What Are The Key Challenges In Scaling Microbial Electrosynthesis?
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microbial Electrosynthesis Background and Objectives
Microbial Electrosynthesis (MES) represents a groundbreaking biotechnological approach that emerged at the intersection of microbiology, electrochemistry, and bioengineering in the early 2000s. This technology harnesses the unique capabilities of electroactive microorganisms to convert electrical energy into valuable chemical compounds, effectively storing renewable electricity in the form of chemical bonds. The evolution of MES has been marked by significant milestones, beginning with the discovery of exoelectrogens capable of accepting electrons from electrodes and progressing to engineered systems that can produce specific target compounds.
The technological trajectory of MES has been characterized by increasing sophistication in electrode materials, reactor designs, and microbial consortia selection. Early systems demonstrated proof-of-concept with simple organic acids production, while recent advances have enabled the synthesis of more complex molecules including alcohols, hydrocarbons, and pharmaceutical precursors. This progression reflects the growing understanding of electron transfer mechanisms between electrodes and microorganisms, as well as improvements in biofilm formation and stability.
Current research trends in MES are focused on enhancing electron transfer rates, improving product selectivity, and increasing production efficiency. The field is witnessing convergence with synthetic biology approaches, where genetic engineering of microorganisms aims to create strains with optimized pathways for specific product formation. Simultaneously, materials science contributions are yielding novel electrode configurations with enhanced biocompatibility and conductivity properties.
The primary technical objectives for advancing MES include achieving higher current densities to improve production rates, developing scalable reactor designs that maintain efficient electron transfer at larger volumes, and identifying robust microbial catalysts capable of withstanding industrial conditions. Additionally, there is significant interest in expanding the product spectrum beyond simple organic compounds to higher-value chemicals and biofuels.
Long-term goals for MES technology encompass its integration into broader carbon capture and utilization strategies, establishing closed-loop systems where CO2 is continuously recycled into valuable products using renewable electricity. This vision positions MES as a potential cornerstone technology for sustainable bioeconomy, offering pathways to carbon-neutral or carbon-negative manufacturing processes.
The ultimate aim is to develop MES systems that can operate continuously at industrial scales with minimal maintenance requirements, competitive production costs, and environmental benefits that exceed conventional chemical synthesis methods. Achieving these objectives would position MES as a transformative technology in addressing climate change while meeting society's chemical and fuel needs through sustainable means.
The technological trajectory of MES has been characterized by increasing sophistication in electrode materials, reactor designs, and microbial consortia selection. Early systems demonstrated proof-of-concept with simple organic acids production, while recent advances have enabled the synthesis of more complex molecules including alcohols, hydrocarbons, and pharmaceutical precursors. This progression reflects the growing understanding of electron transfer mechanisms between electrodes and microorganisms, as well as improvements in biofilm formation and stability.
Current research trends in MES are focused on enhancing electron transfer rates, improving product selectivity, and increasing production efficiency. The field is witnessing convergence with synthetic biology approaches, where genetic engineering of microorganisms aims to create strains with optimized pathways for specific product formation. Simultaneously, materials science contributions are yielding novel electrode configurations with enhanced biocompatibility and conductivity properties.
The primary technical objectives for advancing MES include achieving higher current densities to improve production rates, developing scalable reactor designs that maintain efficient electron transfer at larger volumes, and identifying robust microbial catalysts capable of withstanding industrial conditions. Additionally, there is significant interest in expanding the product spectrum beyond simple organic compounds to higher-value chemicals and biofuels.
Long-term goals for MES technology encompass its integration into broader carbon capture and utilization strategies, establishing closed-loop systems where CO2 is continuously recycled into valuable products using renewable electricity. This vision positions MES as a potential cornerstone technology for sustainable bioeconomy, offering pathways to carbon-neutral or carbon-negative manufacturing processes.
The ultimate aim is to develop MES systems that can operate continuously at industrial scales with minimal maintenance requirements, competitive production costs, and environmental benefits that exceed conventional chemical synthesis methods. Achieving these objectives would position MES as a transformative technology in addressing climate change while meeting society's chemical and fuel needs through sustainable means.
Market Applications and Demand Analysis
Microbial Electrosynthesis (MES) has emerged as a promising technology at the intersection of biotechnology and renewable energy, with significant market potential across multiple sectors. The global market for bio-based chemicals and materials is projected to reach $200 billion by 2025, with MES positioned to capture a growing segment of this market due to its sustainable production capabilities.
The primary market driver for MES technology is the increasing demand for sustainable production methods in the chemical manufacturing industry. As regulatory pressures mount regarding carbon emissions and environmental impact, industries are actively seeking carbon-neutral or carbon-negative production technologies. MES offers a compelling value proposition by utilizing renewable electricity and waste CO2 to produce valuable chemicals and fuels.
In the energy sector, MES presents opportunities for energy storage solutions through the conversion of surplus renewable electricity into storable chemical compounds. This application addresses the intermittency challenges of renewable energy sources like solar and wind power. Market analysis indicates that the global grid-scale energy storage market is growing at a CAGR of 24%, with biochemical storage solutions expected to play an increasingly important role.
The pharmaceutical and fine chemicals industries represent high-value niche markets for MES technology. These sectors prioritize production methods that minimize environmental impact while maintaining high product purity. MES systems can potentially produce pharmaceutical precursors and specialty chemicals with reduced waste streams compared to traditional chemical synthesis methods.
Agricultural applications constitute another significant market segment, particularly for sustainable fertilizer production. As conventional fertilizer manufacturing accounts for approximately 1.4% of global CO2 emissions, MES offers an alternative pathway for nitrogen fixation and fertilizer production with a substantially lower carbon footprint.
Consumer demand trends strongly favor products with verifiable sustainability credentials. Market research indicates that 73% of global consumers are willing to pay premium prices for products with demonstrated environmental benefits. This consumer preference creates market pull for MES-derived products across food additives, cosmetics, and consumer goods sectors.
Regional market analysis reveals varying adoption potential, with Europe leading in regulatory support for bio-based production technologies, while North America shows strength in research investment and commercialization infrastructure. The Asia-Pacific region, particularly China and India, represents the fastest-growing market opportunity due to rapid industrialization coupled with increasing environmental regulations.
Despite these promising market indicators, adoption barriers include cost competitiveness with established petrochemical processes, scaling challenges, and market education requirements. The technology must achieve production costs within 15-30% of conventional methods to gain significant market traction outside of premium product categories.
The primary market driver for MES technology is the increasing demand for sustainable production methods in the chemical manufacturing industry. As regulatory pressures mount regarding carbon emissions and environmental impact, industries are actively seeking carbon-neutral or carbon-negative production technologies. MES offers a compelling value proposition by utilizing renewable electricity and waste CO2 to produce valuable chemicals and fuels.
In the energy sector, MES presents opportunities for energy storage solutions through the conversion of surplus renewable electricity into storable chemical compounds. This application addresses the intermittency challenges of renewable energy sources like solar and wind power. Market analysis indicates that the global grid-scale energy storage market is growing at a CAGR of 24%, with biochemical storage solutions expected to play an increasingly important role.
The pharmaceutical and fine chemicals industries represent high-value niche markets for MES technology. These sectors prioritize production methods that minimize environmental impact while maintaining high product purity. MES systems can potentially produce pharmaceutical precursors and specialty chemicals with reduced waste streams compared to traditional chemical synthesis methods.
Agricultural applications constitute another significant market segment, particularly for sustainable fertilizer production. As conventional fertilizer manufacturing accounts for approximately 1.4% of global CO2 emissions, MES offers an alternative pathway for nitrogen fixation and fertilizer production with a substantially lower carbon footprint.
Consumer demand trends strongly favor products with verifiable sustainability credentials. Market research indicates that 73% of global consumers are willing to pay premium prices for products with demonstrated environmental benefits. This consumer preference creates market pull for MES-derived products across food additives, cosmetics, and consumer goods sectors.
Regional market analysis reveals varying adoption potential, with Europe leading in regulatory support for bio-based production technologies, while North America shows strength in research investment and commercialization infrastructure. The Asia-Pacific region, particularly China and India, represents the fastest-growing market opportunity due to rapid industrialization coupled with increasing environmental regulations.
Despite these promising market indicators, adoption barriers include cost competitiveness with established petrochemical processes, scaling challenges, and market education requirements. The technology must achieve production costs within 15-30% of conventional methods to gain significant market traction outside of premium product categories.
Technical Barriers and Global Research Status
Microbial Electrosynthesis (MES) faces significant technical barriers that currently limit its industrial scalability. The primary challenge lies in the low electron transfer efficiency between electrodes and microorganisms, with current densities typically below 10 mA/cm², far from commercial viability. This inefficiency stems from the limited understanding of extracellular electron transfer mechanisms and the difficulty in engineering microbes with enhanced electron uptake capabilities.
Reactor design presents another major obstacle. Current MES reactors suffer from poor mass transfer, uneven current distribution, and membrane fouling issues. The lack of standardized reactor configurations hampers comparative analysis across research groups and slows technological advancement. Most laboratory demonstrations utilize small-scale reactors (typically <1L), with few successful examples of scaled-up systems exceeding 10L.
Biofilm formation and management on electrodes represent a critical challenge. While biofilms are essential for electron transfer, their uncontrolled growth can lead to increased internal resistance and reduced system performance over time. Researchers have yet to develop effective strategies for maintaining optimal biofilm thickness and activity during long-term operation.
The global research landscape shows concentrated efforts in North America, Europe, and increasingly in China. The United States leads in fundamental research through institutions like Harvard University and UC Berkeley, focusing on genetic engineering of electroactive microorganisms. European research, particularly in Germany and the Netherlands, emphasizes reactor design optimization and system integration with renewable energy sources.
Recent technological breakthroughs include the development of novel electrode materials with enhanced biocompatibility and conductivity. Graphene-based materials and carbon nanotubes have shown promise in laboratory settings, improving electron transfer rates by up to 40% compared to traditional carbon electrodes. However, cost-effective manufacturing of these advanced materials at scale remains problematic.
Product selectivity and yield consistency present ongoing challenges. Current MES systems often produce mixed products, reducing economic efficiency. While acetate production has been relatively well-established, directing electron flow toward higher-value products like butanol or specific fatty acids remains difficult to control at scale.
Energy efficiency represents perhaps the most significant barrier to commercialization. MES systems currently require 2-5 times more energy input than the theoretical minimum, making them economically uncompetitive with conventional chemical synthesis routes. This efficiency gap must be substantially narrowed before industrial adoption becomes feasible.
Reactor design presents another major obstacle. Current MES reactors suffer from poor mass transfer, uneven current distribution, and membrane fouling issues. The lack of standardized reactor configurations hampers comparative analysis across research groups and slows technological advancement. Most laboratory demonstrations utilize small-scale reactors (typically <1L), with few successful examples of scaled-up systems exceeding 10L.
Biofilm formation and management on electrodes represent a critical challenge. While biofilms are essential for electron transfer, their uncontrolled growth can lead to increased internal resistance and reduced system performance over time. Researchers have yet to develop effective strategies for maintaining optimal biofilm thickness and activity during long-term operation.
The global research landscape shows concentrated efforts in North America, Europe, and increasingly in China. The United States leads in fundamental research through institutions like Harvard University and UC Berkeley, focusing on genetic engineering of electroactive microorganisms. European research, particularly in Germany and the Netherlands, emphasizes reactor design optimization and system integration with renewable energy sources.
Recent technological breakthroughs include the development of novel electrode materials with enhanced biocompatibility and conductivity. Graphene-based materials and carbon nanotubes have shown promise in laboratory settings, improving electron transfer rates by up to 40% compared to traditional carbon electrodes. However, cost-effective manufacturing of these advanced materials at scale remains problematic.
Product selectivity and yield consistency present ongoing challenges. Current MES systems often produce mixed products, reducing economic efficiency. While acetate production has been relatively well-established, directing electron flow toward higher-value products like butanol or specific fatty acids remains difficult to control at scale.
Energy efficiency represents perhaps the most significant barrier to commercialization. MES systems currently require 2-5 times more energy input than the theoretical minimum, making them economically uncompetitive with conventional chemical synthesis routes. This efficiency gap must be substantially narrowed before industrial adoption becomes feasible.
Current Scaling Approaches and Solutions
01 Reactor design and configuration for scaling up microbial electrosynthesis
Various reactor designs and configurations have been developed to scale up microbial electrosynthesis processes. These include specialized electrode arrangements, membrane systems, and flow cell designs that optimize electron transfer between electrodes and microorganisms. Advanced reactor configurations incorporate features like improved mass transfer, reduced internal resistance, and enhanced microbial colonization surfaces to increase production efficiency at larger scales.- Reactor design and configuration for scaled microbial electrosynthesis: Various reactor designs and configurations have been developed to scale up microbial electrosynthesis processes. These include specialized electrode arrangements, membrane systems, and flow-through reactors that optimize the interaction between microorganisms and electrodes. Advanced reactor designs incorporate features that enhance mass transfer, reduce internal resistance, and improve overall system efficiency for industrial-scale applications.
- Electrode materials and modifications for enhanced performance: The selection and modification of electrode materials significantly impact the efficiency of scaled microbial electrosynthesis systems. Advanced materials such as carbon-based electrodes, metal oxides, and conductive polymers provide improved biocompatibility, conductivity, and surface area. Surface modifications including nanostructuring, functionalization, and catalyst integration enhance electron transfer rates between microorganisms and electrodes, leading to higher product yields in large-scale operations.
- Microbial strain engineering and community optimization: Engineering microbial strains and optimizing microbial communities are crucial for scaling up electrosynthesis processes. This involves selecting or developing microorganisms with enhanced extracellular electron transfer capabilities, improved substrate utilization, and increased product formation rates. Mixed microbial communities can offer advantages in terms of stability and resilience in industrial-scale operations, while genetic engineering approaches can create strains specifically adapted for large-scale bioelectrochemical systems.
- Process integration and control systems for industrial scaling: Successful scaling of microbial electrosynthesis requires sophisticated process integration and control systems. This includes automated monitoring of key parameters such as pH, temperature, potential, and product formation, along with feedback control mechanisms to maintain optimal conditions. Integration with upstream and downstream processes, such as feedstock preparation and product recovery, is essential for continuous operation at industrial scale. Advanced control algorithms and machine learning approaches can optimize system performance and adapt to changing conditions.
- Economic and sustainability considerations for commercial implementation: Scaling microbial electrosynthesis to commercial levels requires addressing economic and sustainability challenges. This includes reducing capital and operational costs through improved materials, energy efficiency, and process intensification. Integration with renewable energy sources can enhance sustainability and economic viability. Life cycle assessment approaches help optimize resource utilization and minimize environmental impacts. Considerations for waste management, product recovery efficiency, and market competitiveness are essential for successful commercial implementation of microbial electrosynthesis technologies.
02 Electrode materials and modifications for enhanced performance
The selection and modification of electrode materials play a crucial role in scaling up microbial electrosynthesis systems. Novel electrode materials with high conductivity, biocompatibility, and large surface areas improve electron transfer efficiency and microbial attachment. Surface modifications including nanostructuring, functionalization with catalysts, and incorporation of conductive polymers can significantly enhance the performance of scaled-up systems by increasing reaction rates and product yields.Expand Specific Solutions03 Microbial community engineering and optimization
Engineering and optimizing microbial communities is essential for successful scaling of electrosynthesis processes. This involves selecting and adapting electroactive microorganisms with high catalytic activity, developing co-cultures with synergistic metabolic capabilities, and enhancing biofilm formation on electrodes. Genetic engineering approaches are used to improve electron uptake mechanisms, metabolic pathways, and product specificity to maintain performance at industrial scales.Expand Specific Solutions04 Process integration and continuous operation systems
Integrating microbial electrosynthesis with other bioprocesses and developing continuous operation systems are key strategies for industrial scaling. These approaches include coupling electrosynthesis with product separation technologies, integrating with renewable energy sources, and developing cascade systems that utilize intermediates from one process as substrates for another. Continuous operation systems with automated monitoring and control mechanisms help maintain stable performance over extended periods, which is crucial for commercial viability.Expand Specific Solutions05 Techno-economic analysis and sustainability considerations
Comprehensive techno-economic analyses and sustainability assessments are essential for guiding the scaling of microbial electrosynthesis technologies. These evaluations consider factors such as energy efficiency, carbon footprint, production costs, and market competitiveness compared to conventional chemical synthesis methods. Life cycle assessments help identify environmental impacts and resource requirements, while economic models determine capital and operational expenditures to optimize system design and operation for commercial implementation.Expand Specific Solutions
Leading Research Groups and Industrial Players
Microbial Electrosynthesis (MES) is currently in an early development stage, with the market showing promising growth potential but still relatively small. The technology faces significant scaling challenges as it transitions from laboratory to industrial applications. Key players include academic institutions like Tianjin University, Ghent University, and MIT conducting fundamental research, while industrial entities such as SABIC Global Technologies and Dow Global Technologies are exploring commercial applications. Research organizations like Fraunhofer-Gesellschaft and Centre National de la Recherche Scientifique are bridging the gap between academia and industry. Technical maturity varies across applications, with bioelectrochemical systems for waste treatment more advanced than those for chemical production. Major challenges include electrode scaling, microbial community stability, and system integration for continuous operation.
Fraunhofer-Gesellschaft eV
Technical Solution: Fraunhofer-Gesellschaft has developed an integrated scaling approach for microbial electrosynthesis called "ElectroSynth Platform." Their technology addresses multiple scaling challenges simultaneously through a systems engineering approach. The platform features hierarchically structured carbon-based electrodes with nano, micro, and macro-scale features that optimize both electron transfer and mass transport. Their reactor design incorporates membrane-electrode assemblies inspired by fuel cell technology, reducing internal resistance while maintaining separation between reaction environments. A distinguishing feature is their advanced process control system that utilizes machine learning algorithms to predict and prevent performance limitations during scale-up. The platform includes proprietary electrode manufacturing techniques that reduce costs by approximately 60% compared to traditional noble metal catalysts while maintaining comparable performance. Fraunhofer has successfully demonstrated this technology at pilot scale (500L), achieving production rates of target chemicals (primarily acetate and ethanol) that are within 75% of theoretical maximum efficiency.
Strengths: Comprehensive systems engineering approach; cost-effective electrode manufacturing; advanced process control using machine learning; demonstrated at meaningful pilot scale. Weaknesses: Complex integration requires specialized expertise; performance still decreases somewhat at larger scales; requires significant initial capital investment for implementation.
Ghent University
Technical Solution: Ghent University has developed a comprehensive approach to scaling microbial electrosynthesis (MES) focusing on electrode design optimization and biofilm engineering. Their technology employs 3D carbon-based electrodes with increased surface area-to-volume ratios, achieving up to 300% higher microbial colonization compared to traditional flat electrodes. The university's research teams have pioneered selective microbial consortia cultivation techniques that enhance electron transfer rates between electrodes and microorganisms. Their scale-up strategy incorporates modular reactor designs with specialized membrane systems that minimize pH gradients while maintaining separation between anodic and cathodic chambers. Additionally, they've developed advanced monitoring systems using real-time impedance spectroscopy to track biofilm formation and activity, allowing for process optimization during continuous operation.
Strengths: Strong integration of biofilm engineering with electrode design; advanced monitoring capabilities for process optimization; modular approach facilitates incremental scaling. Weaknesses: Higher initial capital costs compared to conventional bioreactors; requires specialized expertise for system maintenance; energy efficiency still needs improvement at larger scales.
Critical Patents and Scientific Breakthroughs
Fast electrical lysis of cells and rapid collection of the contents thereof using capillary electrophoresis
PatentInactiveEP1517752A2
Innovation
- The development of micro-fabricated electrodes that create a maximum voltage drop across a cell's plasma membrane using a single subsecond electrical pulse, allowing for efficient loading of cellular contents into a capillary electrophoresis tube, with the influence of inter-electrode distance, pulse duration, and pulse strength optimized for rapid lysis and collection.
Fabrication-free microfluidic device for scalable, high-volume bacterial electroporation
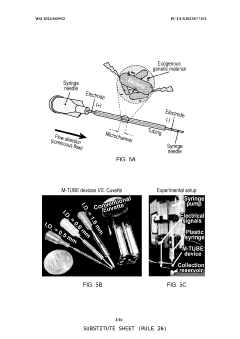
PatentWO2023049932A1
Innovation
- A microfluidic tubing-based electroporation device (M-TUBE) that allows for scalable, high-volume bacterial electroporation without the need for complex fabrication, using hollow tubular conductive elements and insulating structures to create a flow-through electroporation path, enabling flexible and efficient processing of large volumes.
Economic Viability and Cost Analysis
The economic viability of microbial electrosynthesis (MES) remains one of the most significant barriers to its widespread commercial adoption. Current MES systems demonstrate promising conversion efficiencies in laboratory settings, but the capital expenditure required for industrial-scale implementation remains prohibitively high. Electrode materials, particularly those utilizing precious metals or advanced carbon-based structures, contribute substantially to these costs. Initial economic analyses suggest that electrode materials can account for 30-40% of total system costs, with additional significant expenses arising from membrane technologies and control systems.
Operating costs present another major economic challenge. The energy input required to drive electrochemical reactions in MES systems translates to substantial electricity consumption, which directly impacts the cost-effectiveness of the process. Current estimates indicate that electricity costs may represent 25-35% of operational expenses, depending on regional energy prices and grid carbon intensity. Without access to low-cost renewable electricity sources, the economic proposition of MES becomes significantly less attractive compared to conventional chemical synthesis routes.
Product recovery and purification processes add another layer of economic complexity. The dilute nature of MES products in aqueous solutions necessitates energy-intensive separation techniques, which can account for up to 60% of downstream processing costs. This creates a challenging economic equation where the value of the synthesized products must significantly exceed the combined costs of feedstock, electricity, separation, and capital depreciation.
Scale-dependent economics further complicate the pathway to commercialization. Unlike many bioprocesses that benefit substantially from economies of scale, MES systems face unique scaling challenges that can actually increase per-unit production costs at larger scales due to decreased efficiency. Economic modeling suggests that optimal MES reactor sizes may be smaller than traditional chemical processes, potentially limiting the economic benefits typically associated with industrial scaling.
Market competitiveness remains a critical economic consideration. MES-derived products must compete with established petrochemical routes that benefit from decades of optimization and infrastructure development. Current cost analyses indicate that without significant technological breakthroughs or supportive policy frameworks such as carbon pricing, MES products remain 2-5 times more expensive than their conventional counterparts. This price premium represents a substantial barrier to market entry, particularly for commodity chemicals where price sensitivity is high.
Operating costs present another major economic challenge. The energy input required to drive electrochemical reactions in MES systems translates to substantial electricity consumption, which directly impacts the cost-effectiveness of the process. Current estimates indicate that electricity costs may represent 25-35% of operational expenses, depending on regional energy prices and grid carbon intensity. Without access to low-cost renewable electricity sources, the economic proposition of MES becomes significantly less attractive compared to conventional chemical synthesis routes.
Product recovery and purification processes add another layer of economic complexity. The dilute nature of MES products in aqueous solutions necessitates energy-intensive separation techniques, which can account for up to 60% of downstream processing costs. This creates a challenging economic equation where the value of the synthesized products must significantly exceed the combined costs of feedstock, electricity, separation, and capital depreciation.
Scale-dependent economics further complicate the pathway to commercialization. Unlike many bioprocesses that benefit substantially from economies of scale, MES systems face unique scaling challenges that can actually increase per-unit production costs at larger scales due to decreased efficiency. Economic modeling suggests that optimal MES reactor sizes may be smaller than traditional chemical processes, potentially limiting the economic benefits typically associated with industrial scaling.
Market competitiveness remains a critical economic consideration. MES-derived products must compete with established petrochemical routes that benefit from decades of optimization and infrastructure development. Current cost analyses indicate that without significant technological breakthroughs or supportive policy frameworks such as carbon pricing, MES products remain 2-5 times more expensive than their conventional counterparts. This price premium represents a substantial barrier to market entry, particularly for commodity chemicals where price sensitivity is high.
Sustainability Impact and Environmental Regulations
Microbial Electrosynthesis (MES) represents a significant advancement in sustainable technology, offering potential solutions to multiple environmental challenges. The sustainability impact of MES is substantial, as it enables the conversion of electrical energy from renewable sources into valuable chemical compounds using microorganisms as biocatalysts. This process effectively creates a carbon-neutral or even carbon-negative production pathway for chemicals and fuels that traditionally rely on fossil resources.
The environmental benefits of scaling MES include reduced greenhouse gas emissions through carbon capture and utilization, decreased dependence on petrochemical feedstocks, and potential integration with renewable energy systems to utilize excess electricity during peak production periods. Furthermore, MES systems can operate under ambient conditions, requiring less energy input compared to traditional chemical synthesis methods that often demand high temperatures and pressures.
However, the regulatory landscape surrounding MES technology remains complex and evolving. Current environmental regulations were largely developed before the emergence of bioelectrochemical systems, creating potential regulatory gaps or barriers to commercialization. In many jurisdictions, MES falls between established regulatory frameworks for biotechnology, renewable energy, and chemical manufacturing, complicating compliance efforts.
Key regulatory considerations include biosafety protocols for genetically modified microorganisms used in MES, waste management requirements for spent media and biofilms, and standards for integration with existing energy infrastructure. Additionally, life cycle assessment methodologies must be standardized to accurately quantify the environmental benefits of MES compared to conventional production methods.
The international regulatory landscape presents further challenges, with significant variations in approval processes and environmental standards across different regions. This regulatory heterogeneity may impede global scaling efforts and technology transfer, particularly for startups and research institutions with limited resources for regulatory navigation.
Forward-looking policy development is essential to facilitate MES scaling while ensuring environmental protection. This includes establishing clear guidelines for risk assessment, developing specific permits for bioelectrochemical systems, and creating incentive structures that recognize the environmental benefits of MES technology. Carbon pricing mechanisms and renewable energy credits could potentially accelerate commercial adoption by improving economic competitiveness against established production methods.
Collaborative efforts between industry stakeholders, regulatory agencies, and research institutions will be crucial to develop appropriate regulatory frameworks that both protect environmental interests and enable technological innovation in this promising field.
The environmental benefits of scaling MES include reduced greenhouse gas emissions through carbon capture and utilization, decreased dependence on petrochemical feedstocks, and potential integration with renewable energy systems to utilize excess electricity during peak production periods. Furthermore, MES systems can operate under ambient conditions, requiring less energy input compared to traditional chemical synthesis methods that often demand high temperatures and pressures.
However, the regulatory landscape surrounding MES technology remains complex and evolving. Current environmental regulations were largely developed before the emergence of bioelectrochemical systems, creating potential regulatory gaps or barriers to commercialization. In many jurisdictions, MES falls between established regulatory frameworks for biotechnology, renewable energy, and chemical manufacturing, complicating compliance efforts.
Key regulatory considerations include biosafety protocols for genetically modified microorganisms used in MES, waste management requirements for spent media and biofilms, and standards for integration with existing energy infrastructure. Additionally, life cycle assessment methodologies must be standardized to accurately quantify the environmental benefits of MES compared to conventional production methods.
The international regulatory landscape presents further challenges, with significant variations in approval processes and environmental standards across different regions. This regulatory heterogeneity may impede global scaling efforts and technology transfer, particularly for startups and research institutions with limited resources for regulatory navigation.
Forward-looking policy development is essential to facilitate MES scaling while ensuring environmental protection. This includes establishing clear guidelines for risk assessment, developing specific permits for bioelectrochemical systems, and creating incentive structures that recognize the environmental benefits of MES technology. Carbon pricing mechanisms and renewable energy credits could potentially accelerate commercial adoption by improving economic competitiveness against established production methods.
Collaborative efforts between industry stakeholders, regulatory agencies, and research institutions will be crucial to develop appropriate regulatory frameworks that both protect environmental interests and enable technological innovation in this promising field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!