathways for dendrite propagation, ultimately causing short circuits. While sulfides theoretically possess sufficient mechanical strength to suppress dendrite growth, their practical implementation is compromised by these interfacial instabilities.
Processing challenges further complicate sulfide SSE implementation. Their moisture sensitivity necessitates handling in controlled environments, increasing manufacturing complexity and cost. Additionally, the cold sintering processes typically used to fabricate sulfide electrolytes often result in grain boundaries and residual porosity that reduce ionic conductivity and provide pathways for dendrite penetration.
The scalability of sulfide SSE production presents another hurdle. Current synthesis methods like mechanical ball milling are energy-intensive and difficult to scale, while solution-based approaches often introduce impurities that degrade electrochemical performance. The cost of precursor materials, particularly high-purity sulfur compounds, remains prohibitively high for mass production.
Safety concerns also persist despite the solid nature of these electrolytes. Many sulfide SSEs release toxic H2S gas when exposed to moisture or air, creating hazards during manufacturing, recycling, and in case of cell damage. Furthermore, the thermal stability of sulfide interfaces under extreme conditions requires further investigation to ensure safe operation across a wide temperature range.
Mechanically, the nucleation and growth of lithium during cycling creates significant volume changes that the rigid sulfide electrolytes cannot accommodate. This leads to contact loss, void formation, and increased local current densities that accelerate dendrite growth. The problem is particularly pronounced during lithium stripping, where voids form at the interface and persist through subsequent cycles, progressively degrading cell performance.
The brittle nature of sulfide SSEs (with elastic moduli typically between 18-25 GPa) makes them vulnerable to fracture under the stress induced by lithium deposition. These microcracks become pathways for dendrite propagation, ultimately causing short circuits. While sulfides theoretically possess sufficient mechanical strength to suppress dendrite growth, their practical implementation is compromised by these interfacial instabilities.
Processing challenges further complicate sulfide SSE implementation. Their moisture sensitivity necessitates handling in controlled environments, increasing manufacturing complexity and cost. Additionally, the cold sintering processes typically used to fabricate sulfide electrolytes often result in grain boundaries and residual porosity that reduce ionic conductivity and provide pathways for dendrite penetration.
The scalability of sulfide SSE production presents another hurdle. Current synthesis methods like mechanical ball milling are energy-intensive and difficult to scale, while solution-based approaches often introduce impurities that degrade electrochemical performance. The cost of precursor materials, particularly high-purity sulfur compounds, remains prohibitively high for mass production.
Safety concerns also persist despite the solid nature of these electrolytes. Many sulfide SSEs release toxic H2S gas when exposed to moisture or air, creating hazards during manufacturing, recycling, and in case of cell damage. Furthermore, the thermal stability of sulfide interfaces under extreme conditions requires further investigation to ensure safe operation across a wide temperature range.
Existing Approaches to Lithium Nucleation Control
01 Sulfide solid-state electrolyte compositions for lithium metal batteries
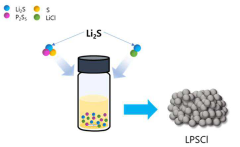
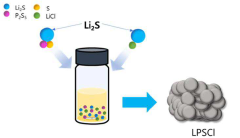
Specific compositions of sulfide-based solid-state electrolytes can be engineered to improve lithium metal nucleation and reduce void formation. These electrolytes typically contain lithium sulfide (Li2S) combined with other components such as P2S5, SiS2, or GeS2 to form glass-ceramic structures. The chemical composition and crystalline structure of these electrolytes significantly influence the lithium ion conductivity and the interface stability with lithium metal, which are critical factors in controlling uniform lithium deposition and preventing void formation.- Sulfide solid-state electrolyte compositions for lithium metal batteries: Specific compositions of sulfide solid-state electrolytes can be engineered to improve lithium metal nucleation and reduce void formation. These electrolytes typically contain lithium sulfide combined with other components such as phosphorus sulfide, silicon sulfide, or germanium sulfide. The chemical composition and structure of these electrolytes affect the interfacial stability with lithium metal, influencing how lithium deposits during charging and preventing dendrite formation and void creation.
- Interface engineering between lithium metal and solid electrolytes: Engineering the interface between lithium metal and sulfide solid-state electrolytes is crucial for controlling nucleation behavior and preventing void formation. This can be achieved through surface modifications, buffer layers, or gradient interfaces that promote uniform lithium deposition. These engineered interfaces help reduce interfacial resistance, improve wetting characteristics, and create favorable nucleation sites for lithium, resulting in more homogeneous lithium deposition and reduced void formation during cycling.
- Pressure and mechanical considerations for void mitigation: Applying controlled pressure during battery operation and considering mechanical properties of the cell components can significantly reduce void formation at the lithium-electrolyte interface. Stack pressure helps maintain intimate contact between the lithium metal and the solid electrolyte, preventing delamination and void formation. The mechanical design of the battery, including compression elements and elastic components, can accommodate volume changes during cycling while maintaining interfacial contact.
- Additives and dopants for improved lithium nucleation: Incorporating specific additives and dopants into sulfide solid-state electrolytes can enhance lithium nucleation behavior and reduce void formation. These additives may include halides, nitrides, or other inorganic compounds that modify the surface energy and lithium ion transport properties at the interface. Some additives create artificial nucleation sites for lithium deposition, while others modify the chemical stability of the electrolyte against lithium metal, resulting in more uniform lithium deposition patterns.
- Temperature control and cycling protocols: Optimized temperature conditions and specific cycling protocols can significantly improve lithium nucleation behavior and reduce void formation with sulfide solid-state electrolytes. Elevated operating temperatures can enhance ion mobility and improve interfacial kinetics, while controlled charging rates and voltage limits can promote uniform lithium deposition. Specialized formation cycles and pulse charging techniques can establish stable interfaces and nucleation patterns that persist through subsequent cycling, minimizing void formation over the battery lifetime.
02 Interface engineering between lithium metal and solid electrolytes
Engineering the interface between lithium metal and sulfide solid-state electrolytes is crucial for controlling lithium nucleation and preventing void formation. Various approaches include applying protective coatings, introducing interlayers, or modifying the surface chemistry of the electrolyte. These interface engineering strategies help to achieve uniform lithium ion flux, reduce local current density variations, and promote homogeneous lithium deposition, thereby mitigating dendrite growth and void formation during battery cycling.Expand Specific Solutions03 Pressure and temperature control during lithium deposition
Applying controlled pressure and temperature during battery operation can significantly improve lithium metal nucleation and reduce void formation with sulfide solid-state electrolytes. Stack pressure helps maintain intimate contact between the lithium metal and the electrolyte, reducing interfacial resistance and promoting uniform lithium deposition. Temperature control affects the kinetics of lithium ion transport and deposition, with optimized temperature profiles enabling more homogeneous nucleation and growth of lithium metal.Expand Specific Solutions04 Additives and dopants for improved lithium nucleation
Incorporating specific additives and dopants into sulfide solid-state electrolytes can enhance lithium nucleation behavior and mitigate void formation. These additives may include halides, oxides, or other inorganic compounds that modify the local electric field distribution, surface energy, or lithium ion transport pathways. Some additives can serve as nucleation sites for lithium deposition, while others may alter the mechanical properties of the electrolyte to better accommodate volume changes during cycling.Expand Specific Solutions05 Advanced manufacturing techniques for solid-state battery assembly
Novel manufacturing and assembly techniques can significantly impact lithium nucleation and void formation in batteries with sulfide solid-state electrolytes. These include specialized methods for electrolyte synthesis, electrode preparation, and cell assembly that ensure uniform interfaces and minimize defects. Advanced techniques such as pulsed laser deposition, atomic layer deposition, or cold sintering can be employed to create high-quality interfaces between lithium metal and the electrolyte, promoting uniform lithium nucleation and reducing the likelihood of void formation during battery operation.Expand Specific Solutions
Leading Research Institutions and Industrial Partners
The lithium metal nucleation and void mitigation with sulfide solid-state electrolytes (SSEs) research field is currently in an early growth phase, with market projections indicating significant expansion as solid-state battery technology matures. Major academic institutions (Harvard, Cornell, Zhejiang University) are collaborating with industry leaders to overcome technical challenges. Companies like LG Energy Solution and EVE Energy are investing heavily in commercialization efforts, while specialized firms such as Solid Energies and Honeycomb Battery are developing proprietary solutions. Research institutes including Shanghai Institute of Ceramics and Battelle Memorial Institute provide critical fundamental research support. The technology remains pre-commercial, with most players focusing on improving interface stability, reducing dendrite formation, and enhancing cycling performance before mass production becomes viable.
Solid Energies, Inc.
Technical Solution: Solid Energies has developed a proprietary "anode-free" cell design that addresses lithium nucleation challenges with sulfide solid-state electrolytes. Their approach eliminates pre-deposited lithium metal anodes, instead relying on in-situ plating of lithium during the first charge cycle. This technique allows for more controlled initial lithium nucleation patterns. The company has engineered specialized sulfide electrolyte compositions with additives that promote uniform lithium wetting and deposition. Their technology incorporates a gradient-structured electrolyte interface where the mechanical properties and ionic conductivity are optimized to maintain contact during cycling. Solid Energies has also developed a unique pressure-regulation system within their cell design that applies consistent stack pressure throughout battery operation, preventing void formation by ensuring the lithium metal maintains contact with the electrolyte surface. Their cells utilize a thin artificial interlayer between the current collector and sulfide electrolyte, composed of lithiophilic materials that serve as preferred nucleation sites for uniform lithium deposition.
Strengths: Their anode-free approach simplifies manufacturing and potentially increases energy density by eliminating excess lithium. The company's focused specialization in solid-state technology allows for rapid iteration and optimization. Weaknesses: As a smaller company, they may have limited manufacturing capabilities compared to larger battery manufacturers, potentially affecting their ability to scale production quickly.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced sulfide solid-state electrolytes with optimized lithium ion conductivity exceeding 10^-3 S/cm at room temperature. Their proprietary technology focuses on controlling the interfacial chemistry between lithium metal anodes and sulfide SSEs through specialized coating layers that promote uniform lithium deposition. The company employs argyrodite-type Li6PS5Cl electrolytes with modified surface properties to reduce interfacial resistance and suppress dendrite formation. Their approach includes introducing artificial interlayers composed of mixed ionic-electronic conductors that facilitate homogeneous lithium-ion flux across the interface, effectively mitigating void formation during cycling. LG has also developed pressure-control systems within their cell designs to maintain intimate contact between lithium metal and the electrolyte, addressing the volume change challenges during cycling.
Strengths: Industry-leading manufacturing capabilities allow for scalable production of sulfide SSEs with consistent quality. Their extensive battery expertise enables practical cell design solutions addressing real-world implementation challenges. Weaknesses: Their sulfide electrolytes still face challenges with air/moisture sensitivity requiring specialized handling environments, increasing production costs and complexity.
Critical Patents in Void Formation Mitigation Strategies
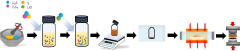
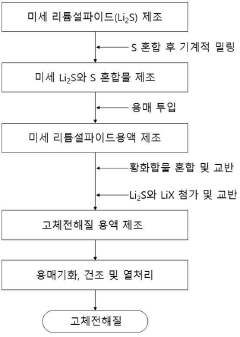
Method for producing a sulfide-based solid electrolyte using micro lithium sulfide and low-toxic solvent, a sulfide-based solid electrolyte prepared thereby, and an all-solid-state battery comprising the same
PatentPendingKR1020240063639A
Innovation
- A method involving the use of fine lithium sulfide and low-toxic solvents, such as cyclopentylmethyl ether (CPME) and 2-methyltetrahydrofuran (2-MeTHF), to produce a sulfide-based solid electrolyte by mechanical milling, mixing, and heat-treating to control particle size and promote nucleation, thereby reducing voids and maintaining solid-solid contact interfaces.
Solid electrolyte film, preparation method and use thereof, and solid state battery
PatentPendingUS20250219132A1
Innovation
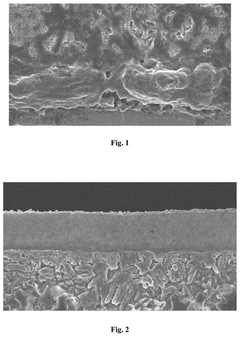
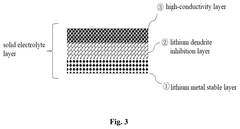
- A solid electrolyte film composed of a lithium metal stable layer, lithium dendrite inhibition layer, and high-conductivity layer, all made of sulfide solid electrolytes, with specific coatings and crystallinity indices to enhance stability and conductivity.
Safety and Performance Benchmarking Methodologies
Establishing standardized safety and performance benchmarking methodologies is crucial for advancing lithium metal nucleation and void mitigation research with sulfide solid-state electrolytes (SSEs). Current evaluation approaches vary significantly across research institutions, making direct comparisons challenging and potentially hindering technological progress.
Safety benchmarking for sulfide SSEs must address their unique characteristics, particularly their reactivity with moisture and air. Standardized protocols should include controlled environment testing, quantitative measurement of hydrogen sulfide generation rates, and systematic evaluation of thermal stability across operating temperature ranges. The development of accelerated aging tests specifically designed for sulfide-based systems is essential for predicting long-term safety performance.
Performance benchmarking requires multi-dimensional evaluation frameworks that capture the complex interplay between lithium metal nucleation behavior and void formation. Critical metrics include interfacial resistance measurements under varying pressure conditions, in-situ visualization of lithium deposition patterns, and quantitative void formation analysis. Standardized cycling protocols with defined current densities, temperature profiles, and pressure conditions would enable meaningful cross-comparison between different sulfide electrolyte compositions.
Electrochemical impedance spectroscopy (EIS) methodologies specifically optimized for sulfide SSE systems need standardization, as traditional liquid electrolyte EIS protocols often prove inadequate. This includes establishing reference electrode configurations that minimize measurement artifacts and enable accurate isolation of interfacial phenomena from bulk transport properties.
Mechanical property evaluation represents another critical benchmarking domain, as the pressure-dependent nature of sulfide SSE performance necessitates standardized mechanical testing protocols. These should include creep behavior assessment, fracture toughness measurement, and quantification of pressure-dependent ionic conductivity across temperature ranges relevant to practical applications.
The integration of multi-modal characterization techniques presents a promising direction for comprehensive benchmarking. Combining operando neutron diffraction, synchrotron X-ray tomography, and electrochemical measurements enables correlation between structural evolution, void formation dynamics, and performance degradation. Establishing standardized protocols for such integrated characterization would significantly advance the field's understanding of failure mechanisms in sulfide SSE systems.
Industry-academic partnerships should be leveraged to develop consensus-based benchmarking standards that balance scientific rigor with practical relevance. These collaborative efforts would accelerate the translation of fundamental insights into commercially viable solutions for next-generation solid-state batteries utilizing sulfide electrolytes.
Safety benchmarking for sulfide SSEs must address their unique characteristics, particularly their reactivity with moisture and air. Standardized protocols should include controlled environment testing, quantitative measurement of hydrogen sulfide generation rates, and systematic evaluation of thermal stability across operating temperature ranges. The development of accelerated aging tests specifically designed for sulfide-based systems is essential for predicting long-term safety performance.
Performance benchmarking requires multi-dimensional evaluation frameworks that capture the complex interplay between lithium metal nucleation behavior and void formation. Critical metrics include interfacial resistance measurements under varying pressure conditions, in-situ visualization of lithium deposition patterns, and quantitative void formation analysis. Standardized cycling protocols with defined current densities, temperature profiles, and pressure conditions would enable meaningful cross-comparison between different sulfide electrolyte compositions.
Electrochemical impedance spectroscopy (EIS) methodologies specifically optimized for sulfide SSE systems need standardization, as traditional liquid electrolyte EIS protocols often prove inadequate. This includes establishing reference electrode configurations that minimize measurement artifacts and enable accurate isolation of interfacial phenomena from bulk transport properties.
Mechanical property evaluation represents another critical benchmarking domain, as the pressure-dependent nature of sulfide SSE performance necessitates standardized mechanical testing protocols. These should include creep behavior assessment, fracture toughness measurement, and quantification of pressure-dependent ionic conductivity across temperature ranges relevant to practical applications.
The integration of multi-modal characterization techniques presents a promising direction for comprehensive benchmarking. Combining operando neutron diffraction, synchrotron X-ray tomography, and electrochemical measurements enables correlation between structural evolution, void formation dynamics, and performance degradation. Establishing standardized protocols for such integrated characterization would significantly advance the field's understanding of failure mechanisms in sulfide SSE systems.
Industry-academic partnerships should be leveraged to develop consensus-based benchmarking standards that balance scientific rigor with practical relevance. These collaborative efforts would accelerate the translation of fundamental insights into commercially viable solutions for next-generation solid-state batteries utilizing sulfide electrolytes.
Environmental Impact and Recycling Considerations
The development and deployment of lithium metal batteries with sulfide solid-state electrolytes (SSEs) necessitates careful consideration of environmental impacts throughout their lifecycle. Sulfide-based SSEs contain elements such as sulfur, phosphorus, germanium, and lithium that require responsible sourcing and management. Mining these materials can lead to habitat disruption, water pollution, and significant carbon emissions. Particularly concerning is the extraction of germanium and phosphorus, which are considered critical materials with limited global reserves.
Manufacturing processes for sulfide SSEs typically involve energy-intensive mechanical milling and heat treatments, contributing to the carbon footprint of these technologies. The high sensitivity of sulfide materials to moisture also necessitates stringent environmental controls during production, requiring additional energy expenditure for maintaining appropriate atmospheric conditions.
During operation, sulfide SSEs offer environmental advantages over conventional liquid electrolyte systems. The elimination of volatile organic compounds and flammable components significantly reduces the risk of toxic emissions during battery operation or in case of accidents. Additionally, the potential for longer cycle life in properly engineered systems could reduce overall material consumption and waste generation compared to conventional lithium-ion batteries.
End-of-life management presents both challenges and opportunities. Sulfide SSEs can release hydrogen sulfide gas when exposed to moisture, requiring specialized handling during recycling or disposal. However, the concentrated nature of valuable elements in solid-state batteries may facilitate more efficient recovery processes compared to conventional batteries with liquid electrolytes.
Emerging recycling technologies specifically designed for solid-state batteries show promise for recovering critical materials. Direct recycling approaches that preserve the crystal structure of sulfide electrolytes could significantly reduce energy requirements compared to pyrometallurgical or hydrometallurgical processes. Research into bioleaching methods using sulfur-metabolizing microorganisms represents an innovative approach to sustainable recycling of sulfide-based materials.
Life cycle assessment studies indicate that the environmental benefits of sulfide SSE batteries are highly dependent on achieving extended cycle life and developing efficient recycling pathways. The potential reduction in battery replacements due to longer lifespans could offset the higher environmental impacts of initial production, resulting in net environmental benefits over conventional technologies when considering full lifecycle impacts.
Manufacturing processes for sulfide SSEs typically involve energy-intensive mechanical milling and heat treatments, contributing to the carbon footprint of these technologies. The high sensitivity of sulfide materials to moisture also necessitates stringent environmental controls during production, requiring additional energy expenditure for maintaining appropriate atmospheric conditions.
During operation, sulfide SSEs offer environmental advantages over conventional liquid electrolyte systems. The elimination of volatile organic compounds and flammable components significantly reduces the risk of toxic emissions during battery operation or in case of accidents. Additionally, the potential for longer cycle life in properly engineered systems could reduce overall material consumption and waste generation compared to conventional lithium-ion batteries.
End-of-life management presents both challenges and opportunities. Sulfide SSEs can release hydrogen sulfide gas when exposed to moisture, requiring specialized handling during recycling or disposal. However, the concentrated nature of valuable elements in solid-state batteries may facilitate more efficient recovery processes compared to conventional batteries with liquid electrolytes.
Emerging recycling technologies specifically designed for solid-state batteries show promise for recovering critical materials. Direct recycling approaches that preserve the crystal structure of sulfide electrolytes could significantly reduce energy requirements compared to pyrometallurgical or hydrometallurgical processes. Research into bioleaching methods using sulfur-metabolizing microorganisms represents an innovative approach to sustainable recycling of sulfide-based materials.
Life cycle assessment studies indicate that the environmental benefits of sulfide SSE batteries are highly dependent on achieving extended cycle life and developing efficient recycling pathways. The potential reduction in battery replacements due to longer lifespans could offset the higher environmental impacts of initial production, resulting in net environmental benefits over conventional technologies when considering full lifecycle impacts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!