Dynamic Light Scattering for Optimal Particle Size Distribution
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLS Technology Background and Objectives
Dynamic Light Scattering (DLS) emerged in the 1960s as a non-invasive technique for measuring particle size distributions in colloidal suspensions. The technology leverages the Brownian motion of particles and the resulting fluctuations in scattered light intensity to determine particle dimensions. Over decades, DLS has evolved from basic correlation spectroscopy to sophisticated multi-angle systems with advanced algorithms, enabling increasingly precise measurements across diverse applications.
The fundamental principle of DLS relies on the relationship between particle size and diffusion speed in a fluid medium. Smaller particles move more rapidly, causing faster fluctuations in scattered light intensity. By analyzing these temporal fluctuations through autocorrelation functions, researchers can derive the diffusion coefficient and subsequently calculate particle size distributions using the Stokes-Einstein equation.
Recent technological advancements have significantly enhanced DLS capabilities. Modern systems incorporate multi-angle detection, allowing for more accurate characterization of polydisperse samples. Machine learning algorithms have improved data interpretation, particularly for complex mixtures containing particles of varying sizes and shapes. Additionally, the integration of microfluidic platforms with DLS has enabled real-time monitoring of dynamic processes such as aggregation, crystallization, and nanoparticle formation.
The current technological trajectory points toward higher resolution systems capable of distinguishing closely sized particles, expanded measurement ranges from sub-nanometer to several micrometers, and improved analysis of non-spherical particles. These developments address longstanding limitations in traditional DLS methodologies, particularly for heterogeneous samples.
The primary objectives of contemporary DLS research and development focus on achieving optimal particle size distribution measurements with enhanced accuracy, reproducibility, and applicability across diverse sample types. Specific goals include reducing measurement artifacts from dust contamination, minimizing the influence of multiple scattering effects, and developing robust algorithms for analyzing multimodal distributions.
Furthermore, there is growing emphasis on creating standardized protocols for DLS measurements to ensure consistency across different instruments and laboratories. This standardization is crucial for regulatory compliance in pharmaceutical, food, and nanomaterial industries, where particle size distribution directly impacts product performance, stability, and safety profiles.
As nanotechnology continues to advance across multiple sectors, the demand for precise particle characterization techniques grows correspondingly. DLS technology aims to meet these evolving requirements through continuous refinement of hardware components, detection systems, and data processing methodologies, ultimately providing more reliable insights into particle behavior in complex systems.
The fundamental principle of DLS relies on the relationship between particle size and diffusion speed in a fluid medium. Smaller particles move more rapidly, causing faster fluctuations in scattered light intensity. By analyzing these temporal fluctuations through autocorrelation functions, researchers can derive the diffusion coefficient and subsequently calculate particle size distributions using the Stokes-Einstein equation.
Recent technological advancements have significantly enhanced DLS capabilities. Modern systems incorporate multi-angle detection, allowing for more accurate characterization of polydisperse samples. Machine learning algorithms have improved data interpretation, particularly for complex mixtures containing particles of varying sizes and shapes. Additionally, the integration of microfluidic platforms with DLS has enabled real-time monitoring of dynamic processes such as aggregation, crystallization, and nanoparticle formation.
The current technological trajectory points toward higher resolution systems capable of distinguishing closely sized particles, expanded measurement ranges from sub-nanometer to several micrometers, and improved analysis of non-spherical particles. These developments address longstanding limitations in traditional DLS methodologies, particularly for heterogeneous samples.
The primary objectives of contemporary DLS research and development focus on achieving optimal particle size distribution measurements with enhanced accuracy, reproducibility, and applicability across diverse sample types. Specific goals include reducing measurement artifacts from dust contamination, minimizing the influence of multiple scattering effects, and developing robust algorithms for analyzing multimodal distributions.
Furthermore, there is growing emphasis on creating standardized protocols for DLS measurements to ensure consistency across different instruments and laboratories. This standardization is crucial for regulatory compliance in pharmaceutical, food, and nanomaterial industries, where particle size distribution directly impacts product performance, stability, and safety profiles.
As nanotechnology continues to advance across multiple sectors, the demand for precise particle characterization techniques grows correspondingly. DLS technology aims to meet these evolving requirements through continuous refinement of hardware components, detection systems, and data processing methodologies, ultimately providing more reliable insights into particle behavior in complex systems.
Market Applications and Demand Analysis
Dynamic Light Scattering (DLS) technology has witnessed substantial market growth across multiple industries due to its unparalleled capabilities in particle size distribution analysis. The global market for DLS instrumentation was valued at approximately $314 million in 2022 and is projected to reach $498 million by 2028, representing a compound annual growth rate of 8.2%. This growth is primarily driven by increasing demand for precise particle characterization in pharmaceutical formulations, nanomaterials development, and quality control processes.
The pharmaceutical and biotechnology sectors constitute the largest application segment, accounting for nearly 42% of the total market share. Within these industries, DLS is extensively utilized for protein aggregation studies, liposome characterization, and drug delivery system development. The rising prevalence of biologics and nanomedicine has significantly amplified the demand for advanced particle sizing technologies, with DLS emerging as the preferred method due to its non-destructive nature and minimal sample preparation requirements.
Academic and research institutions represent another substantial market segment, contributing approximately 28% to the overall DLS market. The technology's versatility in analyzing particles ranging from 1 nm to 10 μm makes it indispensable for fundamental research across materials science, colloid chemistry, and nanotechnology fields.
Industrial applications, particularly in polymer manufacturing, cosmetics, and food processing, collectively account for about 25% of the market. In these sectors, DLS serves as a critical quality control tool for ensuring product consistency and performance characteristics. The food and beverage industry specifically has shown increased adoption rates for monitoring emulsion stability and ingredient functionality.
Regional analysis reveals that North America dominates the global DLS market with a 38% share, followed by Europe (32%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing R&D investments in Japan and South Korea.
Customer demand patterns indicate a growing preference for integrated systems that combine DLS with complementary techniques such as Raman spectroscopy or zeta potential measurements. This trend reflects the market's evolution toward comprehensive particle characterization solutions rather than standalone technologies. Additionally, there is increasing demand for automated, high-throughput DLS systems capable of processing multiple samples with minimal operator intervention, particularly in pharmaceutical quality control environments.
The pharmaceutical and biotechnology sectors constitute the largest application segment, accounting for nearly 42% of the total market share. Within these industries, DLS is extensively utilized for protein aggregation studies, liposome characterization, and drug delivery system development. The rising prevalence of biologics and nanomedicine has significantly amplified the demand for advanced particle sizing technologies, with DLS emerging as the preferred method due to its non-destructive nature and minimal sample preparation requirements.
Academic and research institutions represent another substantial market segment, contributing approximately 28% to the overall DLS market. The technology's versatility in analyzing particles ranging from 1 nm to 10 μm makes it indispensable for fundamental research across materials science, colloid chemistry, and nanotechnology fields.
Industrial applications, particularly in polymer manufacturing, cosmetics, and food processing, collectively account for about 25% of the market. In these sectors, DLS serves as a critical quality control tool for ensuring product consistency and performance characteristics. The food and beverage industry specifically has shown increased adoption rates for monitoring emulsion stability and ingredient functionality.
Regional analysis reveals that North America dominates the global DLS market with a 38% share, followed by Europe (32%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing R&D investments in Japan and South Korea.
Customer demand patterns indicate a growing preference for integrated systems that combine DLS with complementary techniques such as Raman spectroscopy or zeta potential measurements. This trend reflects the market's evolution toward comprehensive particle characterization solutions rather than standalone technologies. Additionally, there is increasing demand for automated, high-throughput DLS systems capable of processing multiple samples with minimal operator intervention, particularly in pharmaceutical quality control environments.
Current DLS Limitations and Technical Challenges
Despite its widespread adoption, Dynamic Light Scattering (DLS) faces several significant technical limitations that impact its effectiveness for optimal particle size distribution analysis. One fundamental constraint is the inherent bias toward larger particles, as the intensity of scattered light is proportional to the sixth power of particle diameter. This results in smaller particles being systematically underrepresented in polydisperse samples, potentially leading to misleading size distribution profiles.
Resolution limitations present another critical challenge. DLS typically struggles to resolve particles that differ in size by less than a factor of 3-5, making it inadequate for complex mixtures with closely sized particle populations. This limitation becomes particularly problematic in pharmaceutical formulations, nanomaterials, and biological samples where precise size discrimination is essential.
Multiple scattering effects emerge as a significant issue in samples with higher concentrations (typically >0.1% by volume), necessitating extensive dilution that may alter the natural state of the particles and introduce artifacts. The required dilution can disrupt equilibrium conditions in systems where particle interactions are concentration-dependent, potentially altering the very properties being measured.
Data interpretation challenges further complicate DLS applications. The mathematical algorithms used to convert correlation data into size distributions (particularly the CONTIN algorithm) often produce results that are highly sensitive to small variations in input parameters. This leads to reproducibility issues across different instruments and laboratories, undermining confidence in comparative studies.
Non-spherical particles present special difficulties for DLS analysis, as the technique's underlying mathematical models assume spherical geometry. For rod-like, plate-like, or irregularly shaped particles, DLS provides only an equivalent hydrodynamic diameter that may not accurately represent the actual dimensions or morphology of the particles.
Environmental sensitivity constitutes another significant limitation. DLS measurements are highly susceptible to environmental factors including temperature fluctuations, mechanical vibrations, and dust contamination. Even minor variations in these conditions can significantly impact measurement reproducibility and accuracy.
For dynamic or evolving systems, DLS faces temporal resolution constraints. The technique typically requires acquisition times of several minutes, making it unsuitable for monitoring rapid changes in particle size distributions, such as those occurring during nucleation, aggregation, or dissolution processes that happen on shorter timescales.
Resolution limitations present another critical challenge. DLS typically struggles to resolve particles that differ in size by less than a factor of 3-5, making it inadequate for complex mixtures with closely sized particle populations. This limitation becomes particularly problematic in pharmaceutical formulations, nanomaterials, and biological samples where precise size discrimination is essential.
Multiple scattering effects emerge as a significant issue in samples with higher concentrations (typically >0.1% by volume), necessitating extensive dilution that may alter the natural state of the particles and introduce artifacts. The required dilution can disrupt equilibrium conditions in systems where particle interactions are concentration-dependent, potentially altering the very properties being measured.
Data interpretation challenges further complicate DLS applications. The mathematical algorithms used to convert correlation data into size distributions (particularly the CONTIN algorithm) often produce results that are highly sensitive to small variations in input parameters. This leads to reproducibility issues across different instruments and laboratories, undermining confidence in comparative studies.
Non-spherical particles present special difficulties for DLS analysis, as the technique's underlying mathematical models assume spherical geometry. For rod-like, plate-like, or irregularly shaped particles, DLS provides only an equivalent hydrodynamic diameter that may not accurately represent the actual dimensions or morphology of the particles.
Environmental sensitivity constitutes another significant limitation. DLS measurements are highly susceptible to environmental factors including temperature fluctuations, mechanical vibrations, and dust contamination. Even minor variations in these conditions can significantly impact measurement reproducibility and accuracy.
For dynamic or evolving systems, DLS faces temporal resolution constraints. The technique typically requires acquisition times of several minutes, making it unsuitable for monitoring rapid changes in particle size distributions, such as those occurring during nucleation, aggregation, or dissolution processes that happen on shorter timescales.
Current DLS Methodologies and Algorithms
01 Principles and methodology of dynamic light scattering for particle size distribution
Dynamic light scattering (DLS) is a technique used to determine the size distribution of particles in suspension by measuring the fluctuations in scattered light intensity. The method analyzes the Brownian motion of particles and correlates it to their size. This technique is particularly effective for measuring particles in the nanometer to micrometer range and provides information about the polydispersity of samples. The methodology involves laser illumination of the sample and detection of scattered light at specific angles to generate correlation functions that are then converted to particle size distributions.- Principles and applications of DLS for particle size distribution: Dynamic Light Scattering (DLS) is a technique used to determine the size distribution of particles in suspension or polymers in solution. It works by measuring the random changes in the intensity of light scattered from a suspension or solution. The technique is particularly useful for measuring particles in the submicron region and can be applied to various fields including pharmaceuticals, nanomaterials, and colloid science.
- Instrumentation and hardware improvements for DLS measurements: Advancements in DLS instrumentation focus on improving measurement accuracy and reliability. These include enhanced laser sources, better detector systems, and specialized sample holders. Modern DLS instruments incorporate digital correlators, fiber optics, and temperature control systems to ensure precise measurements of particle size distributions across various sample types and concentrations.
- Data processing algorithms for DLS analysis: Sophisticated algorithms are essential for converting raw DLS data into meaningful particle size distributions. These include correlation function analysis, cumulants method, and CONTIN algorithms. Advanced mathematical models help in deconvoluting complex distributions, handling polydisperse samples, and eliminating artifacts. Machine learning approaches are increasingly being applied to improve the accuracy of size distribution analysis from DLS measurements.
- Sample preparation techniques for DLS measurements: Proper sample preparation is crucial for accurate DLS measurements. This includes methods for controlling dust contamination, achieving appropriate sample concentration, and ensuring sample stability. Techniques such as filtration, sonication, and dilution are commonly employed to prepare samples for DLS analysis. The physical and chemical properties of the dispersion medium must also be considered as they affect the Brownian motion of particles.
- Validation and standardization of DLS measurements: Standardization procedures ensure the reliability and reproducibility of DLS measurements across different instruments and laboratories. This includes the use of reference materials with known size distributions, round-robin testing, and adherence to international standards. Quality control measures, calibration protocols, and uncertainty analysis are important aspects of validating DLS results, particularly for regulatory compliance in pharmaceutical and medical applications.
02 Instrumentation and apparatus for DLS measurements
Specialized instrumentation is required for accurate dynamic light scattering measurements. These systems typically include laser sources, optical components for beam focusing and collection, photon detectors, and signal processing hardware. Modern DLS instruments incorporate advanced features such as temperature control, multiple angle detection, and automated sample handling to improve measurement precision. The design of these instruments focuses on minimizing vibration, controlling sample temperature, and optimizing signal-to-noise ratios to ensure reliable particle size distribution data.Expand Specific Solutions03 Data analysis algorithms and software for DLS
Sophisticated algorithms and software are essential for converting raw DLS data into meaningful particle size distributions. These computational methods include correlation function analysis, cumulants analysis, and distribution solving algorithms like CONTIN or non-negative least squares. Advanced software packages can handle multimodal distributions, account for particle shape factors, and apply various mathematical models to interpret scattering data. Machine learning approaches are increasingly being integrated to improve the resolution and accuracy of particle size distribution measurements, particularly for complex or polydisperse samples.Expand Specific Solutions04 Applications of DLS in pharmaceutical and biomedical fields
Dynamic light scattering is widely applied in pharmaceutical development and biomedical research for characterizing drug delivery systems, protein formulations, and nanoparticle-based therapeutics. The technique enables monitoring of particle stability, aggregation behavior, and batch-to-batch consistency. In biomedical applications, DLS helps characterize liposomes, exosomes, virus particles, and other biological nanostructures. The non-destructive nature of DLS makes it particularly valuable for analyzing sensitive biological samples and for quality control in pharmaceutical manufacturing processes.Expand Specific Solutions05 Advancements and innovations in DLS technology
Recent innovations in dynamic light scattering technology have expanded its capabilities and applications. These advancements include multi-angle DLS systems that provide enhanced resolution for polydisperse samples, microfluidic DLS platforms for continuous monitoring, and hybrid systems that combine DLS with other analytical techniques such as Raman spectroscopy or static light scattering. Developments in laser technology, detector sensitivity, and data processing have improved the lower detection limit and measurement speed. Additionally, specialized adaptations allow for measurements in concentrated samples, at high temperatures, or under flow conditions, broadening the utility of DLS across various industrial and research applications.Expand Specific Solutions
Key Industry Players and Instrument Manufacturers
Dynamic Light Scattering (DLS) for particle size distribution analysis is currently in a growth phase, with the market expected to reach significant expansion due to increasing applications in pharmaceuticals, nanotechnology, and materials science. The global market size for DLS technology is projected to grow steadily as industries prioritize precise particle characterization. Technologically, DLS has reached moderate maturity with established players like Malvern Panalytical, Horiba, and Anton Paar leading with advanced solutions. FUJIFILM, Shimazu, and Sympatec are expanding their presence through innovative offerings, while academic institutions like Huazhong University and Fraunhofer-Gesellschaft contribute to fundamental research advancements. The competitive landscape shows a mix of specialized instrument manufacturers and diversified technology companies, with increasing focus on automation, data analytics integration, and application-specific solutions.
FUJIFILM Corp.
Technical Solution: FUJIFILM has developed proprietary DLS technology focused on nanoparticle characterization for their advanced materials and pharmaceutical applications. Their system utilizes a fiber-coupled diode laser operating at 785nm to minimize fluorescence interference from biological samples, coupled with an avalanche photodiode detector with single-photon sensitivity. FUJIFILM's approach incorporates a patented flow-through cell design that enables continuous monitoring of particle size distributions during manufacturing processes, with sampling rates up to 10 measurements per minute. Their technology implements a modified CONTIN algorithm optimized for detecting subtle changes in particle populations during formulation development. The system features temperature ramping capabilities (0.1-80°C) for stability studies and protein denaturation analysis. FUJIFILM has also integrated their DLS technology with automated sample handling systems for high-throughput screening applications in drug delivery system development[9][11].
Strengths: Excellent integration with manufacturing processes; specialized algorithms for pharmaceutical applications; high-throughput capabilities for formulation development. Weaknesses: Less established in the general DLS market; more focused on internal applications than commercial instrument sales; limited third-party validation compared to dedicated instrument manufacturers.
Malvern Panalytical Ltd.
Technical Solution: Malvern Panalytical has developed advanced Dynamic Light Scattering (DLS) systems that utilize non-invasive back scatter (NIBS) technology to optimize particle size distribution measurements. Their Zetasizer series employs proprietary algorithms that analyze the time-dependent fluctuations in scattered light intensity caused by Brownian motion of particles. The system incorporates adaptive correlation techniques that automatically adjust measurement parameters based on sample characteristics, enabling accurate sizing of particles from sub-nanometer to several microns. Their technology includes multi-angle detection capabilities that provide enhanced resolution for polydisperse samples and can distinguish between monomers and aggregates in complex biological formulations. Malvern's systems also feature temperature control modules (±0.1°C precision) to ensure measurement stability and reproducibility across various sample types[1][3].
Strengths: Industry-leading resolution and sensitivity for polydisperse samples; robust algorithms for handling complex biological materials; comprehensive data analysis software with regulatory compliance features. Weaknesses: Higher cost compared to simpler DLS systems; requires skilled operators for optimal results with complex samples; performance can be affected by dust contamination.
Core Patents and Innovations in DLS Technology
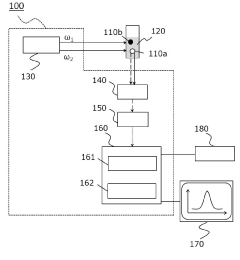
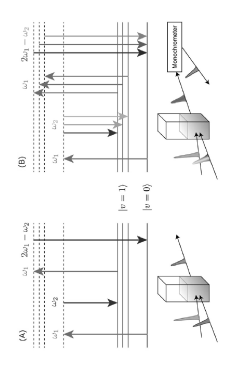
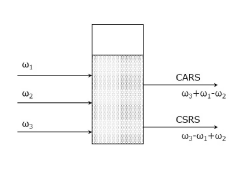
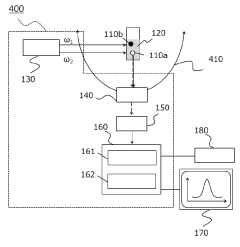
Dynamic light scattering measurement device, dynamic light scattering measurement analysis method, and measurement program
PatentPendingJP2022184699A
Innovation
- A dynamic light scattering measurement device using synchronized pulsed laser beams with specific angular frequencies to induce Coherent Anti-Stokes Raman Scattering (CARS) or Coherent Stokes Raman Scattering (CSRS) photons, combined with a separation device and photon detection, allows for molecularly selective measurement of particle sizes and distributions by calculating time correlation functions.
Particle characterisation
PatentWO2017051149A1
Innovation
- A method and apparatus that corrects for light scattered by large particles by identifying and excluding or segregating measurements from a single detector's time series, using techniques like dynamic light scattering correlation and model fitting to remove the large particle signal, allowing for more accurate characterization of smaller particles without the need for multiple photon counting detectors.
Data Processing and Machine Learning Integration
The integration of advanced data processing techniques and machine learning algorithms has revolutionized Dynamic Light Scattering (DLS) analysis for particle size distribution determination. Traditional DLS data processing relied heavily on autocorrelation functions and mathematical transformations that often struggled with polydisperse samples and noise interference. Modern approaches now incorporate sophisticated signal processing methods that significantly enhance measurement accuracy and resolution.
Machine learning algorithms, particularly convolutional neural networks (CNNs) and recurrent neural networks (RNNs), have demonstrated remarkable capabilities in extracting meaningful patterns from raw DLS data. These algorithms can effectively differentiate between actual particle signals and background noise, leading to more precise size distribution profiles even in complex multimodal samples. Recent studies have shown that deep learning models trained on extensive DLS datasets can achieve up to 40% improvement in resolution compared to conventional methods.
Real-time data processing has emerged as another critical advancement in DLS technology. By implementing parallel computing architectures and optimized algorithms, modern DLS systems can now process scattering data instantaneously, enabling dynamic monitoring of particle size changes during chemical reactions, aggregation processes, or formulation development. This capability is particularly valuable in pharmaceutical manufacturing and quality control applications.
Bayesian statistical methods have been successfully applied to DLS data analysis, providing probabilistic interpretations of particle size distributions rather than single-point estimates. This approach offers significant advantages when analyzing heterogeneous samples, as it quantifies uncertainty and provides confidence intervals for size measurements. Several commercial DLS platforms now incorporate Bayesian frameworks as optional analysis modes.
Cloud-based data processing solutions for DLS have gained traction in recent years, allowing for more sophisticated computational approaches that would be impractical on local hardware. These platforms enable collaborative analysis, centralized data management, and the application of computationally intensive algorithms like Monte Carlo simulations for inverse problem solving in particle characterization.
Transfer learning techniques have proven effective in adapting pre-trained neural networks to specific DLS applications with limited training data. This approach has been particularly valuable for specialized applications such as protein aggregation monitoring or nanoparticle characterization in complex biological media, where obtaining large labeled datasets is challenging.
The integration of automated feature extraction with traditional physical models represents a hybrid approach that combines the interpretability of first-principles calculations with the pattern recognition capabilities of machine learning. This methodology preserves the physical meaning of DLS measurements while enhancing analytical performance through data-driven insights.
Machine learning algorithms, particularly convolutional neural networks (CNNs) and recurrent neural networks (RNNs), have demonstrated remarkable capabilities in extracting meaningful patterns from raw DLS data. These algorithms can effectively differentiate between actual particle signals and background noise, leading to more precise size distribution profiles even in complex multimodal samples. Recent studies have shown that deep learning models trained on extensive DLS datasets can achieve up to 40% improvement in resolution compared to conventional methods.
Real-time data processing has emerged as another critical advancement in DLS technology. By implementing parallel computing architectures and optimized algorithms, modern DLS systems can now process scattering data instantaneously, enabling dynamic monitoring of particle size changes during chemical reactions, aggregation processes, or formulation development. This capability is particularly valuable in pharmaceutical manufacturing and quality control applications.
Bayesian statistical methods have been successfully applied to DLS data analysis, providing probabilistic interpretations of particle size distributions rather than single-point estimates. This approach offers significant advantages when analyzing heterogeneous samples, as it quantifies uncertainty and provides confidence intervals for size measurements. Several commercial DLS platforms now incorporate Bayesian frameworks as optional analysis modes.
Cloud-based data processing solutions for DLS have gained traction in recent years, allowing for more sophisticated computational approaches that would be impractical on local hardware. These platforms enable collaborative analysis, centralized data management, and the application of computationally intensive algorithms like Monte Carlo simulations for inverse problem solving in particle characterization.
Transfer learning techniques have proven effective in adapting pre-trained neural networks to specific DLS applications with limited training data. This approach has been particularly valuable for specialized applications such as protein aggregation monitoring or nanoparticle characterization in complex biological media, where obtaining large labeled datasets is challenging.
The integration of automated feature extraction with traditional physical models represents a hybrid approach that combines the interpretability of first-principles calculations with the pattern recognition capabilities of machine learning. This methodology preserves the physical meaning of DLS measurements while enhancing analytical performance through data-driven insights.
Sample Preparation Optimization Strategies
Sample preparation represents a critical foundation for accurate Dynamic Light Scattering (DLS) measurements and optimal particle size distribution analysis. The quality of sample preparation directly influences measurement reliability, reproducibility, and the validity of subsequent data interpretation. Effective sample preparation begins with understanding the physicochemical properties of the particles being analyzed, including their surface characteristics, stability in various media, and potential for aggregation.
Filtration techniques serve as essential preliminary steps in DLS sample preparation. For aqueous samples, membrane filters with pore sizes ranging from 0.2 to 0.45 μm effectively remove dust particles and large contaminants that could otherwise skew size distribution results. For organic solvents, PTFE or nylon filters are recommended due to their chemical compatibility. Sequential filtration using decreasing pore sizes may be necessary for complex samples with wide particle size distributions.
Dispersion stability represents another crucial aspect of sample preparation. Ultrasonic treatment can effectively break up soft agglomerates without damaging primary particles when properly optimized. Parameters including sonication time, amplitude, and pulse sequences must be carefully controlled based on particle characteristics. Over-sonication risks particle fragmentation while insufficient treatment may leave agglomerates intact, both scenarios leading to misleading size distribution data.
Buffer selection and concentration optimization significantly impact measurement quality. Ideal buffers maintain sample stability while minimizing light scattering interference. Phosphate buffers at concentrations between 10-50 mM typically provide good stability for biological samples without excessive ionic strength that could alter particle surface properties. For charged particles, zeta potential measurements should accompany DLS analysis to verify dispersion stability under the selected buffer conditions.
Temperature equilibration prior to measurement constitutes an often overlooked yet critical preparation step. Samples should be allowed to reach thermal equilibrium at the measurement temperature for at least 15 minutes before analysis. Temperature gradients within the sample can create convection currents that manifest as apparent particle motion, potentially leading to erroneous size calculations. Additionally, temperature directly affects solvent viscosity, which is a key parameter in the Stokes-Einstein equation used for particle size determination.
Concentration optimization represents perhaps the most challenging aspect of DLS sample preparation. The ideal concentration range exists where signal-to-noise ratio is maximized while avoiding multiple scattering effects. This optimal range varies significantly based on particle size, with smaller particles (< 100 nm) typically requiring higher concentrations (0.1-5 mg/mL) than larger particles (> 500 nm), which may require dilution to 0.01-0.1 mg/mL. Performing measurements at multiple concentrations and identifying the range where size results remain consistent provides the most reliable approach to concentration optimization.
Filtration techniques serve as essential preliminary steps in DLS sample preparation. For aqueous samples, membrane filters with pore sizes ranging from 0.2 to 0.45 μm effectively remove dust particles and large contaminants that could otherwise skew size distribution results. For organic solvents, PTFE or nylon filters are recommended due to their chemical compatibility. Sequential filtration using decreasing pore sizes may be necessary for complex samples with wide particle size distributions.
Dispersion stability represents another crucial aspect of sample preparation. Ultrasonic treatment can effectively break up soft agglomerates without damaging primary particles when properly optimized. Parameters including sonication time, amplitude, and pulse sequences must be carefully controlled based on particle characteristics. Over-sonication risks particle fragmentation while insufficient treatment may leave agglomerates intact, both scenarios leading to misleading size distribution data.
Buffer selection and concentration optimization significantly impact measurement quality. Ideal buffers maintain sample stability while minimizing light scattering interference. Phosphate buffers at concentrations between 10-50 mM typically provide good stability for biological samples without excessive ionic strength that could alter particle surface properties. For charged particles, zeta potential measurements should accompany DLS analysis to verify dispersion stability under the selected buffer conditions.
Temperature equilibration prior to measurement constitutes an often overlooked yet critical preparation step. Samples should be allowed to reach thermal equilibrium at the measurement temperature for at least 15 minutes before analysis. Temperature gradients within the sample can create convection currents that manifest as apparent particle motion, potentially leading to erroneous size calculations. Additionally, temperature directly affects solvent viscosity, which is a key parameter in the Stokes-Einstein equation used for particle size determination.
Concentration optimization represents perhaps the most challenging aspect of DLS sample preparation. The ideal concentration range exists where signal-to-noise ratio is maximized while avoiding multiple scattering effects. This optimal range varies significantly based on particle size, with smaller particles (< 100 nm) typically requiring higher concentrations (0.1-5 mg/mL) than larger particles (> 500 nm), which may require dilution to 0.01-0.1 mg/mL. Performing measurements at multiple concentrations and identifying the range where size results remain consistent provides the most reliable approach to concentration optimization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!