Interfacial Buffer Layers For Sulfide Electrolytes In Lithium Metal Cells
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfide Electrolyte Interface Development Background and Objectives
The development of lithium metal batteries represents a significant advancement in energy storage technology, offering theoretical energy densities far exceeding those of conventional lithium-ion batteries. Since the 1970s, researchers have recognized the potential of lithium metal anodes, but their commercial implementation has been hindered by critical safety and performance challenges. The evolution of this technology has been marked by periods of intense research followed by relative dormancy, with renewed interest emerging in the past decade due to increasing demands for higher energy density storage solutions.
Sulfide solid electrolytes have emerged as promising candidates for enabling lithium metal batteries due to their high ionic conductivity, approaching that of liquid electrolytes. These materials, including Li2S-P2S5, Li10GeP2S12 (LGPS), and Li6PS5Cl, demonstrate conductivities in the range of 10^-2 to 10^-3 S/cm at room temperature, significantly outperforming oxide-based alternatives. However, the interface between lithium metal and sulfide electrolytes presents substantial challenges that have impeded practical application.
The primary technical objective in this field is to develop effective interfacial buffer layers that can simultaneously address multiple critical issues: preventing direct chemical reactions between lithium metal and sulfide electrolytes, mitigating dendrite formation, accommodating volume changes during cycling, and maintaining high ionic conductivity while blocking electronic conduction. These buffer layers must function as artificial solid electrolyte interphases (SEIs) with precisely engineered properties.
Historical approaches to interface engineering have evolved from simple passive protection layers to sophisticated multi-functional designs. Early research focused on thin metal films and polymer coatings, while recent advances have explored atomic layer deposition techniques, composite interlayers, and in-situ formed interfaces through chemical pre-treatments of electrolyte surfaces.
The technological trajectory aims to achieve stable cycling of lithium metal cells with sulfide electrolytes for over 1000 cycles with minimal capacity degradation, while maintaining current densities above 3 mA/cm² - metrics necessary for commercial viability. Additionally, researchers seek to develop manufacturing-compatible interface engineering techniques that can be scaled for mass production.
Recent breakthroughs in computational modeling of interfacial phenomena and advanced characterization techniques have accelerated progress in this field. In particular, cryogenic electron microscopy and in-situ neutron diffraction have provided unprecedented insights into interface evolution during battery operation, guiding rational design of buffer layers with optimized composition and structure.
The ultimate goal of this research direction is to enable next-generation energy storage systems with energy densities exceeding 400 Wh/kg at the cell level, representing a transformative advancement for applications ranging from electric vehicles to grid-scale storage.
Sulfide solid electrolytes have emerged as promising candidates for enabling lithium metal batteries due to their high ionic conductivity, approaching that of liquid electrolytes. These materials, including Li2S-P2S5, Li10GeP2S12 (LGPS), and Li6PS5Cl, demonstrate conductivities in the range of 10^-2 to 10^-3 S/cm at room temperature, significantly outperforming oxide-based alternatives. However, the interface between lithium metal and sulfide electrolytes presents substantial challenges that have impeded practical application.
The primary technical objective in this field is to develop effective interfacial buffer layers that can simultaneously address multiple critical issues: preventing direct chemical reactions between lithium metal and sulfide electrolytes, mitigating dendrite formation, accommodating volume changes during cycling, and maintaining high ionic conductivity while blocking electronic conduction. These buffer layers must function as artificial solid electrolyte interphases (SEIs) with precisely engineered properties.
Historical approaches to interface engineering have evolved from simple passive protection layers to sophisticated multi-functional designs. Early research focused on thin metal films and polymer coatings, while recent advances have explored atomic layer deposition techniques, composite interlayers, and in-situ formed interfaces through chemical pre-treatments of electrolyte surfaces.
The technological trajectory aims to achieve stable cycling of lithium metal cells with sulfide electrolytes for over 1000 cycles with minimal capacity degradation, while maintaining current densities above 3 mA/cm² - metrics necessary for commercial viability. Additionally, researchers seek to develop manufacturing-compatible interface engineering techniques that can be scaled for mass production.
Recent breakthroughs in computational modeling of interfacial phenomena and advanced characterization techniques have accelerated progress in this field. In particular, cryogenic electron microscopy and in-situ neutron diffraction have provided unprecedented insights into interface evolution during battery operation, guiding rational design of buffer layers with optimized composition and structure.
The ultimate goal of this research direction is to enable next-generation energy storage systems with energy densities exceeding 400 Wh/kg at the cell level, representing a transformative advancement for applications ranging from electric vehicles to grid-scale storage.
Market Analysis for Advanced Lithium Metal Battery Technologies
The lithium metal battery market is experiencing significant growth, driven by the increasing demand for high-energy-density energy storage solutions across multiple sectors. Current market projections indicate that the global lithium metal battery market will reach approximately $13.4 billion by 2030, with a compound annual growth rate of 18.2% from 2023 to 2030. This growth is primarily fueled by the expanding electric vehicle (EV) industry, which requires batteries with higher energy density and longer cycle life than traditional lithium-ion batteries.
The automotive sector represents the largest market segment for advanced lithium metal batteries, accounting for nearly 45% of the total market share. Major automotive manufacturers are investing heavily in lithium metal battery technology to extend EV driving ranges beyond 400 miles on a single charge. Tesla, Volkswagen, and Toyota have all announced strategic partnerships with battery technology companies focused on lithium metal systems.
Consumer electronics constitutes the second-largest market segment, with demand for longer-lasting portable devices driving adoption. This sector values the potential weight reduction and increased energy density that lithium metal batteries offer compared to conventional lithium-ion technologies.
Grid-scale energy storage represents an emerging market opportunity, particularly as renewable energy integration increases globally. The ability of lithium metal batteries to store large amounts of energy in smaller footprints makes them attractive for utility-scale applications.
Market analysis reveals that interfacial buffer layers for sulfide electrolytes represent a critical technological advancement that could accelerate market adoption. Industry reports indicate that solving the interfacial stability issues between lithium metal anodes and sulfide solid electrolytes could reduce battery costs by up to 30% while improving safety profiles significantly.
Regional analysis shows North America and Asia-Pacific leading market development, with China, South Korea, Japan, and the United States hosting the majority of research initiatives and manufacturing facilities. European markets are growing rapidly due to stringent emissions regulations and governmental support for clean energy technologies.
Key market barriers include safety concerns, manufacturing scalability, and cost considerations. Current lithium metal cells with sulfide electrolytes cost approximately $180-220 per kWh, significantly higher than the $100 per kWh threshold considered necessary for mass-market EV adoption. However, technological advancements in interfacial engineering are projected to reduce these costs by 40-50% within the next five years.
The automotive sector represents the largest market segment for advanced lithium metal batteries, accounting for nearly 45% of the total market share. Major automotive manufacturers are investing heavily in lithium metal battery technology to extend EV driving ranges beyond 400 miles on a single charge. Tesla, Volkswagen, and Toyota have all announced strategic partnerships with battery technology companies focused on lithium metal systems.
Consumer electronics constitutes the second-largest market segment, with demand for longer-lasting portable devices driving adoption. This sector values the potential weight reduction and increased energy density that lithium metal batteries offer compared to conventional lithium-ion technologies.
Grid-scale energy storage represents an emerging market opportunity, particularly as renewable energy integration increases globally. The ability of lithium metal batteries to store large amounts of energy in smaller footprints makes them attractive for utility-scale applications.
Market analysis reveals that interfacial buffer layers for sulfide electrolytes represent a critical technological advancement that could accelerate market adoption. Industry reports indicate that solving the interfacial stability issues between lithium metal anodes and sulfide solid electrolytes could reduce battery costs by up to 30% while improving safety profiles significantly.
Regional analysis shows North America and Asia-Pacific leading market development, with China, South Korea, Japan, and the United States hosting the majority of research initiatives and manufacturing facilities. European markets are growing rapidly due to stringent emissions regulations and governmental support for clean energy technologies.
Key market barriers include safety concerns, manufacturing scalability, and cost considerations. Current lithium metal cells with sulfide electrolytes cost approximately $180-220 per kWh, significantly higher than the $100 per kWh threshold considered necessary for mass-market EV adoption. However, technological advancements in interfacial engineering are projected to reduce these costs by 40-50% within the next five years.
Current Challenges in Sulfide-Based Solid Electrolyte Interfaces
Despite significant advancements in solid-state battery technology, sulfide-based solid electrolytes face critical interfacial challenges that hinder their commercial viability. The primary issue stems from the high chemical reactivity between sulfide electrolytes and lithium metal anodes, resulting in continuous decomposition at the interface. This decomposition forms an interphase layer with poor ionic conductivity, significantly increasing interfacial resistance and degrading battery performance over cycling.
Chemical instability represents another major challenge, as sulfide electrolytes readily react with atmospheric moisture and oxygen, forming toxic hydrogen sulfide gas. This necessitates stringent manufacturing conditions and complicates battery assembly processes. Additionally, the mechanical instability at the lithium metal/sulfide electrolyte interface leads to void formation and loss of physical contact during cycling, further increasing cell impedance.
Volume changes during lithium plating and stripping create substantial mechanical stress at the interface, causing electrolyte fracture and accelerated degradation. The formation of lithium dendrites through sulfide electrolytes remains problematic, as these materials often lack sufficient mechanical strength to suppress dendrite growth, leading to potential short circuits and safety hazards.
Interfacial kinetics present another significant barrier, with charge transfer resistance at the lithium/sulfide interface often dominating the overall cell resistance. The slow lithium-ion transport across this interface limits fast charging capabilities and high-rate performance of sulfide-based solid-state batteries.
Manufacturing challenges compound these issues, as the extreme sensitivity of sulfide materials requires specialized equipment and strictly controlled environments, increasing production costs and complexity. The interface quality is highly dependent on processing conditions, making consistent, high-quality interfaces difficult to achieve at scale.
Current research indicates that the space charge layer formed at the lithium/sulfide interface creates an electric potential barrier that impedes lithium-ion transport. This electrochemical phenomenon further complicates interface optimization efforts. Additionally, the lack of standardized testing protocols for evaluating interfacial properties makes it difficult to compare different buffer layer solutions across research groups.
The complex interplay between chemical, electrochemical, and mechanical phenomena at these interfaces necessitates multidisciplinary approaches to develop effective buffer layers. Understanding and addressing these challenges is crucial for advancing sulfide-based solid-state battery technology toward commercial viability and meeting the growing demand for safer, higher-energy-density energy storage solutions.
Chemical instability represents another major challenge, as sulfide electrolytes readily react with atmospheric moisture and oxygen, forming toxic hydrogen sulfide gas. This necessitates stringent manufacturing conditions and complicates battery assembly processes. Additionally, the mechanical instability at the lithium metal/sulfide electrolyte interface leads to void formation and loss of physical contact during cycling, further increasing cell impedance.
Volume changes during lithium plating and stripping create substantial mechanical stress at the interface, causing electrolyte fracture and accelerated degradation. The formation of lithium dendrites through sulfide electrolytes remains problematic, as these materials often lack sufficient mechanical strength to suppress dendrite growth, leading to potential short circuits and safety hazards.
Interfacial kinetics present another significant barrier, with charge transfer resistance at the lithium/sulfide interface often dominating the overall cell resistance. The slow lithium-ion transport across this interface limits fast charging capabilities and high-rate performance of sulfide-based solid-state batteries.
Manufacturing challenges compound these issues, as the extreme sensitivity of sulfide materials requires specialized equipment and strictly controlled environments, increasing production costs and complexity. The interface quality is highly dependent on processing conditions, making consistent, high-quality interfaces difficult to achieve at scale.
Current research indicates that the space charge layer formed at the lithium/sulfide interface creates an electric potential barrier that impedes lithium-ion transport. This electrochemical phenomenon further complicates interface optimization efforts. Additionally, the lack of standardized testing protocols for evaluating interfacial properties makes it difficult to compare different buffer layer solutions across research groups.
The complex interplay between chemical, electrochemical, and mechanical phenomena at these interfaces necessitates multidisciplinary approaches to develop effective buffer layers. Understanding and addressing these challenges is crucial for advancing sulfide-based solid-state battery technology toward commercial viability and meeting the growing demand for safer, higher-energy-density energy storage solutions.
Current Buffer Layer Solutions for Sulfide Electrolyte Systems
01 Oxide-based buffer layers for sulfide electrolyte interfaces
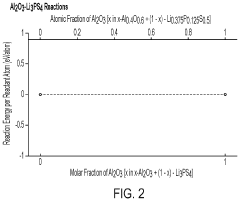
Oxide-based materials can be used as buffer layers between sulfide solid electrolytes and electrodes to improve interface stability. These oxide layers help prevent unwanted reactions between the sulfide electrolytes and electrode materials, reducing interfacial resistance and enhancing electrochemical performance. The buffer layers can be composed of various metal oxides that are chemically compatible with both the sulfide electrolyte and electrode materials, creating a stable interface that prevents degradation during cycling.- Oxide-based buffer layers for sulfide electrolyte interfaces: Oxide-based materials can be used as buffer layers at the interface between sulfide solid electrolytes and electrodes to improve stability. These oxide layers help prevent direct contact between the sulfide electrolyte and electrode materials, reducing interfacial resistance and suppressing unwanted side reactions. Common oxide materials include lithium-containing oxides, aluminum oxide, and zirconium oxide, which can effectively mitigate the chemical and electrochemical degradation at the interface while maintaining good ionic conductivity.
- Polymer-based interfacial layers for sulfide electrolytes: Polymer-based interfacial layers can be applied between sulfide solid electrolytes and electrodes to enhance interface stability. These polymer layers act as flexible buffers that accommodate volume changes during cycling and reduce mechanical stress at the interface. Additionally, they can be designed to have good adhesion properties and ionic conductivity while preventing direct contact between reactive components. Functionalized polymers with specific chemical groups can further improve compatibility with sulfide electrolytes.
- Composite buffer layers with gradient compositions: Composite buffer layers with gradient compositions can effectively bridge the chemical and mechanical differences between sulfide electrolytes and electrode materials. These layers typically feature a gradual transition in composition, structure, or properties from one interface to another, minimizing abrupt property changes that could lead to instability. The gradient design helps distribute stress, enhance ion transport across interfaces, and reduce interfacial resistance while maintaining good electrochemical performance.
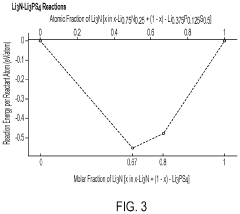
- Artificial SEI (Solid Electrolyte Interphase) formation techniques: Artificial SEI formation techniques involve deliberately creating a stable interfacial layer between sulfide electrolytes and electrodes before cell assembly. These engineered interfaces can be formed through various methods including atomic layer deposition, solution processing, or in-situ chemical reactions. The artificial SEI layers are designed to be ionically conductive while electronically insulating, preventing continuous electrolyte decomposition and stabilizing the interface during cycling. This approach significantly improves the long-term stability of sulfide-based solid-state batteries.
- Nanoscale coating strategies for interface modification: Nanoscale coating strategies involve applying ultrathin layers of stabilizing materials at sulfide electrolyte interfaces to enhance compatibility without significantly increasing resistance. These nanoscale coatings, typically ranging from a few nanometers to tens of nanometers in thickness, can be applied using techniques such as sputtering, vapor deposition, or solution-based methods. The thin nature of these coatings allows for effective passivation of reactive sites while maintaining good ionic transport properties, resulting in improved interface stability and battery performance.
02 Polymer-based interfacial layers for sulfide electrolytes
Polymer-based interfacial layers can be applied between sulfide solid electrolytes and electrodes to enhance interface stability. These polymer layers act as flexible buffers that accommodate volume changes during cycling while preventing direct contact between reactive components. The polymer interfaces can be functionalized to improve adhesion and ion transport properties, reducing interfacial resistance and enhancing the overall stability of the electrochemical system. These materials are particularly effective at preventing dendrite formation and mitigating degradation reactions at the interface.Expand Specific Solutions03 Artificial SEI formation techniques for sulfide electrolytes
Artificial solid electrolyte interphase (SEI) layers can be engineered to improve the stability of sulfide electrolyte interfaces. These layers are designed to mimic the natural SEI that forms during battery operation but with controlled composition and properties. By pre-forming these protective layers through various deposition techniques, the interface stability can be significantly enhanced, preventing continuous electrolyte decomposition and electrode degradation. The artificial SEI layers can incorporate specific additives that promote favorable ion transport while blocking unwanted side reactions.Expand Specific Solutions04 Composite buffer layers with gradient compositions
Composite buffer layers with gradient compositions can be designed to provide a smooth transition between sulfide electrolytes and electrode materials. These gradient structures gradually change in composition from one interface to another, reducing abrupt property changes that can lead to mechanical stress and chemical incompatibility. The gradual transition helps to match the chemical potentials and mechanical properties across the interface, resulting in improved stability and reduced interfacial resistance. These composite layers can combine multiple materials to achieve optimal performance characteristics.Expand Specific Solutions05 Atomic layer deposition techniques for ultrathin buffer layers
Atomic layer deposition (ALD) can be used to create ultrathin, conformal buffer layers at sulfide electrolyte interfaces with precise thickness control. These nanometer-scale coatings provide effective protection while minimizing the additional resistance introduced by the buffer layer. The ALD process allows for uniform coverage even on complex geometries, ensuring complete protection of the interface. The resulting ultrathin layers can significantly improve the cycling stability and rate capability of batteries using sulfide solid electrolytes by preventing interfacial degradation reactions while maintaining fast ion transport.Expand Specific Solutions
Leading Companies and Research Institutions in Solid-State Battery Field
The lithium metal cell market with sulfide electrolytes is currently in an early growth phase, characterized by intensive R&D efforts focused on interfacial buffer layers to address stability challenges. The global market is projected to expand significantly as part of the broader $213 billion battery industry by 2030. Leading players include established corporations like LG Energy Solution, LG Chem, and Panasonic, alongside research-focused entities such as Korea Electronics Technology Institute and various universities (Monash, Zhejiang, Beijing Institute of Technology). Technical maturity remains moderate, with companies like PolyPlus Battery and EVE Energy making notable advances in protective layer technologies. The competitive landscape features collaboration between academic institutions and industrial partners to overcome electrolyte-electrode interface degradation issues that currently limit commercial viability.
LG Chem Ltd.
Technical Solution: LG Chem has developed a comprehensive approach to interfacial buffer layers for sulfide electrolytes in lithium metal cells, focusing on composite protective layers. Their technology utilizes a dual-layer structure consisting of an inorganic Li3PO4 layer combined with a polymer-based protective coating. This configuration effectively prevents direct contact between the lithium metal anode and sulfide solid electrolyte, mitigating interfacial reactions that lead to capacity fading. The company's research has demonstrated that these buffer layers can reduce interfacial resistance by approximately 60% compared to unprotected interfaces while maintaining stable cycling performance over 500+ cycles. LG Chem has also pioneered the use of lithium nitride-based buffer layers that form a stable SEI (Solid Electrolyte Interphase) with sulfide electrolytes, showing exceptional compatibility with materials like Li6PS5Cl and Li10GeP2S12.
Strengths: Superior interfacial stability with reduced resistance, excellent cycle life enhancement, and compatibility with various sulfide electrolyte compositions. The dual-layer approach provides both mechanical protection and chemical stability. Weaknesses: Manufacturing complexity of multi-layer structures increases production costs, and some buffer materials may have limited temperature stability ranges.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has developed an innovative approach to interfacial buffer layers for sulfide-based solid-state batteries using a gradient composition strategy. Their technology employs a buffer layer with gradually changing composition from the lithium metal anode to the sulfide electrolyte, creating a smooth transition zone that minimizes interfacial stress and chemical incompatibility. The buffer system incorporates lithium-conducting oxides (such as LLZO or LATP) near the lithium metal interface that gradually blend into sulfide-compatible materials toward the electrolyte side. This gradient approach has been shown to reduce interfacial resistance by up to 70% compared to direct lithium-sulfide electrolyte contacts. Panasonic's research demonstrates that these buffer layers can effectively prevent lithium dendrite penetration while maintaining high ionic conductivity (>10^-4 S/cm) across the interface, enabling high-rate capability in all-solid-state batteries with sulfide electrolytes.
Strengths: The gradient composition approach provides excellent mechanical and chemical compatibility across interfaces, significantly reducing resistance while maintaining high ionic conductivity. Weaknesses: Complex manufacturing process for creating gradient structures may limit mass production scalability, and the multi-component nature of the buffer layer introduces potential long-term stability challenges.
Key Patents and Scientific Breakthroughs in Interfacial Engineering
Stable lithium metal sulfide coatings for solid-state batteries
PatentPendingUS20230369586A1
Innovation
- Incorporating a lithium metal sulfide interfacial layer with specific properties, such as high ionic conductivity and chemical stability, between the anode and solid-state electrolyte to prevent reactive degradation, which can be deposited using techniques like chemical vapor deposition or physical vapor deposition.
Lithium secondary battery and preparation method therefor
PatentPendingUS20250158011A1
Innovation
- A multilayer structure is created using magnetron sputtering to deposit LiPON and Li3PO4 thin film layers on either side of a sulfide solid electrolyte layer, followed by high-temperature calcination to form a positive active material layer, and finally, an aluminum current collector layer and metal lithium layer are added to enhance interfacial compatibility and stability.
Safety and Performance Metrics for Solid-State Battery Interfaces
The safety and performance of solid-state battery interfaces represent critical metrics for evaluating the viability of sulfide electrolyte systems in lithium metal cells. These interfaces, particularly those incorporating buffer layers, must meet stringent safety standards while delivering optimal electrochemical performance to enable commercial adoption.
Safety metrics for sulfide-based solid-state battery interfaces include thermal stability, chemical compatibility, and mechanical integrity. Thermal runaway resistance is paramount, with buffer layers needing to maintain stability at temperatures ranging from -40°C to over 100°C during both operation and storage conditions. Chemical stability metrics focus on preventing harmful side reactions between the sulfide electrolyte and lithium metal, which can lead to hydrogen sulfide gas evolution—a significant safety hazard requiring quantitative measurement protocols.
Mechanical stability metrics evaluate the buffer layer's ability to accommodate volume changes during cycling while maintaining interfacial contact. This includes measurements of interfacial adhesion strength, typically assessed through peel tests or nanoindentation techniques, with target values exceeding 1 MPa to ensure long-term cycling stability.
Performance metrics are equally critical and include ionic conductivity, interfacial resistance, and cycling efficiency. Effective buffer layers must maintain high lithium-ion conductivity (>10^-4 S/cm) while minimizing electronic conductivity to prevent short circuits. Interfacial resistance, measured through electrochemical impedance spectroscopy, should remain below 10 Ω·cm² to enable high-rate capability.
Coulombic efficiency serves as a key performance indicator, with values above 99.9% necessary for practical applications. This metric directly correlates with cycle life and capacity retention, where buffer layers must enable at least 1000 cycles with less than 20% capacity degradation to meet commercial requirements.
Dendrite suppression capability represents another crucial metric, quantified through critical current density measurements. Effective buffer layers should enable operation at current densities exceeding 3 mA/cm² without lithium dendrite penetration, significantly higher than conventional liquid electrolyte systems.
Standardized testing protocols for these metrics remain under development, with organizations like the Battery Standards Testing Council working to establish industry-wide benchmarks. These efforts aim to create uniform evaluation methods that enable direct comparison between different buffer layer technologies and accelerate commercialization of sulfide-based solid-state batteries with enhanced safety profiles and superior performance characteristics.
Safety metrics for sulfide-based solid-state battery interfaces include thermal stability, chemical compatibility, and mechanical integrity. Thermal runaway resistance is paramount, with buffer layers needing to maintain stability at temperatures ranging from -40°C to over 100°C during both operation and storage conditions. Chemical stability metrics focus on preventing harmful side reactions between the sulfide electrolyte and lithium metal, which can lead to hydrogen sulfide gas evolution—a significant safety hazard requiring quantitative measurement protocols.
Mechanical stability metrics evaluate the buffer layer's ability to accommodate volume changes during cycling while maintaining interfacial contact. This includes measurements of interfacial adhesion strength, typically assessed through peel tests or nanoindentation techniques, with target values exceeding 1 MPa to ensure long-term cycling stability.
Performance metrics are equally critical and include ionic conductivity, interfacial resistance, and cycling efficiency. Effective buffer layers must maintain high lithium-ion conductivity (>10^-4 S/cm) while minimizing electronic conductivity to prevent short circuits. Interfacial resistance, measured through electrochemical impedance spectroscopy, should remain below 10 Ω·cm² to enable high-rate capability.
Coulombic efficiency serves as a key performance indicator, with values above 99.9% necessary for practical applications. This metric directly correlates with cycle life and capacity retention, where buffer layers must enable at least 1000 cycles with less than 20% capacity degradation to meet commercial requirements.
Dendrite suppression capability represents another crucial metric, quantified through critical current density measurements. Effective buffer layers should enable operation at current densities exceeding 3 mA/cm² without lithium dendrite penetration, significantly higher than conventional liquid electrolyte systems.
Standardized testing protocols for these metrics remain under development, with organizations like the Battery Standards Testing Council working to establish industry-wide benchmarks. These efforts aim to create uniform evaluation methods that enable direct comparison between different buffer layer technologies and accelerate commercialization of sulfide-based solid-state batteries with enhanced safety profiles and superior performance characteristics.
Manufacturing Scalability of Advanced Buffer Layer Technologies
The scalability of advanced buffer layer technologies represents a critical challenge in transitioning from laboratory-scale demonstrations to commercial production of lithium metal batteries with sulfide electrolytes. Current manufacturing processes for interfacial buffer layers often involve complex deposition techniques such as atomic layer deposition (ALD), physical vapor deposition (PVD), or chemical vapor deposition (CVD), which present significant hurdles for mass production.
These sophisticated deposition methods typically require high vacuum environments, precise temperature control, and specialized equipment that limit throughput and increase production costs. For instance, ALD processes, while offering excellent thickness control and conformality, operate at deposition rates of only a few angstroms per minute, making them impractical for high-volume manufacturing without substantial parallelization.
Solution-based coating methods offer more promising scalability pathways. Techniques such as spray coating, spin coating, and doctor blade processes can be integrated into roll-to-roll manufacturing lines, potentially enabling continuous production of buffer layer-coated materials. However, these methods currently face challenges in achieving the nanometer-scale thickness uniformity and pinhole-free coverage that vacuum-based techniques provide.
Recent innovations in manufacturing approaches include the development of solvent-free dry coating techniques and plasma-enhanced deposition methods that can operate at atmospheric pressure. These advancements could significantly reduce the capital equipment costs and increase throughput rates for buffer layer deposition. Additionally, emerging technologies like spatial ALD show promise for combining the precision of traditional ALD with dramatically improved deposition rates.
Material supply chains represent another critical factor affecting manufacturing scalability. Many advanced buffer layer materials incorporate rare earth elements or specialty chemicals with limited global production capacity. Developing buffer layer compositions based on earth-abundant materials will be essential for large-scale deployment.
Cost modeling analyses indicate that buffer layer processing currently contributes significantly to overall cell production costs, with estimates ranging from $5-15/kWh depending on the specific technology. To achieve cost parity with conventional lithium-ion batteries, these processing costs must be reduced by at least 60-70% through manufacturing innovations and economies of scale.
Regulatory considerations also impact manufacturing scalability, particularly regarding environmental permits for facilities using certain precursor chemicals and solvents. Developing greener processing routes that minimize hazardous materials usage will facilitate faster deployment of production capacity across global markets.
These sophisticated deposition methods typically require high vacuum environments, precise temperature control, and specialized equipment that limit throughput and increase production costs. For instance, ALD processes, while offering excellent thickness control and conformality, operate at deposition rates of only a few angstroms per minute, making them impractical for high-volume manufacturing without substantial parallelization.
Solution-based coating methods offer more promising scalability pathways. Techniques such as spray coating, spin coating, and doctor blade processes can be integrated into roll-to-roll manufacturing lines, potentially enabling continuous production of buffer layer-coated materials. However, these methods currently face challenges in achieving the nanometer-scale thickness uniformity and pinhole-free coverage that vacuum-based techniques provide.
Recent innovations in manufacturing approaches include the development of solvent-free dry coating techniques and plasma-enhanced deposition methods that can operate at atmospheric pressure. These advancements could significantly reduce the capital equipment costs and increase throughput rates for buffer layer deposition. Additionally, emerging technologies like spatial ALD show promise for combining the precision of traditional ALD with dramatically improved deposition rates.
Material supply chains represent another critical factor affecting manufacturing scalability. Many advanced buffer layer materials incorporate rare earth elements or specialty chemicals with limited global production capacity. Developing buffer layer compositions based on earth-abundant materials will be essential for large-scale deployment.
Cost modeling analyses indicate that buffer layer processing currently contributes significantly to overall cell production costs, with estimates ranging from $5-15/kWh depending on the specific technology. To achieve cost parity with conventional lithium-ion batteries, these processing costs must be reduced by at least 60-70% through manufacturing innovations and economies of scale.
Regulatory considerations also impact manufacturing scalability, particularly regarding environmental permits for facilities using certain precursor chemicals and solvents. Developing greener processing routes that minimize hazardous materials usage will facilitate faster deployment of production capacity across global markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!