Microbial Electrosynthesis For Ammonia Production
SEP 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microbial Electrosynthesis Ammonia Production Background and Objectives
Microbial Electrosynthesis (MES) represents a groundbreaking biotechnological approach that harnesses the metabolic capabilities of microorganisms to convert electrical energy into valuable chemical compounds. The concept emerged in the early 2000s as researchers began exploring the interface between microbiology and electrochemistry, recognizing the potential of certain microorganisms to accept electrons from electrodes and utilize this energy for biosynthetic processes.
The evolution of MES technology has been marked by significant milestones, including the discovery of electrotrophs—microorganisms capable of directly accepting electrons from solid-state electrodes—and the development of biocompatible electrode materials that enhance electron transfer efficiency. Recent years have witnessed an expansion of MES applications beyond simple organic compounds to more complex molecules, with ammonia production representing a particularly promising frontier.
Ammonia synthesis via MES addresses critical limitations of the conventional Haber-Bosch process, which currently dominates industrial ammonia production but consumes approximately 1-2% of global energy and generates substantial greenhouse gas emissions. The traditional process requires high temperature (400-500°C) and pressure (100-200 atm) conditions, whereas MES offers the potential for ambient-temperature, atmospheric-pressure ammonia synthesis using renewable electricity.
The technical objectives for MES-based ammonia production encompass several dimensions. Primary goals include achieving commercially viable production rates exceeding 50 mg NH₃/L/h, developing electrode materials with enhanced durability and electron transfer capabilities, and engineering microbial strains with improved ammonia synthesis pathways. Additional objectives focus on optimizing reactor designs for continuous operation and scaling up laboratory prototypes to industrial capacities.
Research trends indicate growing interest in hybrid systems that combine biological and electrochemical components, as well as the integration of renewable energy sources to power MES processes. The field is moving toward more sophisticated control systems that can dynamically adjust operational parameters based on microbial activity and production rates.
The convergence of synthetic biology, materials science, and electrochemical engineering is expected to accelerate progress in this domain. Genetic modification of microorganisms to enhance nitrogen fixation capabilities and electron uptake mechanisms represents a particularly promising avenue for technological advancement. Simultaneously, innovations in electrode materials, including carbon-based nanomaterials and metal-organic frameworks, are expanding the possibilities for efficient electron transfer.
The ultimate vision for MES-based ammonia production is to develop a sustainable, decentralized manufacturing platform that can operate using intermittent renewable energy sources, thereby revolutionizing fertilizer production and energy storage paradigms globally.
The evolution of MES technology has been marked by significant milestones, including the discovery of electrotrophs—microorganisms capable of directly accepting electrons from solid-state electrodes—and the development of biocompatible electrode materials that enhance electron transfer efficiency. Recent years have witnessed an expansion of MES applications beyond simple organic compounds to more complex molecules, with ammonia production representing a particularly promising frontier.
Ammonia synthesis via MES addresses critical limitations of the conventional Haber-Bosch process, which currently dominates industrial ammonia production but consumes approximately 1-2% of global energy and generates substantial greenhouse gas emissions. The traditional process requires high temperature (400-500°C) and pressure (100-200 atm) conditions, whereas MES offers the potential for ambient-temperature, atmospheric-pressure ammonia synthesis using renewable electricity.
The technical objectives for MES-based ammonia production encompass several dimensions. Primary goals include achieving commercially viable production rates exceeding 50 mg NH₃/L/h, developing electrode materials with enhanced durability and electron transfer capabilities, and engineering microbial strains with improved ammonia synthesis pathways. Additional objectives focus on optimizing reactor designs for continuous operation and scaling up laboratory prototypes to industrial capacities.
Research trends indicate growing interest in hybrid systems that combine biological and electrochemical components, as well as the integration of renewable energy sources to power MES processes. The field is moving toward more sophisticated control systems that can dynamically adjust operational parameters based on microbial activity and production rates.
The convergence of synthetic biology, materials science, and electrochemical engineering is expected to accelerate progress in this domain. Genetic modification of microorganisms to enhance nitrogen fixation capabilities and electron uptake mechanisms represents a particularly promising avenue for technological advancement. Simultaneously, innovations in electrode materials, including carbon-based nanomaterials and metal-organic frameworks, are expanding the possibilities for efficient electron transfer.
The ultimate vision for MES-based ammonia production is to develop a sustainable, decentralized manufacturing platform that can operate using intermittent renewable energy sources, thereby revolutionizing fertilizer production and energy storage paradigms globally.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing a significant shift towards sustainable production methods, with microbial electrosynthesis (MES) emerging as a promising technology. Currently, the conventional Haber-Bosch process dominates ammonia production, accounting for approximately 180 million tons annually and consuming 1-2% of global energy while generating substantial CO2 emissions. This creates a compelling market opportunity for greener alternatives like MES.
Market demand for sustainable ammonia is being driven by multiple factors. The fertilizer industry, which consumes 80% of produced ammonia, faces increasing pressure to reduce its carbon footprint. Additionally, ammonia's potential as a carbon-free energy carrier and fuel is gaining recognition, with projections suggesting this application could expand the market by 30-40% by 2050.
Policy frameworks worldwide are accelerating this transition. The European Green Deal, carbon pricing mechanisms, and renewable energy mandates are creating favorable conditions for sustainable ammonia technologies. Countries with ambitious net-zero targets, including Japan, Australia, and several European nations, are actively investing in green ammonia infrastructure and production facilities.
Investment trends reflect growing market confidence in sustainable ammonia technologies. Venture capital funding in this sector has increased threefold since 2018, with particular interest in biological production methods. Major industrial players like Yara, BASF, and CF Industries have announced significant investments in green ammonia projects, signaling industry-wide recognition of the market potential.
Regional market dynamics vary considerably. Europe leads in policy support and early adoption, while Asia-Pacific represents the largest growth market due to agricultural demands and industrial development. North America offers strong research infrastructure and investment capital, particularly for biotechnology solutions like MES.
Economic analysis indicates that while MES for ammonia production currently faces cost challenges compared to conventional methods, the gap is narrowing. Production costs for MES-based ammonia are projected to decrease by 40-60% over the next decade as the technology matures and scales. Additionally, as carbon pricing becomes more widespread, the economic case for biological ammonia synthesis will strengthen significantly.
Customer segments for MES-derived ammonia include agricultural suppliers seeking sustainable fertilizers, energy companies exploring ammonia as a hydrogen carrier, and industrial users requiring carbon-neutral chemical feedstocks. Each segment presents distinct requirements regarding price sensitivity, purity specifications, and supply chain considerations.
Market demand for sustainable ammonia is being driven by multiple factors. The fertilizer industry, which consumes 80% of produced ammonia, faces increasing pressure to reduce its carbon footprint. Additionally, ammonia's potential as a carbon-free energy carrier and fuel is gaining recognition, with projections suggesting this application could expand the market by 30-40% by 2050.
Policy frameworks worldwide are accelerating this transition. The European Green Deal, carbon pricing mechanisms, and renewable energy mandates are creating favorable conditions for sustainable ammonia technologies. Countries with ambitious net-zero targets, including Japan, Australia, and several European nations, are actively investing in green ammonia infrastructure and production facilities.
Investment trends reflect growing market confidence in sustainable ammonia technologies. Venture capital funding in this sector has increased threefold since 2018, with particular interest in biological production methods. Major industrial players like Yara, BASF, and CF Industries have announced significant investments in green ammonia projects, signaling industry-wide recognition of the market potential.
Regional market dynamics vary considerably. Europe leads in policy support and early adoption, while Asia-Pacific represents the largest growth market due to agricultural demands and industrial development. North America offers strong research infrastructure and investment capital, particularly for biotechnology solutions like MES.
Economic analysis indicates that while MES for ammonia production currently faces cost challenges compared to conventional methods, the gap is narrowing. Production costs for MES-based ammonia are projected to decrease by 40-60% over the next decade as the technology matures and scales. Additionally, as carbon pricing becomes more widespread, the economic case for biological ammonia synthesis will strengthen significantly.
Customer segments for MES-derived ammonia include agricultural suppliers seeking sustainable fertilizers, energy companies exploring ammonia as a hydrogen carrier, and industrial users requiring carbon-neutral chemical feedstocks. Each segment presents distinct requirements regarding price sensitivity, purity specifications, and supply chain considerations.
Current Status and Challenges in Microbial Electrosynthesis
Microbial Electrosynthesis (MES) for ammonia production represents a cutting-edge biotechnological approach that combines electrochemistry with microbial metabolism to convert nitrogen gas into ammonia under ambient conditions. Currently, this technology exists primarily at laboratory scale, with several research groups demonstrating proof-of-concept systems achieving ammonia production rates ranging from 0.05 to 1.2 g NH₃-N/L/day. These rates, while promising, remain significantly lower than conventional Haber-Bosch process yields, which typically produce ammonia at industrial scales exceeding 3,000 tons per day.
The global research landscape shows concentrated efforts in North America, Europe, and East Asia, with China emerging as a particularly active player in recent publications. Academic institutions currently dominate the field, though increasing interest from industrial partners has been observed in the past three years, particularly from renewable energy and agricultural technology sectors seeking sustainable nitrogen fixation methods.
Key technical challenges limiting widespread implementation include low faradaic efficiency, typically below 30% in most reported systems. This inefficiency stems from competing reactions at the cathode surface and the inherent energy losses in biological systems. Additionally, the selection and engineering of appropriate electroactive microorganisms capable of efficiently catalyzing nitrogen reduction remains problematic, with most current systems relying on mixed microbial consortia with unpredictable performance characteristics.
Electrode materials present another significant hurdle, as researchers struggle to develop surfaces that simultaneously support robust biofilm formation while maintaining electrical conductivity and resistance to biofouling. Carbon-based materials modified with nitrogen-doped structures have shown promise but suffer from degradation during extended operation periods.
Scale-up challenges are particularly pronounced, with laboratory reactors typically operating at volumes below 1 liter. The transition to pilot-scale systems (10-100L) has revealed issues with mass transfer limitations, uneven current distribution, and difficulties maintaining stable microbial communities over extended operational periods exceeding 30 days.
Economic viability remains questionable under current technological parameters. Energy consumption in MES systems for ammonia production typically ranges from 60-120 kWh/kg NH₃, significantly higher than the theoretical minimum of 12.7 kWh/kg NH₃. This energy requirement, coupled with low production rates, currently positions MES-based ammonia production as economically uncompetitive with conventional methods, which operate at approximately 10-12 kWh/kg NH₃ in modern plants.
Regulatory frameworks for bioelectrochemical systems remain underdeveloped in most jurisdictions, creating uncertainty for potential commercial applications. Additionally, the interdisciplinary nature of the technology requires expertise spanning microbiology, electrochemistry, and chemical engineering, creating workforce development challenges for organizations seeking to advance this technology.
The global research landscape shows concentrated efforts in North America, Europe, and East Asia, with China emerging as a particularly active player in recent publications. Academic institutions currently dominate the field, though increasing interest from industrial partners has been observed in the past three years, particularly from renewable energy and agricultural technology sectors seeking sustainable nitrogen fixation methods.
Key technical challenges limiting widespread implementation include low faradaic efficiency, typically below 30% in most reported systems. This inefficiency stems from competing reactions at the cathode surface and the inherent energy losses in biological systems. Additionally, the selection and engineering of appropriate electroactive microorganisms capable of efficiently catalyzing nitrogen reduction remains problematic, with most current systems relying on mixed microbial consortia with unpredictable performance characteristics.
Electrode materials present another significant hurdle, as researchers struggle to develop surfaces that simultaneously support robust biofilm formation while maintaining electrical conductivity and resistance to biofouling. Carbon-based materials modified with nitrogen-doped structures have shown promise but suffer from degradation during extended operation periods.
Scale-up challenges are particularly pronounced, with laboratory reactors typically operating at volumes below 1 liter. The transition to pilot-scale systems (10-100L) has revealed issues with mass transfer limitations, uneven current distribution, and difficulties maintaining stable microbial communities over extended operational periods exceeding 30 days.
Economic viability remains questionable under current technological parameters. Energy consumption in MES systems for ammonia production typically ranges from 60-120 kWh/kg NH₃, significantly higher than the theoretical minimum of 12.7 kWh/kg NH₃. This energy requirement, coupled with low production rates, currently positions MES-based ammonia production as economically uncompetitive with conventional methods, which operate at approximately 10-12 kWh/kg NH₃ in modern plants.
Regulatory frameworks for bioelectrochemical systems remain underdeveloped in most jurisdictions, creating uncertainty for potential commercial applications. Additionally, the interdisciplinary nature of the technology requires expertise spanning microbiology, electrochemistry, and chemical engineering, creating workforce development challenges for organizations seeking to advance this technology.
Current Microbial Electrosynthesis Approaches for Ammonia
01 Microbial electrosynthesis systems for ammonia production
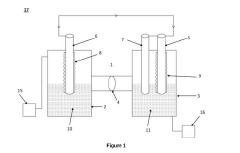
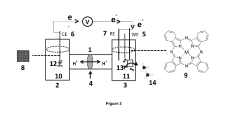
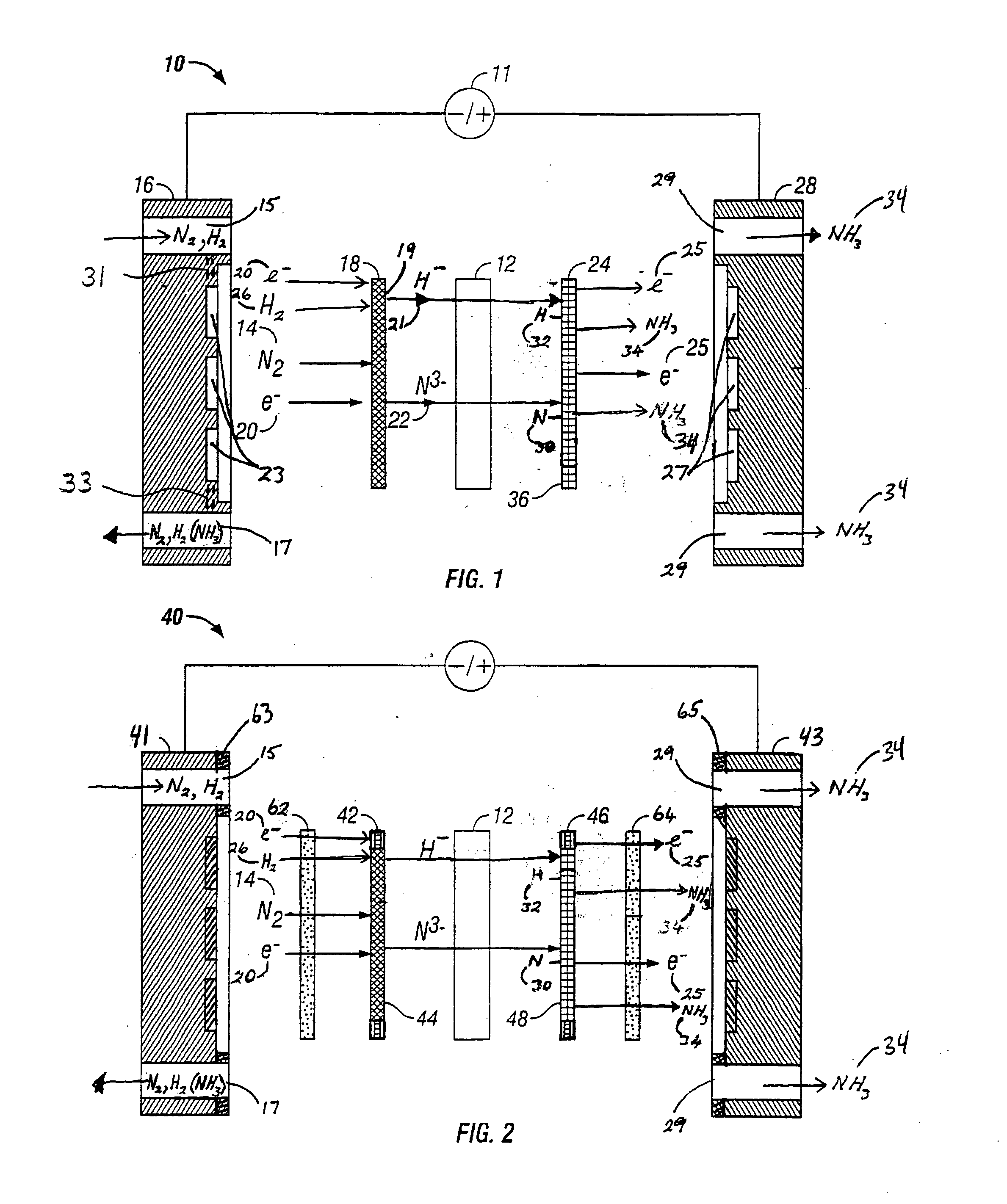
Microbial electrosynthesis systems utilize electroactive microorganisms to convert electrical energy into chemical energy for ammonia synthesis. These systems typically consist of bioelectrochemical cells where microorganisms act as biocatalysts at the cathode, reducing nitrogen to ammonia using electrons supplied by an external power source. The integration of specialized electrode materials and optimized reactor designs enhances electron transfer efficiency and ammonia production rates.- Bioelectrochemical systems for ammonia synthesis: Bioelectrochemical systems utilize microorganisms as biocatalysts to convert electrical energy into chemical energy for ammonia production. These systems typically employ electroactive bacteria that can accept electrons from cathodes and use them to reduce nitrogen to ammonia under ambient conditions. This approach offers advantages over traditional Haber-Bosch process by operating at lower temperatures and pressures, resulting in reduced energy consumption and environmental impact.
- Electrode materials and modifications for microbial electrosynthesis: Advanced electrode materials and modifications play a crucial role in enhancing the efficiency of microbial electrosynthesis for ammonia production. Electrodes can be modified with catalysts, conductive polymers, or nanostructured materials to improve electron transfer between the electrode and microorganisms. These modifications can increase the surface area for microbial colonization, enhance biofilm formation, and improve the overall performance of the bioelectrochemical system for ammonia synthesis.
- Nitrogen-fixing microorganisms for electrosynthesis: Specific nitrogen-fixing microorganisms can be employed in electrosynthesis systems to convert atmospheric nitrogen into ammonia. These microorganisms possess nitrogenase enzymes that catalyze the reduction of nitrogen to ammonia when provided with electrons from an electrode. Selection and engineering of appropriate microbial strains can significantly improve the efficiency and selectivity of the ammonia production process. Some systems utilize pure cultures while others employ mixed microbial consortia to achieve optimal performance.
- Reactor design and operating conditions: The design of bioelectrochemical reactors and their operating conditions significantly impact the efficiency of microbial electrosynthesis for ammonia production. Key parameters include electrode spacing, membrane selection, electrolyte composition, pH control, temperature regulation, and nitrogen supply methods. Innovative reactor configurations such as three-chamber systems, fluidized bed reactors, or membrane-based designs can enhance mass transfer, reduce energy consumption, and improve ammonia yield and production rates.
- Integration with renewable energy sources: Microbial electrosynthesis systems for ammonia production can be integrated with renewable energy sources such as solar, wind, or hydroelectric power. This integration allows for sustainable ammonia production using green electricity, further reducing the carbon footprint compared to conventional methods. Such systems can serve as energy storage solutions by converting excess renewable electricity into ammonia, which can be used as a carbon-neutral fuel or chemical feedstock. This approach addresses both energy storage challenges and sustainable chemical production needs.
02 Nitrogen-fixing microorganisms for electrosynthesis
Specific nitrogen-fixing microorganisms are employed in electrosynthesis systems to facilitate ammonia production. These include genetically engineered bacteria with enhanced nitrogen fixation capabilities or naturally occurring diazotrophs that can efficiently convert atmospheric nitrogen to ammonia under electrochemical conditions. The selection and cultivation of appropriate microbial communities significantly impacts the efficiency and sustainability of the ammonia production process.Expand Specific Solutions03 Electrode materials and catalysts for microbial ammonia synthesis
Advanced electrode materials and catalysts play a crucial role in microbial electrosynthesis of ammonia. Novel materials such as carbon-based electrodes modified with metal nanoparticles, conductive polymers, or biocompatible coatings enhance electron transfer between electrodes and microorganisms. These specialized materials create favorable interfaces for microbial attachment and metabolic activity, thereby improving the overall efficiency of the bioelectrochemical ammonia production process.Expand Specific Solutions04 Reactor design and process optimization for electrosynthetic ammonia production
Innovative reactor designs and process optimization strategies are essential for efficient microbial electrosynthesis of ammonia. These include membrane-based systems that separate reaction chambers, continuous flow reactors that enhance mass transfer, and modular designs that allow for scalability. Process parameters such as pH, temperature, nitrogen source concentration, and applied potential are carefully controlled to maximize ammonia yield while minimizing energy consumption and side reactions.Expand Specific Solutions05 Integration with renewable energy sources for sustainable ammonia production
Microbial electrosynthesis systems for ammonia production can be integrated with renewable energy sources to create sustainable production pathways. These integrated systems utilize intermittent renewable electricity from solar or wind power to drive the bioelectrochemical processes. Advanced control systems manage fluctuating power inputs, while energy storage components ensure continuous operation. This integration reduces the carbon footprint of ammonia production and provides a viable alternative to conventional Haber-Bosch process.Expand Specific Solutions
Leading Organizations in Microbial Electrosynthesis Research
Microbial Electrosynthesis for Ammonia Production is emerging as a promising green technology in the early commercialization phase. The market is projected to grow significantly as industries seek sustainable alternatives to the energy-intensive Haber-Bosch process. The competitive landscape features diverse players including academic institutions (MIT, University of Tokyo, Zhejiang University), chemical companies (BASF, Nissan Chemical, Ajinomoto), and energy corporations (Siemens, CHN Energy). Research institutions like Korea Institute of Energy Research and National Institute of Clean & Low Carbon Energy are advancing fundamental technologies, while companies like GenCell and Enlighten Innovations are developing commercial applications. The technology remains in development with varying maturity levels across different implementation approaches, indicating substantial room for innovation and market differentiation.
Technical University of Denmark
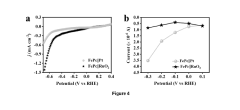
Technical Solution: Technical University of Denmark (DTU) has developed an advanced microbial electrosynthesis system for ammonia production that combines specialized electroactive microorganisms with innovative reactor designs. Their approach utilizes a consortium of nitrogen-fixing and electroactive bacteria that work synergistically to convert atmospheric nitrogen to ammonia using renewable electricity. The system features a distinctive three-chamber bioelectrochemical reactor with specialized ion-selective membranes that separate the anode, cathode, and collection chambers while facilitating targeted ion transport. DTU researchers have engineered electrode materials with nanoscale architectures that enhance microbial attachment and electron transfer efficiency, incorporating conductive polymers and carbon nanotubes that increase surface area and conductivity. Their system achieves ammonia production rates of approximately 6.8 mg NH3/L/h with Faradaic efficiencies reaching 55% under optimized conditions. DTU has also developed proprietary microbial communities through adaptive laboratory evolution that demonstrate enhanced tolerance to ammonia toxicity and improved electron uptake capabilities, allowing for sustained operation over extended periods without significant performance degradation.
Strengths: Utilizes renewable electricity sources, enabling sustainable ammonia production with minimal carbon footprint. System demonstrates remarkable stability with consistent performance maintained over 1000+ hours of operation. Weaknesses: Current production rates remain below industrial requirements, limiting immediate commercial application. System complexity increases capital costs and maintenance requirements compared to conventional electrochemical approaches.
Siemens AG
Technical Solution: Siemens AG has developed an innovative microbial electrosynthesis system for ammonia production that combines bioelectrochemical processes with advanced automation and control technologies. Their approach utilizes a hybrid system where specialized electroactive microorganisms work in conjunction with electrocatalysts to reduce nitrogen to ammonia using renewable electricity. The system features a proprietary reactor design with multiple electrode configurations that optimize electron transfer to both biological and chemical catalytic sites. Siemens has engineered carbon-based electrodes with hierarchical porous structures modified with nitrogen-doped graphene and metal nanoparticles that enhance microbial colonization while providing additional catalytic activity. Their integrated process achieves ammonia production rates of approximately 8.7 mg NH3/L/h with Faradaic efficiencies reaching 58% under optimized conditions. Siemens' technology incorporates advanced digital monitoring and control systems that continuously adjust operating parameters based on real-time performance data, maintaining optimal conditions for both microbial activity and electrochemical reactions. The system operates at near-ambient conditions (temperature <40°C, pressure <5 bar), significantly reducing energy requirements compared to conventional ammonia synthesis processes.
Strengths: Seamless integration with renewable energy sources through advanced power management systems, enabling dynamic operation that adapts to variable electricity inputs. Sophisticated automation and control systems that optimize performance and reduce operational complexity. Weaknesses: Higher capital costs compared to purely biological or purely electrochemical systems. Requires specialized expertise for maintenance and operation, potentially limiting deployment in certain regions.
Key Patents and Innovations in Bioelectrochemical Ammonia Production
Electrochemical cell for generating ammonia
PatentPendingIN202211074333A
Innovation
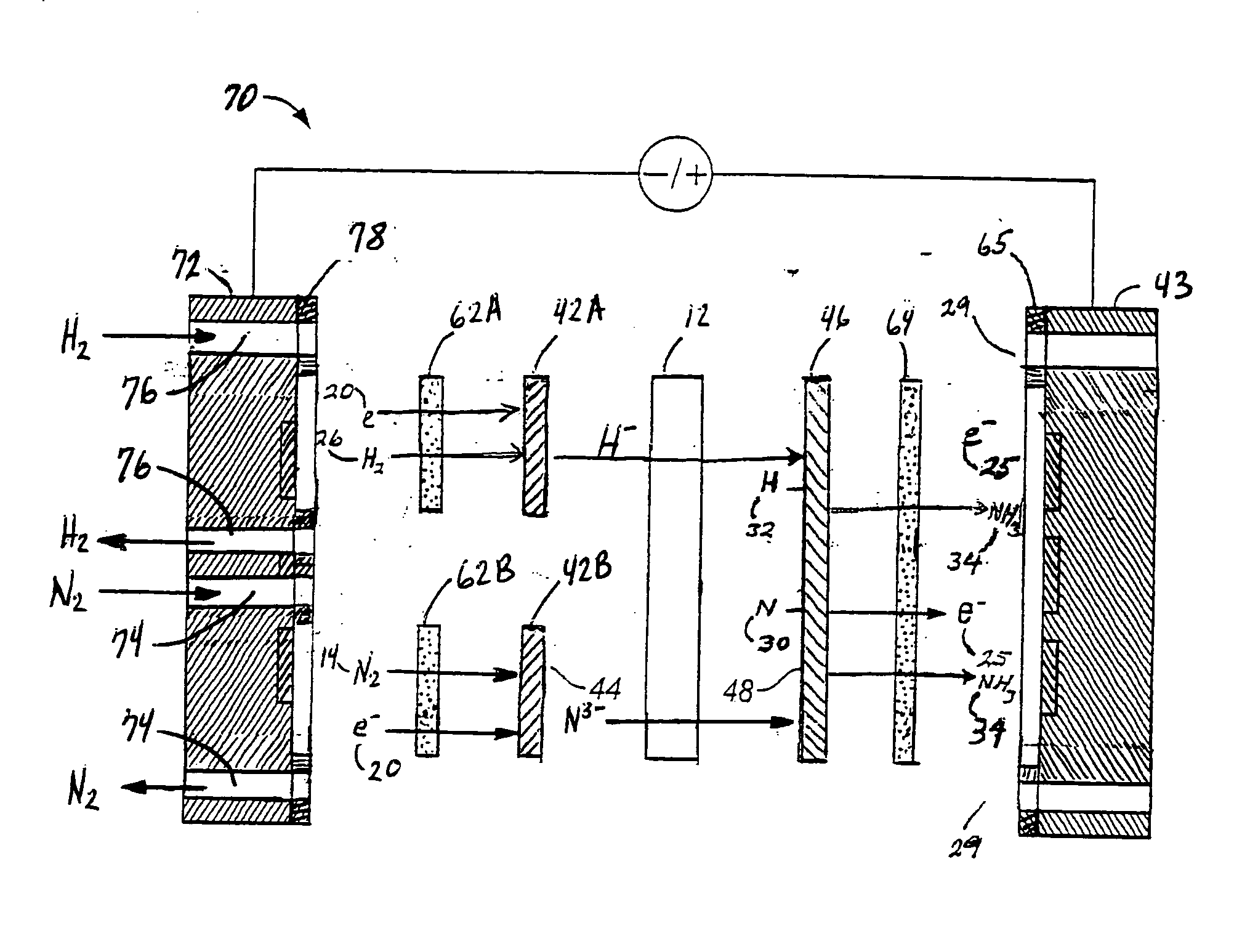
- An electrochemical cell system with a cathode electrode coated with a transition metal-based catalyst layer, such as Iron (Fe), Cobalt (Co), or Copper (Cu) phthalocyanine, and an anode electrode coated with Ruthenium (IV) oxide, using sodium tetrafluoroborate as the catholyte and potassium hydroxide as the anolyte, which improves nitrogen reduction reaction efficiency and oxygen evolution reaction kinetics.
Electrochemical synthesis of ammonia
PatentInactiveUS20060049063A1
Innovation
- An anodic electrochemical method using molten salts with dissolved nitride ions and a porous anode structure to oxidize nitride ions and react them with adsorbed hydrogen atoms to form ammonia, allowing for the production of ammonia at lower temperatures and pressures with improved conversion efficiencies.
Environmental Impact Assessment of Bioelectrochemical Ammonia Production
The environmental impact assessment of bioelectrochemical ammonia production reveals significant advantages over conventional Haber-Bosch processes. Traditional ammonia synthesis consumes approximately 1-2% of global energy and generates substantial greenhouse gas emissions, with 1.9 tons of CO2 released per ton of ammonia produced. In contrast, microbial electrosynthesis (MES) systems demonstrate potential for carbon-neutral or even carbon-negative production when powered by renewable electricity sources.
Life cycle assessments indicate that bioelectrochemical ammonia production could reduce carbon footprints by 60-90% compared to conventional methods, depending on the electricity source. When utilizing solar or wind power, the process approaches true sustainability, with minimal environmental impact during operation. The elimination of high-temperature, high-pressure conditions further reduces the overall energy intensity and associated emissions.
Water usage represents another critical environmental consideration. While conventional ammonia production requires significant water for cooling and steam generation, bioelectrochemical systems operate at ambient conditions with substantially lower water requirements. Studies estimate water consumption reductions of 30-50% in MES systems, contributing to improved water conservation in regions facing scarcity challenges.
Regarding land use impacts, bioelectrochemical systems present a mixed profile. They require less direct industrial land area than conventional plants but may need additional space for renewable energy infrastructure if powered by dedicated solar or wind installations. However, the decentralized nature of MES technology allows for distributed production closer to agricultural application points, potentially reducing transportation emissions and associated environmental impacts.
Waste generation and management constitute important environmental factors. MES systems produce minimal hazardous waste compared to conventional processes, which generate spent catalysts and various chemical byproducts. The biological components in MES reactors are generally biodegradable, though proper disposal protocols for exhausted biofilms and electrode materials require further development to ensure complete environmental safety.
Potential ecological risks from bioelectrochemical ammonia production include the release of genetically modified microorganisms (if employed) and the discharge of electrolytes containing trace metals from electrodes. Current research indicates these risks are manageable through proper containment systems and operational protocols, but long-term monitoring programs would be essential for commercial implementations to ensure environmental protection.
The scalability of environmental benefits presents a significant consideration. While laboratory and pilot-scale systems demonstrate impressive environmental performance, maintaining these advantages at industrial scales requires careful engineering and system integration. Optimizing electrode materials, microbial communities, and reactor designs will be crucial to preserving environmental benefits during commercial scaling.
Life cycle assessments indicate that bioelectrochemical ammonia production could reduce carbon footprints by 60-90% compared to conventional methods, depending on the electricity source. When utilizing solar or wind power, the process approaches true sustainability, with minimal environmental impact during operation. The elimination of high-temperature, high-pressure conditions further reduces the overall energy intensity and associated emissions.
Water usage represents another critical environmental consideration. While conventional ammonia production requires significant water for cooling and steam generation, bioelectrochemical systems operate at ambient conditions with substantially lower water requirements. Studies estimate water consumption reductions of 30-50% in MES systems, contributing to improved water conservation in regions facing scarcity challenges.
Regarding land use impacts, bioelectrochemical systems present a mixed profile. They require less direct industrial land area than conventional plants but may need additional space for renewable energy infrastructure if powered by dedicated solar or wind installations. However, the decentralized nature of MES technology allows for distributed production closer to agricultural application points, potentially reducing transportation emissions and associated environmental impacts.
Waste generation and management constitute important environmental factors. MES systems produce minimal hazardous waste compared to conventional processes, which generate spent catalysts and various chemical byproducts. The biological components in MES reactors are generally biodegradable, though proper disposal protocols for exhausted biofilms and electrode materials require further development to ensure complete environmental safety.
Potential ecological risks from bioelectrochemical ammonia production include the release of genetically modified microorganisms (if employed) and the discharge of electrolytes containing trace metals from electrodes. Current research indicates these risks are manageable through proper containment systems and operational protocols, but long-term monitoring programs would be essential for commercial implementations to ensure environmental protection.
The scalability of environmental benefits presents a significant consideration. While laboratory and pilot-scale systems demonstrate impressive environmental performance, maintaining these advantages at industrial scales requires careful engineering and system integration. Optimizing electrode materials, microbial communities, and reactor designs will be crucial to preserving environmental benefits during commercial scaling.
Scalability and Industrial Implementation Challenges
The transition from laboratory-scale microbial electrosynthesis (MES) systems for ammonia production to industrial implementation faces significant challenges. Current MES setups typically operate at volumes of 0.1-1L, whereas industrial applications would require reactors in the range of thousands of liters. This scale-up introduces complex engineering problems related to electrode surface area optimization, uniform electric field distribution, and maintaining consistent microbial activity throughout larger reactors.
Energy efficiency represents a critical barrier to commercial viability. Laboratory-scale MES systems for ammonia production currently demonstrate Faradaic efficiencies between 15-30%, significantly lower than the 60-80% efficiency required for economic feasibility at industrial scale. Power consumption, typically 10-15 kWh per kg of ammonia in optimized lab conditions, must be reduced to compete with the Haber-Bosch process's 8-10 kWh per kg benchmark.
Material constraints further complicate industrial implementation. Electrode materials that perform well in laboratory settings often face durability issues in continuous operation. Platinum and other noble metal catalysts that show promising performance are prohibitively expensive for large-scale deployment. Alternative materials like carbon-based electrodes offer cost advantages but suffer from lower catalytic activity and faster degradation rates, with typical lifespans of 3-6 months versus the 2-5 years needed for industrial viability.
Process stability presents another significant challenge. MES systems rely on living microorganisms that are sensitive to environmental fluctuations. Industrial-scale operations experience greater temperature gradients, pH variations, and potential contamination risks that can compromise microbial performance. Current systems maintain optimal performance for approximately 2-4 weeks before requiring intervention, whereas industrial applications would need operational stability for months.
Reactor design optimization remains underdeveloped for large-scale MES ammonia production. The ideal configuration must balance electrode spacing, electrolyte circulation, gas exchange, and microbial growth requirements. Current designs achieve ammonia production rates of 0.1-0.5 g NH₃/L/day, whereas economically viable industrial implementation would require at least 1-2 g NH₃/L/day.
Integration with existing industrial infrastructure presents additional challenges. MES systems for ammonia production would need to interface with renewable energy sources to maximize sustainability benefits, requiring sophisticated control systems to manage variable power inputs. Furthermore, downstream processing of the produced ammonia must meet industrial purity standards of >99.5% while minimizing energy consumption and product loss.
Energy efficiency represents a critical barrier to commercial viability. Laboratory-scale MES systems for ammonia production currently demonstrate Faradaic efficiencies between 15-30%, significantly lower than the 60-80% efficiency required for economic feasibility at industrial scale. Power consumption, typically 10-15 kWh per kg of ammonia in optimized lab conditions, must be reduced to compete with the Haber-Bosch process's 8-10 kWh per kg benchmark.
Material constraints further complicate industrial implementation. Electrode materials that perform well in laboratory settings often face durability issues in continuous operation. Platinum and other noble metal catalysts that show promising performance are prohibitively expensive for large-scale deployment. Alternative materials like carbon-based electrodes offer cost advantages but suffer from lower catalytic activity and faster degradation rates, with typical lifespans of 3-6 months versus the 2-5 years needed for industrial viability.
Process stability presents another significant challenge. MES systems rely on living microorganisms that are sensitive to environmental fluctuations. Industrial-scale operations experience greater temperature gradients, pH variations, and potential contamination risks that can compromise microbial performance. Current systems maintain optimal performance for approximately 2-4 weeks before requiring intervention, whereas industrial applications would need operational stability for months.
Reactor design optimization remains underdeveloped for large-scale MES ammonia production. The ideal configuration must balance electrode spacing, electrolyte circulation, gas exchange, and microbial growth requirements. Current designs achieve ammonia production rates of 0.1-0.5 g NH₃/L/day, whereas economically viable industrial implementation would require at least 1-2 g NH₃/L/day.
Integration with existing industrial infrastructure presents additional challenges. MES systems for ammonia production would need to interface with renewable energy sources to maximize sustainability benefits, requiring sophisticated control systems to manage variable power inputs. Furthermore, downstream processing of the produced ammonia must meet industrial purity standards of >99.5% while minimizing energy consumption and product loss.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!