Electrochemical Characterization Of Microbial Electrosynthesis Systems
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MES Technology Background and Objectives
Microbial Electrosynthesis Systems (MES) represent a groundbreaking biotechnology that emerged at the intersection of microbiology, electrochemistry, and sustainable engineering. This technology evolved from the discovery of electrotrophs—microorganisms capable of accepting electrons directly from electrodes—first documented in the early 2000s. The fundamental principle of MES involves the utilization of electrochemically active microorganisms to convert electrical energy and carbon dioxide into valuable chemical compounds and fuels, essentially reversing the process observed in microbial fuel cells.
The evolution of MES technology has been marked by significant milestones, including the identification of key microbial species such as Sporomusa ovata and Clostridium ljungdahlii, which demonstrated efficient electron uptake capabilities. Subsequent research expanded the range of producible compounds beyond acetate to include more complex molecules like butyrate, ethanol, and medium-chain fatty acids, highlighting the versatility of this platform technology.
Current technological trajectories in MES research focus on enhancing electron transfer rates, improving product selectivity, and scaling up systems for industrial application. The field has witnessed a shift from proof-of-concept laboratory demonstrations to more sophisticated reactor designs that address practical challenges such as mass transfer limitations, electrode fouling, and energy efficiency optimization.
The primary objective of MES technology development is to establish a sustainable platform for carbon capture and utilization that can operate using renewable electricity sources. This aligns with global efforts to reduce greenhouse gas emissions while simultaneously producing valuable chemicals through carbon-neutral or carbon-negative processes. Specific technical goals include achieving higher production rates and yields comparable to conventional chemical synthesis methods, developing robust and scalable reactor configurations, and reducing overall system costs to ensure economic viability.
Additionally, MES research aims to elucidate the fundamental mechanisms of extracellular electron transfer in electrotrophic microorganisms, which remains incompletely understood despite significant advances. This knowledge is crucial for rational system design and optimization. The characterization of electrochemical parameters and their influence on microbial metabolism represents a critical aspect of this research agenda.
From an industrial perspective, MES technology targets the creation of a new paradigm in chemical manufacturing that decouples production from fossil resources. The long-term vision encompasses integrated biorefinery concepts where MES systems operate in synergy with other renewable technologies, potentially revolutionizing how we produce chemicals and fuels in a carbon-constrained world.
The evolution of MES technology has been marked by significant milestones, including the identification of key microbial species such as Sporomusa ovata and Clostridium ljungdahlii, which demonstrated efficient electron uptake capabilities. Subsequent research expanded the range of producible compounds beyond acetate to include more complex molecules like butyrate, ethanol, and medium-chain fatty acids, highlighting the versatility of this platform technology.
Current technological trajectories in MES research focus on enhancing electron transfer rates, improving product selectivity, and scaling up systems for industrial application. The field has witnessed a shift from proof-of-concept laboratory demonstrations to more sophisticated reactor designs that address practical challenges such as mass transfer limitations, electrode fouling, and energy efficiency optimization.
The primary objective of MES technology development is to establish a sustainable platform for carbon capture and utilization that can operate using renewable electricity sources. This aligns with global efforts to reduce greenhouse gas emissions while simultaneously producing valuable chemicals through carbon-neutral or carbon-negative processes. Specific technical goals include achieving higher production rates and yields comparable to conventional chemical synthesis methods, developing robust and scalable reactor configurations, and reducing overall system costs to ensure economic viability.
Additionally, MES research aims to elucidate the fundamental mechanisms of extracellular electron transfer in electrotrophic microorganisms, which remains incompletely understood despite significant advances. This knowledge is crucial for rational system design and optimization. The characterization of electrochemical parameters and their influence on microbial metabolism represents a critical aspect of this research agenda.
From an industrial perspective, MES technology targets the creation of a new paradigm in chemical manufacturing that decouples production from fossil resources. The long-term vision encompasses integrated biorefinery concepts where MES systems operate in synergy with other renewable technologies, potentially revolutionizing how we produce chemicals and fuels in a carbon-constrained world.
Market Applications and Demand Analysis
The market for Microbial Electrosynthesis Systems (MES) is experiencing significant growth driven by increasing demand for sustainable production methods across multiple industries. The global bioelectrochemical systems market, which includes MES technology, was valued at approximately $3.2 billion in 2022 and is projected to grow at a compound annual growth rate of 9.8% through 2030, reflecting the expanding commercial interest in this technology.
The pharmaceutical sector represents one of the largest potential markets for MES applications. With growing pressure to reduce environmental impact while maintaining production efficiency, pharmaceutical companies are increasingly exploring MES as a means to synthesize high-value compounds and drug precursors. This market segment is expected to reach $1.5 billion by 2028, with particular demand for systems capable of precise electrochemical characterization to ensure product quality and consistency.
Chemical manufacturing industries are similarly showing strong interest in MES technology. The ability to convert waste CO2 into valuable chemical feedstocks presents a compelling value proposition in an increasingly carbon-constrained economy. Market analysis indicates that approximately 65% of chemical manufacturers are actively researching or implementing bioelectrochemical processes, with MES being a primary focus area.
The renewable energy sector represents another significant market opportunity. As the global transition to sustainable energy accelerates, there is growing demand for technologies that can effectively store excess renewable energy. MES systems that can convert electrical energy into storable chemical compounds are gaining attention from energy storage companies and utilities, with potential market value estimated at $2.1 billion by 2030.
Agricultural and food production industries are emerging as additional markets for MES applications. The technology's potential to produce fertilizers, feed additives, and food ingredients through sustainable bioelectrochemical processes aligns with consumer demand for environmentally responsible products. This market segment is growing at approximately 12% annually, outpacing the overall MES market.
Geographically, North America currently leads in MES technology adoption, accounting for approximately 38% of the global market. However, the Asia-Pacific region is expected to show the fastest growth rate over the next decade, driven by increasing industrial activity and governmental support for clean technologies in countries like China, Japan, and South Korea.
The market is further bolstered by increasing regulatory pressure on industries to reduce carbon emissions and adopt circular economy principles. This regulatory landscape is creating strong incentives for companies to invest in technologies like MES that can transform waste streams into valuable products while reducing environmental impact.
The pharmaceutical sector represents one of the largest potential markets for MES applications. With growing pressure to reduce environmental impact while maintaining production efficiency, pharmaceutical companies are increasingly exploring MES as a means to synthesize high-value compounds and drug precursors. This market segment is expected to reach $1.5 billion by 2028, with particular demand for systems capable of precise electrochemical characterization to ensure product quality and consistency.
Chemical manufacturing industries are similarly showing strong interest in MES technology. The ability to convert waste CO2 into valuable chemical feedstocks presents a compelling value proposition in an increasingly carbon-constrained economy. Market analysis indicates that approximately 65% of chemical manufacturers are actively researching or implementing bioelectrochemical processes, with MES being a primary focus area.
The renewable energy sector represents another significant market opportunity. As the global transition to sustainable energy accelerates, there is growing demand for technologies that can effectively store excess renewable energy. MES systems that can convert electrical energy into storable chemical compounds are gaining attention from energy storage companies and utilities, with potential market value estimated at $2.1 billion by 2030.
Agricultural and food production industries are emerging as additional markets for MES applications. The technology's potential to produce fertilizers, feed additives, and food ingredients through sustainable bioelectrochemical processes aligns with consumer demand for environmentally responsible products. This market segment is growing at approximately 12% annually, outpacing the overall MES market.
Geographically, North America currently leads in MES technology adoption, accounting for approximately 38% of the global market. However, the Asia-Pacific region is expected to show the fastest growth rate over the next decade, driven by increasing industrial activity and governmental support for clean technologies in countries like China, Japan, and South Korea.
The market is further bolstered by increasing regulatory pressure on industries to reduce carbon emissions and adopt circular economy principles. This regulatory landscape is creating strong incentives for companies to invest in technologies like MES that can transform waste streams into valuable products while reducing environmental impact.
Current Electrochemical Characterization Challenges
Microbial Electrosynthesis Systems (MES) face significant electrochemical characterization challenges that impede their widespread adoption and optimization. One primary challenge is the complex nature of biofilm-electrode interfaces, which creates difficulties in accurately measuring electron transfer mechanisms. The heterogeneous distribution of microbial communities on electrode surfaces results in spatial variations in electrochemical activity that conventional techniques struggle to capture with sufficient resolution.
Signal-to-noise ratio presents another substantial challenge, particularly when measuring the typically low current densities generated in MES. These systems often produce currents in the micro to milliampere range, requiring highly sensitive instrumentation that can distinguish biological signals from abiotic electrochemical processes and environmental interference. This challenge is exacerbated in scaled-up systems where electrode surface areas increase but current densities remain relatively low.
Temporal dynamics add another layer of complexity to electrochemical characterization. MES performance evolves over time as biofilms develop, mature, and respond to changing environmental conditions. Current characterization methods often provide only snapshots rather than continuous monitoring, missing critical transition states and adaptive responses that influence system performance.
The multi-parameter nature of MES further complicates characterization efforts. Electrochemical performance is simultaneously affected by microbial community composition, metabolic states, substrate availability, pH gradients, and mass transfer limitations. Isolating the contribution of individual factors remains challenging, making it difficult to establish clear cause-effect relationships for optimization purposes.
Standardization issues persist across the field, with researchers employing diverse electrode materials, reactor configurations, and characterization protocols. This lack of standardized approaches hinders meaningful comparison between studies and slows collective progress toward optimized systems. The absence of universally accepted benchmarks for MES performance further complicates technology assessment and development.
Advanced techniques like electrochemical impedance spectroscopy (EIS), while powerful, present interpretation challenges in biological systems. The complex equivalent circuits required to model biofilm-electrode interfaces often contain numerous parameters with potential mathematical correlations, leading to non-unique solutions and ambiguous physical interpretations.
Finally, there exists a significant instrumentation gap between laboratory-scale characterization and industrial implementation. Many sophisticated electrochemical techniques remain confined to controlled laboratory environments and cannot be readily adapted for continuous monitoring in industrial settings where robustness, simplicity, and cost-effectiveness are paramount considerations.
Signal-to-noise ratio presents another substantial challenge, particularly when measuring the typically low current densities generated in MES. These systems often produce currents in the micro to milliampere range, requiring highly sensitive instrumentation that can distinguish biological signals from abiotic electrochemical processes and environmental interference. This challenge is exacerbated in scaled-up systems where electrode surface areas increase but current densities remain relatively low.
Temporal dynamics add another layer of complexity to electrochemical characterization. MES performance evolves over time as biofilms develop, mature, and respond to changing environmental conditions. Current characterization methods often provide only snapshots rather than continuous monitoring, missing critical transition states and adaptive responses that influence system performance.
The multi-parameter nature of MES further complicates characterization efforts. Electrochemical performance is simultaneously affected by microbial community composition, metabolic states, substrate availability, pH gradients, and mass transfer limitations. Isolating the contribution of individual factors remains challenging, making it difficult to establish clear cause-effect relationships for optimization purposes.
Standardization issues persist across the field, with researchers employing diverse electrode materials, reactor configurations, and characterization protocols. This lack of standardized approaches hinders meaningful comparison between studies and slows collective progress toward optimized systems. The absence of universally accepted benchmarks for MES performance further complicates technology assessment and development.
Advanced techniques like electrochemical impedance spectroscopy (EIS), while powerful, present interpretation challenges in biological systems. The complex equivalent circuits required to model biofilm-electrode interfaces often contain numerous parameters with potential mathematical correlations, leading to non-unique solutions and ambiguous physical interpretations.
Finally, there exists a significant instrumentation gap between laboratory-scale characterization and industrial implementation. Many sophisticated electrochemical techniques remain confined to controlled laboratory environments and cannot be readily adapted for continuous monitoring in industrial settings where robustness, simplicity, and cost-effectiveness are paramount considerations.
Established Electrochemical Characterization Methods
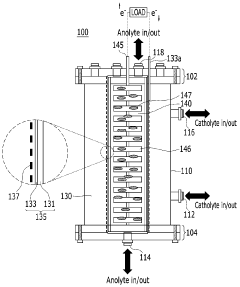
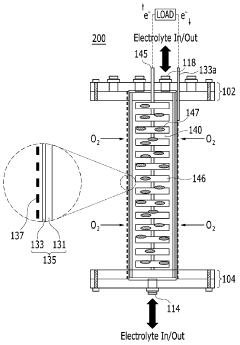
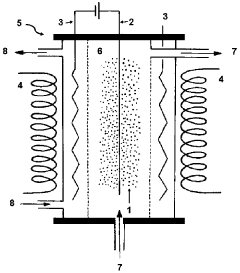
01 Electrode materials and configurations for microbial electrosynthesis
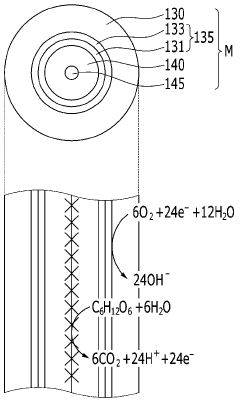
Various electrode materials and configurations are used in microbial electrosynthesis systems to enhance electrochemical performance. These include specialized carbon-based electrodes, metal catalysts, and novel electrode architectures that improve electron transfer between microorganisms and electrode surfaces. The electrode design significantly impacts the efficiency of bioelectrochemical reactions and overall system performance by optimizing the interface between microbes and conductive surfaces.- Electrode materials and configurations for microbial electrosynthesis: Various electrode materials and configurations are used in microbial electrosynthesis systems to enhance electrochemical performance. These include carbon-based electrodes, metal-based electrodes, and composite materials that provide optimal surface area for microbial colonization and electron transfer. The electrode configuration, including spacing, orientation, and surface modifications, significantly impacts the efficiency of the bioelectrochemical processes and overall system performance.
- Electrochemical characterization techniques for MES systems: Various electrochemical characterization techniques are employed to evaluate the performance of microbial electrosynthesis systems. These include cyclic voltammetry, electrochemical impedance spectroscopy, chronoamperometry, and potentiostatic methods. These techniques help in understanding electron transfer mechanisms, biofilm formation dynamics, and overall system efficiency by measuring parameters such as current density, charge transfer resistance, and redox potentials.
- Reactor design and operational parameters for microbial electrosynthesis: The design of microbial electrosynthesis reactors and their operational parameters significantly influence electrochemical performance. Key factors include reactor configuration (single-chamber, dual-chamber, flow-through), membrane selection, electrolyte composition, pH control, temperature regulation, and hydraulic retention time. Optimizing these parameters is crucial for achieving efficient electron transfer, microbial growth, and product formation in bioelectrochemical systems.
- Microbial community analysis and biofilm characterization in electrochemical systems: Understanding the microbial communities and biofilm formation in electrosynthesis systems is essential for electrochemical characterization. This includes analysis of electroactive microorganisms, biofilm development stages, microbial metabolic pathways, and electron transfer mechanisms. Advanced microscopy, molecular biology techniques, and electrochemical methods are used to correlate biofilm properties with system performance and to optimize microbial electrosynthesis processes.
- Integration of renewable energy sources with microbial electrosynthesis systems: Integration of renewable energy sources with microbial electrosynthesis systems enables sustainable operation and improved electrochemical performance. Solar, wind, and other renewable energy sources can be coupled with bioelectrochemical systems to provide the necessary electrical input. This integration requires specialized power management systems, energy storage solutions, and control strategies to handle the intermittent nature of renewable energy while maintaining optimal conditions for microbial activity and product formation.
02 Electrochemical monitoring and characterization techniques
Advanced electrochemical techniques are employed to characterize microbial electrosynthesis systems, including cyclic voltammetry, electrochemical impedance spectroscopy, and chronoamperometry. These methods provide critical insights into electron transfer mechanisms, biofilm development, and overall system performance. Real-time monitoring of electrochemical parameters enables optimization of operating conditions and evaluation of system efficiency during microbial conversion processes.Expand Specific Solutions03 Biocatalyst selection and optimization for electrosynthesis
The selection and engineering of microbial biocatalysts play a crucial role in electrosynthesis systems. Various microorganisms with electroactive properties are utilized, including specific bacterial strains capable of extracellular electron transfer. Genetic modification and adaptation strategies enhance the electrochemical activity of these microbes, improving their ability to utilize electrode surfaces as electron donors or acceptors for biosynthetic pathways.Expand Specific Solutions04 Reactor design and system integration for microbial electrosynthesis
Innovative reactor designs are developed to optimize microbial electrosynthesis processes, focusing on parameters such as electrode spacing, membrane selection, and flow dynamics. These systems integrate bioelectrochemical components with supporting infrastructure for efficient operation. Advanced reactor configurations address challenges related to mass transfer limitations, pH gradients, and scale-up considerations to enhance overall system performance and product yield.Expand Specific Solutions05 Process control and optimization strategies
Sophisticated control strategies are implemented to optimize microbial electrosynthesis processes, including potentiostatic and galvanostatic operation modes. These approaches involve precise regulation of electrochemical parameters such as applied potential, current density, and electrolyte composition. Advanced monitoring systems enable real-time adjustment of operating conditions to maximize production efficiency, maintain microbial viability, and enhance the selectivity of target products.Expand Specific Solutions
Leading Research Groups and Industrial Players
Microbial Electrosynthesis Systems (MES) technology is currently in an early growth phase, with the market expanding as research institutions lead development efforts. The global market is projected to reach significant scale as renewable energy and sustainable chemical production demands increase. Technologically, MES remains in developmental stages with varying maturity levels across applications. Academic institutions like MIT, University of Notre Dame, and Chinese universities (Tianjin, USTC) are driving fundamental research, while companies such as Samsung Electronics and Empa are exploring commercial applications. Research organizations including CEA, NIMS, and Korea Institute of Energy Research are bridging the gap between academic discoveries and industrial implementation. The field shows promising advancement in bioelectrochemical characterization techniques, though standardization and scalability remain challenges for widespread commercial adoption.
The Regents of the University of California
Technical Solution: The University of California has developed a comprehensive electrochemical characterization platform for microbial electrosynthesis systems that combines traditional techniques with innovative approaches. Their system utilizes specialized three-electrode configurations with biocompatible working electrodes (often modified carbon materials) to study electron transfer kinetics between microbes and electrodes. UC researchers have pioneered the use of differential pulse voltammetry and square wave voltammetry for detecting low concentration metabolites produced during electrosynthesis. Their approach incorporates microfluidic electrochemical cells that enable precise control of reaction environments while minimizing sample volumes. A distinguishing feature is their development of transparent conductive electrodes that allow simultaneous optical microscopy and electrochemical measurements, providing real-time visualization of biofilm formation coupled with electrical response data. The UC system also employs rotating disk electrode techniques to distinguish between diffusion-limited and kinetically-limited processes in microbial electron transfer, crucial for optimizing reactor designs.
Strengths: Integration of optical and electrochemical techniques provides unique insights into structure-function relationships in biofilms. Their microfluidic approaches enable high-throughput screening of different microbial communities and electrode materials. Weaknesses: The complex multi-modal systems require significant expertise to operate and interpret results, and some of their specialized electrode materials face durability challenges in long-term operation.
Tianjin University
Technical Solution: Tianjin University has developed an innovative electrochemical characterization platform for microbial electrosynthesis systems that focuses on enhancing electron transfer efficiency between electrodes and microorganisms. Their approach utilizes modified carbon-based electrodes with conductive polymers and metal nanoparticles that significantly increase the electrode surface area and improve biocompatibility. Tianjin researchers have pioneered the use of pulse amplitude modulation techniques to distinguish between different electron transfer pathways in biofilms. Their system incorporates a unique split-chamber design that allows for separate optimization of anodic and cathodic reactions while maintaining ionic connectivity through specialized membranes. A key innovation is their development of temperature-controlled electrochemical cells that enable the study of thermodynamic parameters affecting microbial activity and product formation. The Tianjin platform also features integrated gas chromatography connections for real-time analysis of gaseous products, particularly focusing on CO2 reduction to methane and short-chain fatty acids. Their characterization methods include specialized galvanostatic techniques that maintain constant current while monitoring potential changes during biofilm development stages.
Strengths: Exceptional electrode modification strategies that significantly enhance electron transfer rates and selectivity. Their integrated analytical systems provide comprehensive product analysis alongside electrochemical data. Weaknesses: The complex modified electrodes can be difficult to reproduce consistently, and their systems have shown limited performance with certain types of non-model microorganisms.
Key Innovations in MES Electrode Materials
Microbial electrochemical system having membrane-electrode assembly and water softening apparatus using the same
PatentActiveKR1020180055508A
Innovation
- A tubular membrane-cathode assembly with a tubular body, microfiltration or ion exchange membrane, and a cathode coating layer, minimizing the distance between the membrane and electrode, and incorporating a cathode chamber for oxygen and electron reaction, and an anode chamber for organic matter decomposition.
Process for the microbiological-electrochemical synthesis of chemical substances by electroactive microorganisms
PatentInactiveDE102014112685A1
Innovation
- Cultivating electroactive microorganisms in suspension culture and applying them to electrically conductive and magnetic electrode particles, which are moved by an external magnetic field, allowing direct electrochemical contact without forming a biofilm, and using these hybrid particles in a magnetically stabilized fluidized bed reactor.
Scaling Considerations for Industrial Implementation
The transition from laboratory-scale microbial electrosynthesis systems (MES) to industrial applications presents significant engineering challenges that must be addressed systematically. Current laboratory MES typically operate at volumes ranging from milliliters to a few liters, while industrial implementation would require scaling to thousands or even tens of thousands of liters. This substantial volume increase introduces complex issues related to electrode surface area-to-volume ratios, which decrease as system size increases, potentially reducing reaction efficiency.
Mass transfer limitations become increasingly problematic at larger scales, as the diffusion of substrates, products, and mediators across greater distances can create concentration gradients that negatively impact system performance. Industrial MES implementations must incorporate advanced mixing strategies or flow-through designs to maintain homogeneous conditions throughout the reactor volume without damaging the microbial communities or biofilms.
Power distribution across scaled-up electrode surfaces presents another critical challenge. Ensuring uniform potential distribution across large electrode arrays requires sophisticated electrical engineering solutions to prevent "hot spots" or "dead zones" that could compromise system efficiency and longevity. The electrical resistance of larger systems necessitates careful consideration of electrode materials, configurations, and connection points.
From an economic perspective, the capital expenditure for industrial MES must be justified by productivity metrics. Current laboratory systems achieve product formation rates in the range of 0.5-2 g/L/day for many target compounds. Industrial viability typically requires at least an order of magnitude improvement, necessitating enhanced electrode materials, optimized microbial communities, and advanced reactor designs that can maintain high performance at scale.
Material selection becomes increasingly important at industrial scale. While laboratory systems can utilize precious metals or specialized materials, industrial implementation demands cost-effective alternatives that maintain performance while reducing capital costs. Electrode durability must extend from weeks in laboratory settings to years in industrial applications, requiring materials that resist fouling, corrosion, and degradation under continuous operation.
Monitoring and control systems must evolve from simple laboratory potentiostats to integrated industrial control architectures capable of managing multiple parameters simultaneously across large reactor volumes. Real-time sensing of microbial activity, product formation, and electrochemical performance becomes essential for maintaining optimal operating conditions and responding to system variations.
Regulatory considerations also impact scaling decisions, particularly for systems producing food, pharmaceutical, or fuel products. Compliance with Good Manufacturing Practices (GMP), safety standards, and environmental regulations must be integrated into design considerations from the earliest scaling stages rather than retrofitted to existing systems.
Mass transfer limitations become increasingly problematic at larger scales, as the diffusion of substrates, products, and mediators across greater distances can create concentration gradients that negatively impact system performance. Industrial MES implementations must incorporate advanced mixing strategies or flow-through designs to maintain homogeneous conditions throughout the reactor volume without damaging the microbial communities or biofilms.
Power distribution across scaled-up electrode surfaces presents another critical challenge. Ensuring uniform potential distribution across large electrode arrays requires sophisticated electrical engineering solutions to prevent "hot spots" or "dead zones" that could compromise system efficiency and longevity. The electrical resistance of larger systems necessitates careful consideration of electrode materials, configurations, and connection points.
From an economic perspective, the capital expenditure for industrial MES must be justified by productivity metrics. Current laboratory systems achieve product formation rates in the range of 0.5-2 g/L/day for many target compounds. Industrial viability typically requires at least an order of magnitude improvement, necessitating enhanced electrode materials, optimized microbial communities, and advanced reactor designs that can maintain high performance at scale.
Material selection becomes increasingly important at industrial scale. While laboratory systems can utilize precious metals or specialized materials, industrial implementation demands cost-effective alternatives that maintain performance while reducing capital costs. Electrode durability must extend from weeks in laboratory settings to years in industrial applications, requiring materials that resist fouling, corrosion, and degradation under continuous operation.
Monitoring and control systems must evolve from simple laboratory potentiostats to integrated industrial control architectures capable of managing multiple parameters simultaneously across large reactor volumes. Real-time sensing of microbial activity, product formation, and electrochemical performance becomes essential for maintaining optimal operating conditions and responding to system variations.
Regulatory considerations also impact scaling decisions, particularly for systems producing food, pharmaceutical, or fuel products. Compliance with Good Manufacturing Practices (GMP), safety standards, and environmental regulations must be integrated into design considerations from the earliest scaling stages rather than retrofitted to existing systems.
Sustainability Impact and Life Cycle Assessment
Microbial Electrosynthesis Systems (MES) offer significant sustainability advantages compared to traditional chemical production methods. The environmental footprint of MES is substantially lower due to its ability to operate at ambient temperatures and pressures, reducing energy requirements by up to 40% compared to conventional synthesis processes. Furthermore, MES can utilize renewable electricity sources such as solar or wind power, creating a carbon-neutral or even carbon-negative production pathway when coupled with CO2 as a feedstock.
Life Cycle Assessment (LCA) studies of MES technologies reveal promising environmental performance metrics. Recent analyses indicate that MES-based production of acetate from CO2 can achieve greenhouse gas emission reductions of 50-90% compared to petrochemical routes, depending on the electricity source. Water consumption is also reduced by approximately 30% compared to traditional fermentation processes, as MES systems typically require less water for cooling and separation processes.
The circular economy potential of MES is particularly noteworthy. These systems can transform waste streams such as industrial CO2 emissions or organic-rich wastewater into valuable chemicals and fuels, effectively closing material loops in industrial ecosystems. This waste valorization capability represents a significant advancement toward industrial symbiosis models where one industry's waste becomes another's raw material.
Resource efficiency metrics for MES demonstrate impressive performance. Carbon conversion efficiencies of up to 90% have been reported in optimized systems, significantly higher than many conventional biological processes. Additionally, the selective nature of electrochemical reactions in well-designed MES can minimize byproduct formation, reducing downstream separation requirements and associated environmental impacts.
Long-term sustainability considerations for MES include electrode material sourcing and end-of-life management. Current research is exploring bio-based carbon materials and recycled metals to replace rare or toxic electrode components. Modular design approaches are being implemented to facilitate component replacement and recycling, extending system lifespans by up to 50% compared to first-generation designs.
Social sustainability dimensions of MES technologies include potential decentralization of chemical production, enabling local manufacturing with reduced transportation impacts. This distributed production model could strengthen community resilience and create new green chemistry job opportunities in regions transitioning away from fossil fuel economies.
Life Cycle Assessment (LCA) studies of MES technologies reveal promising environmental performance metrics. Recent analyses indicate that MES-based production of acetate from CO2 can achieve greenhouse gas emission reductions of 50-90% compared to petrochemical routes, depending on the electricity source. Water consumption is also reduced by approximately 30% compared to traditional fermentation processes, as MES systems typically require less water for cooling and separation processes.
The circular economy potential of MES is particularly noteworthy. These systems can transform waste streams such as industrial CO2 emissions or organic-rich wastewater into valuable chemicals and fuels, effectively closing material loops in industrial ecosystems. This waste valorization capability represents a significant advancement toward industrial symbiosis models where one industry's waste becomes another's raw material.
Resource efficiency metrics for MES demonstrate impressive performance. Carbon conversion efficiencies of up to 90% have been reported in optimized systems, significantly higher than many conventional biological processes. Additionally, the selective nature of electrochemical reactions in well-designed MES can minimize byproduct formation, reducing downstream separation requirements and associated environmental impacts.
Long-term sustainability considerations for MES include electrode material sourcing and end-of-life management. Current research is exploring bio-based carbon materials and recycled metals to replace rare or toxic electrode components. Modular design approaches are being implemented to facilitate component replacement and recycling, extending system lifespans by up to 50% compared to first-generation designs.
Social sustainability dimensions of MES technologies include potential decentralization of chemical production, enabling local manufacturing with reduced transportation impacts. This distributed production model could strengthen community resilience and create new green chemistry job opportunities in regions transitioning away from fossil fuel economies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!