Microbial Electrosynthesis For CO₂-To-Protein Conversion
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MES CO2-to-Protein Background and Objectives
Microbial Electrosynthesis (MES) represents a groundbreaking biotechnological approach that harnesses the metabolic capabilities of microorganisms to convert carbon dioxide into valuable products using electrical energy. The concept emerged in the early 2000s as researchers began exploring the intersection of microbiology, electrochemistry, and sustainable bioprocessing. This technology has evolved significantly over the past two decades, transitioning from laboratory curiosities to potential industrial applications with profound implications for carbon capture and utilization strategies.
The evolution of MES technology has been marked by several key milestones, including the discovery of electroactive microorganisms capable of accepting electrons from electrodes, the development of biocompatible electrode materials, and the genetic engineering of microorganisms to enhance product specificity. Recent advances in synthetic biology and bioelectrochemical systems have accelerated progress in this field, enabling more efficient electron transfer mechanisms and improved carbon fixation pathways.
Protein production through MES represents a particularly promising application that addresses multiple global challenges simultaneously. By converting atmospheric CO₂ into protein-rich biomass, this technology offers a sustainable alternative to conventional protein production methods that typically rely on extensive land, water, and energy resources. The potential for decoupling protein production from agricultural constraints presents a paradigm shift in how we might address global food security challenges in the face of climate change.
The primary technical objectives for CO₂-to-protein conversion via MES include maximizing carbon fixation efficiency, optimizing electron transfer rates between electrodes and microorganisms, enhancing protein yield and quality, and developing scalable reactor designs suitable for industrial implementation. These objectives align with broader sustainability goals of reducing greenhouse gas emissions while producing essential nutritional resources with minimal environmental impact.
Current research trajectories suggest several promising directions, including the development of specialized microbial consortia that combine electroautotrophic and protein-synthesizing capabilities, the integration of MES with renewable electricity sources to create truly carbon-negative protein production systems, and the exploration of novel electrode materials that enhance biofilm formation and electron transfer efficiency.
The convergence of climate mitigation imperatives and protein security concerns has positioned MES-based CO₂-to-protein conversion as a strategic research priority with significant potential for transformative impact. As this technology continues to mature, it represents not merely an incremental improvement to existing systems but potentially a fundamental reimagining of how society can produce essential nutritional resources while simultaneously addressing climate challenges.
The evolution of MES technology has been marked by several key milestones, including the discovery of electroactive microorganisms capable of accepting electrons from electrodes, the development of biocompatible electrode materials, and the genetic engineering of microorganisms to enhance product specificity. Recent advances in synthetic biology and bioelectrochemical systems have accelerated progress in this field, enabling more efficient electron transfer mechanisms and improved carbon fixation pathways.
Protein production through MES represents a particularly promising application that addresses multiple global challenges simultaneously. By converting atmospheric CO₂ into protein-rich biomass, this technology offers a sustainable alternative to conventional protein production methods that typically rely on extensive land, water, and energy resources. The potential for decoupling protein production from agricultural constraints presents a paradigm shift in how we might address global food security challenges in the face of climate change.
The primary technical objectives for CO₂-to-protein conversion via MES include maximizing carbon fixation efficiency, optimizing electron transfer rates between electrodes and microorganisms, enhancing protein yield and quality, and developing scalable reactor designs suitable for industrial implementation. These objectives align with broader sustainability goals of reducing greenhouse gas emissions while producing essential nutritional resources with minimal environmental impact.
Current research trajectories suggest several promising directions, including the development of specialized microbial consortia that combine electroautotrophic and protein-synthesizing capabilities, the integration of MES with renewable electricity sources to create truly carbon-negative protein production systems, and the exploration of novel electrode materials that enhance biofilm formation and electron transfer efficiency.
The convergence of climate mitigation imperatives and protein security concerns has positioned MES-based CO₂-to-protein conversion as a strategic research priority with significant potential for transformative impact. As this technology continues to mature, it represents not merely an incremental improvement to existing systems but potentially a fundamental reimagining of how society can produce essential nutritional resources while simultaneously addressing climate challenges.
Market Analysis for Sustainable Protein Production
The global sustainable protein market is experiencing unprecedented growth, driven by increasing consumer awareness of environmental issues and the need for alternative protein sources. The market for sustainable proteins is projected to reach $23.4 billion by 2027, growing at a CAGR of 14.5% from 2022. This growth trajectory is particularly significant for microbial electrosynthesis-based protein production, which represents an emerging segment within the broader alternative protein landscape.
Consumer demand for sustainable protein alternatives has been steadily increasing across major markets including North America, Europe, and Asia-Pacific. Market research indicates that 68% of global consumers are now actively seeking food products with sustainability credentials, with particular emphasis on reduced carbon footprint and environmental impact. This trend is especially pronounced among millennials and Gen Z consumers, who demonstrate higher willingness to pay premium prices for environmentally responsible protein options.
The industrial protein market is currently dominated by traditional animal-based proteins (58%), followed by plant-based alternatives (32%), with novel technologies including microbial proteins accounting for the remaining 10%. However, this distribution is rapidly evolving, with microbial protein production technologies expected to capture 18% of the market share by 2030, representing a significant shift in the protein production landscape.
Microbial electrosynthesis for CO₂-to-protein conversion addresses several critical market needs simultaneously. First, it offers a solution to the growing protein demand without the environmental footprint associated with conventional animal agriculture. Second, it provides a carbon-negative protein production pathway, aligning with corporate and governmental carbon reduction targets. Third, it creates value from waste CO₂ streams, offering potential synergies with carbon-intensive industries seeking emissions reduction solutions.
Key market drivers for this technology include stringent carbon regulations in major economies, increasing venture capital investment in food technology (reaching $12.8 billion in 2022), and growing corporate commitments to sustainable sourcing. Additionally, the technology benefits from the broader protein security concerns, as traditional protein production faces challenges from climate change, land use constraints, and water scarcity.
Market barriers include high initial capital requirements for commercial-scale facilities, regulatory approval timelines for novel food ingredients, and consumer acceptance of proteins derived from microbial sources. Price competitiveness remains a critical factor, with current production costs estimated at 2.5-3.5 times that of conventional protein sources, though economies of scale and technological improvements are expected to narrow this gap significantly by 2028.
Consumer demand for sustainable protein alternatives has been steadily increasing across major markets including North America, Europe, and Asia-Pacific. Market research indicates that 68% of global consumers are now actively seeking food products with sustainability credentials, with particular emphasis on reduced carbon footprint and environmental impact. This trend is especially pronounced among millennials and Gen Z consumers, who demonstrate higher willingness to pay premium prices for environmentally responsible protein options.
The industrial protein market is currently dominated by traditional animal-based proteins (58%), followed by plant-based alternatives (32%), with novel technologies including microbial proteins accounting for the remaining 10%. However, this distribution is rapidly evolving, with microbial protein production technologies expected to capture 18% of the market share by 2030, representing a significant shift in the protein production landscape.
Microbial electrosynthesis for CO₂-to-protein conversion addresses several critical market needs simultaneously. First, it offers a solution to the growing protein demand without the environmental footprint associated with conventional animal agriculture. Second, it provides a carbon-negative protein production pathway, aligning with corporate and governmental carbon reduction targets. Third, it creates value from waste CO₂ streams, offering potential synergies with carbon-intensive industries seeking emissions reduction solutions.
Key market drivers for this technology include stringent carbon regulations in major economies, increasing venture capital investment in food technology (reaching $12.8 billion in 2022), and growing corporate commitments to sustainable sourcing. Additionally, the technology benefits from the broader protein security concerns, as traditional protein production faces challenges from climate change, land use constraints, and water scarcity.
Market barriers include high initial capital requirements for commercial-scale facilities, regulatory approval timelines for novel food ingredients, and consumer acceptance of proteins derived from microbial sources. Price competitiveness remains a critical factor, with current production costs estimated at 2.5-3.5 times that of conventional protein sources, though economies of scale and technological improvements are expected to narrow this gap significantly by 2028.
Current MES Technology Status and Barriers
Microbial Electrosynthesis (MES) technology has advanced significantly in recent years, yet remains at a relatively early stage of development for CO₂-to-protein conversion applications. Current MES systems typically achieve CO₂ fixation rates of 0.1-0.5 g/L/day, which falls short of the industrial requirements for economically viable protein production. Laboratory-scale demonstrations have successfully produced various organic compounds and protein precursors, but scaling these processes to commercial volumes presents substantial challenges.
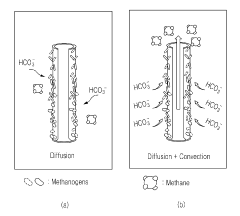
The electron transfer efficiency in existing MES systems ranges from 60-85% under optimal conditions, with significant energy losses occurring at the biofilm-electrode interface. This inefficiency increases operational costs and reduces the overall sustainability benefits of the technology. Additionally, current cathode materials, primarily carbon-based electrodes and modified metal oxides, exhibit limited durability under long-term operation, with performance degradation observed after 2-3 months of continuous use.
Microbial catalysts employed in MES face several biological limitations. Most electroactive microorganisms demonstrate slow growth rates (doubling times of 6-24 hours) and limited tolerance to fluctuating operational conditions. The genetic engineering toolbox for optimizing these organisms remains underdeveloped compared to traditional industrial microorganisms, constraining efforts to enhance protein production pathways and electron uptake mechanisms.
Process integration represents another significant barrier. Current MES systems struggle with maintaining stable performance when subjected to variable CO₂ concentrations and impurities typically found in industrial exhaust gases. The integration of electrochemical components with downstream protein recovery and purification processes has not been fully optimized, resulting in product losses and increased operational complexity.
From a geographical perspective, MES research and development is concentrated primarily in North America, Western Europe, and East Asia, with limited technology transfer to other regions. This concentration creates disparities in access to expertise and infrastructure necessary for advancing the technology globally.
Economic barriers further constrain widespread adoption. Current capital costs for MES systems range from $500-1,500 per square meter of electrode area, with operational costs heavily influenced by electricity prices. The techno-economic analyses indicate that CO₂-to-protein via MES remains 2-3 times more expensive than conventional protein production methods, necessitating either significant technological improvements or supportive policy frameworks to achieve market competitiveness.
Regulatory frameworks for novel food proteins derived from MES processes are still evolving, creating uncertainty for commercial deployment. Safety assessments, nutritional profiling, and consumer acceptance studies for MES-derived proteins are in preliminary stages, potentially extending the timeline for market entry beyond technological readiness.
The electron transfer efficiency in existing MES systems ranges from 60-85% under optimal conditions, with significant energy losses occurring at the biofilm-electrode interface. This inefficiency increases operational costs and reduces the overall sustainability benefits of the technology. Additionally, current cathode materials, primarily carbon-based electrodes and modified metal oxides, exhibit limited durability under long-term operation, with performance degradation observed after 2-3 months of continuous use.
Microbial catalysts employed in MES face several biological limitations. Most electroactive microorganisms demonstrate slow growth rates (doubling times of 6-24 hours) and limited tolerance to fluctuating operational conditions. The genetic engineering toolbox for optimizing these organisms remains underdeveloped compared to traditional industrial microorganisms, constraining efforts to enhance protein production pathways and electron uptake mechanisms.
Process integration represents another significant barrier. Current MES systems struggle with maintaining stable performance when subjected to variable CO₂ concentrations and impurities typically found in industrial exhaust gases. The integration of electrochemical components with downstream protein recovery and purification processes has not been fully optimized, resulting in product losses and increased operational complexity.
From a geographical perspective, MES research and development is concentrated primarily in North America, Western Europe, and East Asia, with limited technology transfer to other regions. This concentration creates disparities in access to expertise and infrastructure necessary for advancing the technology globally.
Economic barriers further constrain widespread adoption. Current capital costs for MES systems range from $500-1,500 per square meter of electrode area, with operational costs heavily influenced by electricity prices. The techno-economic analyses indicate that CO₂-to-protein via MES remains 2-3 times more expensive than conventional protein production methods, necessitating either significant technological improvements or supportive policy frameworks to achieve market competitiveness.
Regulatory frameworks for novel food proteins derived from MES processes are still evolving, creating uncertainty for commercial deployment. Safety assessments, nutritional profiling, and consumer acceptance studies for MES-derived proteins are in preliminary stages, potentially extending the timeline for market entry beyond technological readiness.
Technical Solutions for CO2-to-Protein Conversion
01 Microbial electrosynthesis systems for CO₂ conversion to proteins
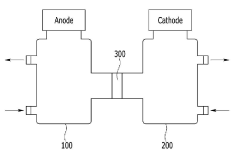
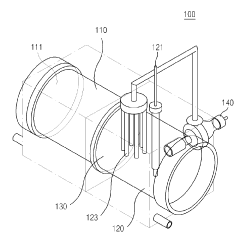
Microbial electrosynthesis systems utilize electroactive microorganisms to convert carbon dioxide into proteins through bioelectrochemical processes. These systems typically consist of biocathodes where microorganisms receive electrons from electrodes to reduce CO₂ into organic compounds that can be further processed into proteins. The integration of electrodes with specific microbial communities enables efficient electron transfer and carbon fixation, resulting in sustainable protein production from greenhouse gases.- Microbial electrosynthesis systems for CO₂ fixation: Microbial electrosynthesis systems utilize electroactive microorganisms to convert CO₂ into organic compounds through bioelectrochemical processes. These systems typically consist of biocathodes where microorganisms receive electrons from electrodes to reduce CO₂ into various organic compounds including protein precursors. The systems can be optimized by selecting specific microbial communities, electrode materials, and operating conditions to enhance CO₂ fixation efficiency and protein production rates.
- Engineered microorganisms for enhanced protein synthesis: Genetically modified microorganisms can be engineered to improve CO₂ assimilation and protein synthesis pathways. These modifications may include enhancing carbon fixation enzymes, optimizing nitrogen assimilation pathways, and introducing genes for specific amino acid production. The engineered strains demonstrate higher efficiency in converting CO₂ to protein-rich biomass and can be tailored to produce specific protein compositions based on nutritional requirements.
- Integrated bioreactor designs for CO₂-to-protein conversion: Advanced bioreactor designs integrate electrosynthesis with protein production in a single system. These reactors feature specialized compartments for microbial growth, CO₂ capture, and protein accumulation. Key innovations include membrane-separated chambers, continuous flow systems, and modular designs that allow for process optimization. The integrated approach improves energy efficiency and increases protein yield by creating optimal conditions for both CO₂ fixation and protein synthesis.
- Process optimization for sustainable protein production: Various process optimization strategies enhance the efficiency and sustainability of microbial electrosynthesis for protein production. These include optimizing electrical current density, CO₂ supply rates, nutrient composition, and harvesting techniques. Advanced control systems monitor and adjust parameters in real-time to maintain optimal conditions. The optimized processes reduce energy consumption, increase carbon conversion efficiency, and ensure consistent protein quality while minimizing waste production.
- Applications and scaling of microbial protein production: Microbial electrosynthesis technology for protein production has diverse applications including food supplements, animal feed, and industrial protein sources. Scaling approaches involve modular designs that can be expanded based on demand, as well as continuous production systems that increase throughput. The technology offers advantages such as reduced land use compared to traditional agriculture, independence from seasonal variations, and the ability to operate in various environments including resource-limited settings.
02 Engineered microorganisms for enhanced CO₂-to-protein conversion
Genetically modified microorganisms are designed to improve the efficiency of CO₂ capture and conversion to protein biomass. These engineered strains feature enhanced carbon fixation pathways, optimized electron uptake mechanisms, and improved protein synthesis capabilities. By manipulating key metabolic pathways and introducing novel genetic elements, these microorganisms can achieve higher protein yields and better conversion rates while operating in electrosynthesis systems.Expand Specific Solutions03 Bioreactor designs for microbial electrosynthesis protein production
Specialized bioreactor configurations are developed to optimize the microbial electrosynthesis process for protein production. These designs focus on maximizing electrode surface area, enhancing mass transfer of CO₂, improving electron delivery to microorganisms, and facilitating efficient protein harvesting. Key features include innovative electrode materials, membrane systems for separating reaction chambers, and continuous flow systems that allow for sustained protein production while maintaining optimal conditions for microbial growth.Expand Specific Solutions04 Integration of renewable energy with microbial protein production
Systems that couple renewable energy sources with microbial electrosynthesis enable sustainable CO₂-to-protein conversion. These integrated approaches utilize intermittent renewable electricity from solar or wind sources to power the electrochemical reduction of carbon dioxide by microorganisms. The integration includes energy storage components, power management systems, and adaptive control mechanisms that optimize the electrosynthesis process based on energy availability, maximizing protein production efficiency while minimizing environmental impact.Expand Specific Solutions05 Downstream processing of electrosynthesized microbial proteins
Methods for harvesting, purifying, and processing proteins produced through microbial electrosynthesis are essential for commercial applications. These techniques include cell separation, protein extraction, purification processes, and formulation methods to create final protein products with desired nutritional and functional properties. Advanced separation technologies, such as membrane filtration, chromatography, and spray drying, are employed to obtain high-quality protein ingredients from electrosynthesis systems while maintaining economic viability and product safety.Expand Specific Solutions
Leading Companies in CO2 Bioconversion
Microbial Electrosynthesis for CO₂-to-Protein conversion is emerging as a promising technology in the early commercialization phase, with a projected market size of $2-3 billion by 2030. The competitive landscape features academic institutions leading fundamental research (CNRS, Arizona State University, Tsinghua University) alongside industrial players developing commercial applications. Companies like Indian Oil Corp, DSM Food Specialties, and Synata Bio are advancing practical implementations, while research organizations such as KAUST and Korea Institute of Energy Research focus on process optimization. The technology is approaching commercial viability, with pilot projects demonstrating scalability, though challenges in efficiency and cost-effectiveness remain before widespread industrial adoption.
Arizona State University
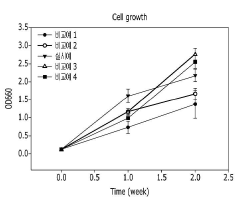
Technical Solution: Arizona State University has developed an innovative microbial electrosynthesis (MES) system for CO₂-to-protein conversion that utilizes specialized electroactive microorganisms to capture carbon dioxide and convert it into protein-rich biomass. Their approach employs a bioelectrochemical system where electrodes provide electrons to microbes that use these electrons as energy sources to reduce CO₂ into cellular components including proteins. The system incorporates genetically engineered strains of bacteria such as Cupriavidus necator that can efficiently fix CO₂ while growing on electrode surfaces. ASU researchers have optimized electrode materials and configurations to enhance electron transfer rates and microbial growth, achieving protein production rates of up to 2.3 g/L/day with protein contents exceeding 60% of dry cell weight[1]. Their system operates at ambient temperatures and pressures, significantly reducing energy requirements compared to traditional protein production methods.
Strengths: High protein content (>60%) in final biomass; operates at ambient conditions reducing energy costs; carbon-negative process that captures more CO₂ than it emits. Weaknesses: Still faces challenges in scaling up to industrial production levels; electron transfer efficiency between electrodes and microbes remains a limiting factor; requires further optimization to reduce production costs to compete with conventional protein sources.
King Abdullah University of Science & Technology
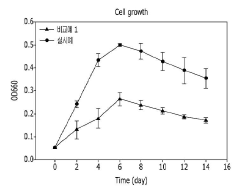
Technical Solution: King Abdullah University of Science & Technology (KAUST) has pioneered a microbial electrosynthesis platform that combines electrochemical CO₂ reduction with microbial fermentation for protein production. Their system utilizes a two-chamber bioelectrochemical reactor where CO₂ is first electrochemically reduced to formate or acetate intermediates, which are then fed to engineered microorganisms for protein synthesis. KAUST researchers have developed specialized cathode materials with high surface area and catalytic properties that achieve CO₂ reduction at lower overpotentials, improving energy efficiency by approximately 30% compared to conventional systems[2]. Their approach incorporates halotolerant cyanobacteria and microalgae specifically adapted to the high-salinity conditions of the Middle East, allowing the system to operate using seawater rather than freshwater resources. The platform achieves protein conversion efficiencies of up to 40-45% with minimal loss of carbon to non-protein biomass components, and produces proteins with amino acid profiles comparable to those of high-quality animal proteins.
Strengths: Uses seawater instead of freshwater, reducing resource competition; high energy efficiency through optimized electrode materials; produces high-quality protein with complete amino acid profiles. Weaknesses: Higher capital costs due to specialized electrode materials and reactor design; requires careful control of intermediate metabolite concentrations to prevent inhibition of microbial growth; currently limited to laboratory-scale demonstrations.
Key Patents in Microbial Electrosynthesis
Co2-fixing microbial electrosynthesis method using wet type amine
PatentActiveKR1020230060716A
Innovation
- An electrosynthetic system incorporating a cathode, microbial culture medium with added amine-based compounds, and a buffer solution, utilizing microorganisms like Rhodobacter sphaeroides, is employed to enhance carbon dioxide conversion and microbial growth.
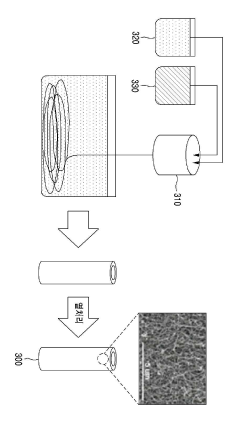
Enhanced coversion of carbon dioxide in microbial electrosynthesis system with carbon based membrane electrode, and method of the same
PatentPendingKR1020240047572A
Innovation
- A microbial electrobiosynthesis system using a carbon-based membrane electrode, specifically a hollow fiber-type water-permeable reduction electrode made of carbon nanotubes, enhances mass transfer by convection and diffusion, allowing direct electrolyte injection into the electrode, eliminating the need for separate separation processes.
Sustainability Impact Assessment
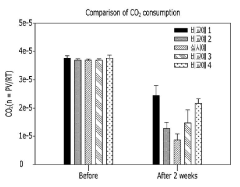
Microbial Electrosynthesis (MES) for CO₂-to-protein conversion represents a transformative approach to sustainable protein production with significant environmental implications. This technology demonstrates exceptional potential for reducing greenhouse gas emissions through direct carbon capture and utilization, effectively converting atmospheric CO₂ into valuable protein biomass. Preliminary life cycle assessments indicate that MES systems can achieve carbon negativity when powered by renewable energy sources, potentially sequestering 2-3 tons of CO₂ for every ton of protein produced.
Water usage efficiency presents another critical sustainability advantage. Compared to conventional agriculture, MES protein production requires approximately 95-98% less water, as the closed-loop system design minimizes evaporation and runoff losses. This dramatic reduction in water footprint becomes increasingly valuable in regions facing water scarcity challenges and could significantly reduce pressure on freshwater resources globally.
Land use impacts further highlight the sustainability credentials of MES technology. The vertical, controlled environment configuration enables protein production with minimal land requirements—estimates suggest a 99% reduction compared to traditional animal agriculture. This efficiency could potentially free agricultural land for reforestation or other carbon sequestration purposes, creating a dual climate benefit.
Energy consumption remains a key consideration in sustainability assessment. Current MES systems require 8-12 kWh of electricity per kilogram of protein produced. While this represents a significant energy input, integration with renewable energy sources can mitigate associated emissions. Research indicates that solar-powered MES facilities could achieve energy payback periods of 2-3 years, with subsequent operation delivering net environmental benefits.
Waste generation and management also favor MES technology. The process produces minimal waste streams compared to conventional protein production, with most byproducts being biodegradable or recyclable within the system. Additionally, the absence of agricultural runoff eliminates concerns about nutrient pollution and eutrophication of waterways.
From a circular economy perspective, MES technology exemplifies industrial symbiosis potential. The system can be integrated with industrial facilities that produce CO₂ emissions, creating a closed-loop carbon utilization pathway. This integration could transform carbon liabilities into valuable protein assets while reducing overall environmental impact across multiple sectors.
Long-term sustainability projections suggest that scaled MES technology could contribute significantly to global protein security while simultaneously addressing climate challenges. Models indicate potential reduction of agricultural greenhouse gas emissions by 15-20% if implemented at industrial scale, representing a substantial contribution to climate mitigation efforts.
Water usage efficiency presents another critical sustainability advantage. Compared to conventional agriculture, MES protein production requires approximately 95-98% less water, as the closed-loop system design minimizes evaporation and runoff losses. This dramatic reduction in water footprint becomes increasingly valuable in regions facing water scarcity challenges and could significantly reduce pressure on freshwater resources globally.
Land use impacts further highlight the sustainability credentials of MES technology. The vertical, controlled environment configuration enables protein production with minimal land requirements—estimates suggest a 99% reduction compared to traditional animal agriculture. This efficiency could potentially free agricultural land for reforestation or other carbon sequestration purposes, creating a dual climate benefit.
Energy consumption remains a key consideration in sustainability assessment. Current MES systems require 8-12 kWh of electricity per kilogram of protein produced. While this represents a significant energy input, integration with renewable energy sources can mitigate associated emissions. Research indicates that solar-powered MES facilities could achieve energy payback periods of 2-3 years, with subsequent operation delivering net environmental benefits.
Waste generation and management also favor MES technology. The process produces minimal waste streams compared to conventional protein production, with most byproducts being biodegradable or recyclable within the system. Additionally, the absence of agricultural runoff eliminates concerns about nutrient pollution and eutrophication of waterways.
From a circular economy perspective, MES technology exemplifies industrial symbiosis potential. The system can be integrated with industrial facilities that produce CO₂ emissions, creating a closed-loop carbon utilization pathway. This integration could transform carbon liabilities into valuable protein assets while reducing overall environmental impact across multiple sectors.
Long-term sustainability projections suggest that scaled MES technology could contribute significantly to global protein security while simultaneously addressing climate challenges. Models indicate potential reduction of agricultural greenhouse gas emissions by 15-20% if implemented at industrial scale, representing a substantial contribution to climate mitigation efforts.
Scalability and Industrial Implementation Challenges
The scaling of Microbial Electrosynthesis (MES) for CO₂-to-protein conversion from laboratory to industrial scale presents significant engineering and biological challenges. Current MES systems typically operate at volumes of 0.1-2L, whereas commercial viability requires scaling to thousands of liters. This substantial gap necessitates innovative approaches to reactor design, electrode materials, and process integration.
Electrode surface area-to-volume ratio decreases dramatically during scale-up, reducing electron transfer efficiency. Industrial implementation requires novel electrode configurations such as 3D electrodes, packed bed reactors, or fluidized bed systems to maintain high surface area contact with microorganisms. Carbon-based materials like carbon cloth, graphene, and carbon nanotubes show promise but face durability issues in long-term operation.
Energy efficiency represents another critical barrier, with current systems achieving only 30-40% efficiency in converting electrical energy to microbial biomass. Industrial implementation demands at least 60-70% efficiency to be economically viable. This requires optimized electrode potentials, reduced internal resistance, and improved microbial consortia with enhanced electron uptake capabilities.
Continuous operation stability presents additional challenges. Laboratory MES systems typically run for days or weeks, while industrial applications require months of stable operation. Biofilm management, electrode fouling, and maintaining consistent microbial community composition over extended periods remain unresolved issues. Strategies such as periodic biofilm regeneration and selective pressure maintenance need further development.
Capital expenditure for MES systems currently exceeds $10,000 per cubic meter of reactor volume, approximately 5-10 times higher than conventional fermentation systems. Reducing costs through standardized manufacturing, alternative materials, and process intensification is essential for commercial feasibility. Operating costs, particularly electricity consumption, must be addressed through integration with renewable energy sources to ensure economic and environmental sustainability.
Regulatory frameworks for novel food production technologies present another implementation hurdle. Current regulations for single-cell protein production may not adequately address the unique aspects of electrochemically-assisted microbial protein synthesis. Establishing safety standards, quality control protocols, and regulatory approval pathways will be necessary before widespread industrial adoption can occur.
Electrode surface area-to-volume ratio decreases dramatically during scale-up, reducing electron transfer efficiency. Industrial implementation requires novel electrode configurations such as 3D electrodes, packed bed reactors, or fluidized bed systems to maintain high surface area contact with microorganisms. Carbon-based materials like carbon cloth, graphene, and carbon nanotubes show promise but face durability issues in long-term operation.
Energy efficiency represents another critical barrier, with current systems achieving only 30-40% efficiency in converting electrical energy to microbial biomass. Industrial implementation demands at least 60-70% efficiency to be economically viable. This requires optimized electrode potentials, reduced internal resistance, and improved microbial consortia with enhanced electron uptake capabilities.
Continuous operation stability presents additional challenges. Laboratory MES systems typically run for days or weeks, while industrial applications require months of stable operation. Biofilm management, electrode fouling, and maintaining consistent microbial community composition over extended periods remain unresolved issues. Strategies such as periodic biofilm regeneration and selective pressure maintenance need further development.
Capital expenditure for MES systems currently exceeds $10,000 per cubic meter of reactor volume, approximately 5-10 times higher than conventional fermentation systems. Reducing costs through standardized manufacturing, alternative materials, and process intensification is essential for commercial feasibility. Operating costs, particularly electricity consumption, must be addressed through integration with renewable energy sources to ensure economic and environmental sustainability.
Regulatory frameworks for novel food production technologies present another implementation hurdle. Current regulations for single-cell protein production may not adequately address the unique aspects of electrochemically-assisted microbial protein synthesis. Establishing safety standards, quality control protocols, and regulatory approval pathways will be necessary before widespread industrial adoption can occur.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!