Microbial Electrosynthesis Of Long-Chain Fatty Acids
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microbial Electrosynthesis Background and Objectives
Microbial electrosynthesis (MES) represents a groundbreaking biotechnological approach that harnesses the metabolic capabilities of microorganisms to convert electrical energy into valuable chemical compounds. The concept emerged in the early 2000s as researchers began exploring the interface between microbiology and electrochemistry, with significant advancements occurring over the past decade. This technology builds upon the natural ability of certain microorganisms to accept electrons from external sources and utilize them in their metabolic pathways.
The evolution of MES technology has been marked by several key milestones, including the discovery of electroactive microorganisms, development of biocompatible electrode materials, and optimization of bioelectrochemical systems. Initially focused on simple compounds like hydrogen and methane, the field has progressively expanded toward more complex molecules, with long-chain fatty acids (LCFAs) representing a frontier with immense potential for sustainable bioproduction.
Long-chain fatty acids are particularly valuable targets for MES due to their diverse applications in biofuels, food additives, pharmaceuticals, and cosmetics. Traditional production methods for LCFAs rely heavily on petroleum-based feedstocks or agricultural resources, raising concerns about sustainability and competition with food production. MES offers a promising alternative by utilizing renewable electricity, potentially from solar or wind sources, and carbon dioxide as primary inputs.
Recent technological advances have significantly improved the efficiency and specificity of MES processes. These include the development of specialized electrode materials with enhanced biocompatibility, genetic engineering of microorganisms for improved electron uptake, and innovative reactor designs that optimize electron transfer between electrodes and microbial cells. The integration of synthetic biology approaches has further expanded the potential product spectrum beyond naturally occurring compounds.
The primary objective of MES research for LCFA production is to establish economically viable and environmentally sustainable bioprocesses that can compete with conventional production methods. This involves addressing several key challenges, including improving electron transfer efficiency, enhancing product specificity, increasing production rates, and developing scalable reactor designs suitable for industrial implementation.
Additional objectives include identifying and characterizing novel electroactive microorganisms capable of LCFA synthesis, understanding the fundamental mechanisms of extracellular electron transfer, and developing predictive models to guide process optimization. The ultimate goal is to create a carbon-neutral or carbon-negative production platform that converts renewable electricity and waste carbon dioxide into high-value fatty acids, thereby contributing to circular bioeconomy initiatives and climate change mitigation efforts.
The evolution of MES technology has been marked by several key milestones, including the discovery of electroactive microorganisms, development of biocompatible electrode materials, and optimization of bioelectrochemical systems. Initially focused on simple compounds like hydrogen and methane, the field has progressively expanded toward more complex molecules, with long-chain fatty acids (LCFAs) representing a frontier with immense potential for sustainable bioproduction.
Long-chain fatty acids are particularly valuable targets for MES due to their diverse applications in biofuels, food additives, pharmaceuticals, and cosmetics. Traditional production methods for LCFAs rely heavily on petroleum-based feedstocks or agricultural resources, raising concerns about sustainability and competition with food production. MES offers a promising alternative by utilizing renewable electricity, potentially from solar or wind sources, and carbon dioxide as primary inputs.
Recent technological advances have significantly improved the efficiency and specificity of MES processes. These include the development of specialized electrode materials with enhanced biocompatibility, genetic engineering of microorganisms for improved electron uptake, and innovative reactor designs that optimize electron transfer between electrodes and microbial cells. The integration of synthetic biology approaches has further expanded the potential product spectrum beyond naturally occurring compounds.
The primary objective of MES research for LCFA production is to establish economically viable and environmentally sustainable bioprocesses that can compete with conventional production methods. This involves addressing several key challenges, including improving electron transfer efficiency, enhancing product specificity, increasing production rates, and developing scalable reactor designs suitable for industrial implementation.
Additional objectives include identifying and characterizing novel electroactive microorganisms capable of LCFA synthesis, understanding the fundamental mechanisms of extracellular electron transfer, and developing predictive models to guide process optimization. The ultimate goal is to create a carbon-neutral or carbon-negative production platform that converts renewable electricity and waste carbon dioxide into high-value fatty acids, thereby contributing to circular bioeconomy initiatives and climate change mitigation efforts.
Market Analysis for Bio-based Long-Chain Fatty Acids
The global market for bio-based long-chain fatty acids (LCFAs) is experiencing significant growth, driven by increasing consumer demand for sustainable and environmentally friendly products. The market value was estimated at approximately $5.2 billion in 2022 and is projected to reach $8.7 billion by 2028, representing a compound annual growth rate (CAGR) of 8.9% during the forecast period.
The food and beverage industry remains the largest consumer of bio-based LCFAs, accounting for nearly 35% of the total market share. This dominance is attributed to the rising consumer preference for natural ingredients and clean-label products. The cosmetics and personal care sector follows closely, with a market share of around 28%, as manufacturers increasingly replace petroleum-based ingredients with bio-based alternatives to meet consumer demands for sustainable beauty products.
Regionally, Europe leads the market with approximately 32% share, followed by North America (27%) and Asia-Pacific (25%). The European market is primarily driven by stringent regulations promoting bio-based products and reducing carbon footprints. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, fueled by rapid industrialization, increasing disposable income, and growing awareness about sustainable products.
The microbial electrosynthesis of LCFAs represents a disruptive technology in this market landscape. Traditional methods of LCFA production, including plant extraction and chemical synthesis, face challenges related to land use, resource consumption, and environmental impact. Microbial electrosynthesis offers a promising alternative with potential advantages in production efficiency, reduced environmental footprint, and independence from agricultural feedstocks.
Market analysis indicates that industries are increasingly willing to pay premium prices for sustainably produced LCFAs. A survey conducted among 150 major consumer goods companies revealed that 73% are actively seeking bio-based alternatives to conventional fatty acids, with 62% specifically interested in microbially produced variants.
The competitive landscape is characterized by both established players diversifying their production methods and startups focusing exclusively on novel biotechnological approaches. Major chemical companies like BASF, Cargill, and DuPont have initiated R&D programs in microbial production of LCFAs, while biotechnology startups such as Genomatica and Lygos have secured significant venture capital funding to commercialize their microbial production platforms.
Key market drivers include increasing regulatory pressure on conventional production methods, growing consumer awareness about sustainability, and the potential for cost reduction through technological advancement. However, challenges such as scaling up production, achieving cost parity with conventional methods, and addressing technical limitations in microbial productivity remain significant barriers to widespread market adoption.
The food and beverage industry remains the largest consumer of bio-based LCFAs, accounting for nearly 35% of the total market share. This dominance is attributed to the rising consumer preference for natural ingredients and clean-label products. The cosmetics and personal care sector follows closely, with a market share of around 28%, as manufacturers increasingly replace petroleum-based ingredients with bio-based alternatives to meet consumer demands for sustainable beauty products.
Regionally, Europe leads the market with approximately 32% share, followed by North America (27%) and Asia-Pacific (25%). The European market is primarily driven by stringent regulations promoting bio-based products and reducing carbon footprints. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, fueled by rapid industrialization, increasing disposable income, and growing awareness about sustainable products.
The microbial electrosynthesis of LCFAs represents a disruptive technology in this market landscape. Traditional methods of LCFA production, including plant extraction and chemical synthesis, face challenges related to land use, resource consumption, and environmental impact. Microbial electrosynthesis offers a promising alternative with potential advantages in production efficiency, reduced environmental footprint, and independence from agricultural feedstocks.
Market analysis indicates that industries are increasingly willing to pay premium prices for sustainably produced LCFAs. A survey conducted among 150 major consumer goods companies revealed that 73% are actively seeking bio-based alternatives to conventional fatty acids, with 62% specifically interested in microbially produced variants.
The competitive landscape is characterized by both established players diversifying their production methods and startups focusing exclusively on novel biotechnological approaches. Major chemical companies like BASF, Cargill, and DuPont have initiated R&D programs in microbial production of LCFAs, while biotechnology startups such as Genomatica and Lygos have secured significant venture capital funding to commercialize their microbial production platforms.
Key market drivers include increasing regulatory pressure on conventional production methods, growing consumer awareness about sustainability, and the potential for cost reduction through technological advancement. However, challenges such as scaling up production, achieving cost parity with conventional methods, and addressing technical limitations in microbial productivity remain significant barriers to widespread market adoption.
Current Challenges in MES LCFA Production
Despite the promising potential of Microbial Electrosynthesis (MES) for long-chain fatty acid (LCFA) production, several significant challenges currently impede its widespread industrial implementation. The primary obstacle remains the low production rates and yields compared to conventional chemical synthesis methods. Current MES systems typically achieve LCFA production rates in the range of 0.1-0.5 g/L/day, which falls substantially short of the industrial viability threshold of 1-2 g/L/day.
Energy efficiency represents another critical challenge, as the conversion of electrical energy to chemical energy in LCFAs often exhibits efficiencies below 30%. This inefficiency stems from competing metabolic pathways, electron losses to alternative acceptors, and the inherent thermodynamic limitations of biological systems operating at ambient temperatures and pressures.
The selectivity of the process poses additional difficulties, with many MES systems producing a mixture of fatty acids of varying chain lengths rather than specific target LCFAs. This lack of product specificity necessitates costly downstream separation processes, further reducing economic viability. Current separation technologies for LCFA recovery from fermentation broths remain energy-intensive and inefficient at the dilute concentrations typically achieved.
Microbial catalyst limitations constitute a significant barrier, as many electroactive microorganisms capable of accepting electrons from electrodes lack the metabolic pathways necessary for efficient LCFA synthesis. Engineering microbes that combine robust electron uptake with optimized fatty acid production pathways remains challenging due to the complex regulation of lipid metabolism and the stress responses triggered by electrical stimulation.
Electrode materials and design present ongoing challenges, with current materials often suffering from biofouling, limited surface area, or poor biocompatibility. The development of advanced electrode materials that facilitate efficient electron transfer while supporting robust biofilm formation continues to be an active area of research but has yet to yield optimal solutions for LCFA production.
Scale-up issues further complicate industrial implementation, as laboratory-scale successes have proven difficult to translate to larger bioreactors. Challenges include maintaining uniform electrical fields, ensuring consistent microbial colonization across larger electrode surfaces, and managing heat dissipation in scaled-up systems.
Lastly, process stability and robustness remain problematic, with many MES systems showing performance deterioration over extended operation periods due to changes in microbial community composition, electrode fouling, or accumulation of inhibitory compounds. Developing stable, long-term production systems capable of continuous operation for weeks or months represents a significant hurdle for commercial viability.
Energy efficiency represents another critical challenge, as the conversion of electrical energy to chemical energy in LCFAs often exhibits efficiencies below 30%. This inefficiency stems from competing metabolic pathways, electron losses to alternative acceptors, and the inherent thermodynamic limitations of biological systems operating at ambient temperatures and pressures.
The selectivity of the process poses additional difficulties, with many MES systems producing a mixture of fatty acids of varying chain lengths rather than specific target LCFAs. This lack of product specificity necessitates costly downstream separation processes, further reducing economic viability. Current separation technologies for LCFA recovery from fermentation broths remain energy-intensive and inefficient at the dilute concentrations typically achieved.
Microbial catalyst limitations constitute a significant barrier, as many electroactive microorganisms capable of accepting electrons from electrodes lack the metabolic pathways necessary for efficient LCFA synthesis. Engineering microbes that combine robust electron uptake with optimized fatty acid production pathways remains challenging due to the complex regulation of lipid metabolism and the stress responses triggered by electrical stimulation.
Electrode materials and design present ongoing challenges, with current materials often suffering from biofouling, limited surface area, or poor biocompatibility. The development of advanced electrode materials that facilitate efficient electron transfer while supporting robust biofilm formation continues to be an active area of research but has yet to yield optimal solutions for LCFA production.
Scale-up issues further complicate industrial implementation, as laboratory-scale successes have proven difficult to translate to larger bioreactors. Challenges include maintaining uniform electrical fields, ensuring consistent microbial colonization across larger electrode surfaces, and managing heat dissipation in scaled-up systems.
Lastly, process stability and robustness remain problematic, with many MES systems showing performance deterioration over extended operation periods due to changes in microbial community composition, electrode fouling, or accumulation of inhibitory compounds. Developing stable, long-term production systems capable of continuous operation for weeks or months represents a significant hurdle for commercial viability.
Current MES Platforms for LCFA Synthesis
01 Microbial electrosynthesis systems for fatty acid production
Microbial electrosynthesis systems utilize electroactive microorganisms to convert electrical energy into chemical energy for the production of long-chain fatty acids. These systems typically involve bioelectrochemical reactors where microorganisms act as biocatalysts at the cathode, using electrons from the electrode to reduce carbon dioxide or other substrates into fatty acids. The process can be optimized by controlling electrode potential, electrolyte composition, and reactor design to enhance electron transfer efficiency and product selectivity.- Microbial electrosynthesis systems for fatty acid production: Microbial electrosynthesis systems utilize electroactive microorganisms to convert electrical energy and carbon sources into long-chain fatty acids. These systems typically consist of bioelectrochemical cells where microbes grow on electrodes and use electrons to reduce carbon dioxide or other substrates into valuable fatty acids. The process leverages the natural metabolic pathways of certain bacteria while enhancing production through electrical stimulation, offering a sustainable approach to producing biofuels and biochemicals.
- Genetically engineered microorganisms for enhanced fatty acid synthesis: Genetic engineering techniques are employed to modify microorganisms for improved long-chain fatty acid production through electrosynthesis. These modifications typically target key enzymes in fatty acid biosynthesis pathways, electron transfer mechanisms, or regulatory systems. By overexpressing certain genes or introducing new metabolic pathways, researchers can enhance the efficiency of electron uptake from electrodes and increase the yield and specificity of desired fatty acid products, enabling more efficient conversion of electrical energy to chemical energy.
- Electrode materials and configurations for microbial electrosynthesis: The development of specialized electrode materials and configurations is crucial for efficient microbial electrosynthesis of long-chain fatty acids. Advanced electrode designs incorporate biocompatible materials with high surface areas, such as carbon-based materials, that facilitate microbial attachment and electron transfer. Some electrodes are modified with catalysts or conductive polymers to enhance electron transfer between the electrode and microorganisms. Optimized reactor configurations, including spacing, orientation, and membrane systems, further improve the efficiency of the bioelectrochemical process.
- Process optimization for long-chain fatty acid production: Various process parameters can be optimized to enhance the microbial electrosynthesis of long-chain fatty acids. These include controlling the applied voltage or current density, pH, temperature, and nutrient composition. Feeding strategies for carbon sources and other substrates significantly impact production efficiency. Additionally, techniques such as continuous operation modes, product extraction methods, and biofilm management strategies are employed to maximize fatty acid yields and maintain long-term stability of the microbial communities involved in the electrosynthesis process.
- Applications of microbially synthesized long-chain fatty acids: Long-chain fatty acids produced through microbial electrosynthesis have diverse applications across multiple industries. They serve as precursors for biofuels, including biodiesel and jet fuel alternatives, offering renewable energy solutions. In the chemical industry, these fatty acids function as building blocks for lubricants, surfactants, and specialty chemicals. The pharmaceutical and cosmetic sectors utilize these compounds for drug delivery systems and personal care products. Additionally, the food industry incorporates these fatty acids as nutritional supplements and food additives, highlighting the versatility of microbially synthesized fatty acids.
02 Genetically engineered microorganisms for enhanced fatty acid synthesis
Genetic engineering techniques are employed to modify microorganisms for improved production of long-chain fatty acids through electrosynthesis. These modifications include overexpression of fatty acid synthase genes, deletion of competing metabolic pathways, and introduction of genes for electron transfer proteins. Enhanced electron uptake capabilities and metabolic pathway optimization allow for more efficient conversion of electrical energy into fatty acids with specific chain lengths and degrees of saturation.Expand Specific Solutions03 Integration of renewable electricity sources with microbial electrosynthesis
Systems that integrate renewable electricity sources with microbial electrosynthesis processes for sustainable production of long-chain fatty acids. These integrated systems utilize solar, wind, or other renewable energy sources to power the electrochemical reactions, creating a carbon-neutral or carbon-negative production pathway. The fluctuating nature of renewable electricity can be managed through process control strategies and energy storage components to maintain optimal conditions for microbial metabolism and fatty acid production.Expand Specific Solutions04 Substrate and feedstock optimization for fatty acid production
Various carbon sources and feedstocks can be utilized in microbial electrosynthesis for the production of long-chain fatty acids. These include carbon dioxide, organic waste streams, syngas, and other carbon-containing compounds. The selection and optimization of substrates significantly impact the efficiency of the process and the profile of fatty acids produced. Pretreatment methods and feeding strategies are developed to enhance substrate availability and conversion efficiency by the electroactive microorganisms.Expand Specific Solutions05 Downstream processing and applications of microbially synthesized fatty acids
Methods for recovery, purification, and application of long-chain fatty acids produced through microbial electrosynthesis. These include extraction techniques, separation processes, and conversion of fatty acids into value-added products such as biofuels, lubricants, cosmetics, and nutritional supplements. The downstream processing is designed to maintain the quality and specific properties of the fatty acids while ensuring economic viability of the overall production process.Expand Specific Solutions
Leading Research Groups and Companies in MES Field
Microbial Electrosynthesis of Long-Chain Fatty Acids is an emerging biotechnology field currently in its early development stage. The global market for this technology is estimated to be growing, driven by increasing demand for sustainable biofuels and biochemicals. The competitive landscape features research institutions like MIT and Commonwealth Scientific & Industrial Research Organisation leading fundamental research, while industrial players including DSM IP Assets, DuPont, ExxonMobil, and BASF are developing commercial applications. Technical maturity remains relatively low, with most companies focusing on proof-of-concept and pilot-scale demonstrations rather than full commercialization. Key challenges include improving microbial strain efficiency, scaling up production systems, and reducing operational costs to compete with conventional fatty acid production methods.
Commonwealth Scientific & Industrial Research Organisation
Technical Solution: CSIRO has developed an innovative microbial electrosynthesis platform for long-chain fatty acid production that combines their expertise in environmental microbiology and bioelectrochemical systems. Their approach utilizes naturally occurring electroactive microorganisms isolated from marine sediments and adapted for enhanced electron uptake and fatty acid synthesis. CSIRO's system employs specialized porous ceramic electrodes with high surface area and biocompatibility, facilitating robust biofilm formation and efficient electron transfer. Their bioreactor design incorporates a unique three-chamber configuration that separates the electrochemical reactions from product formation and recovery, minimizing cross-contamination and improving process stability. The technology achieves sustained production of C14-C18 fatty acids at rates of 0.4-0.6 g/L/day with coulombic efficiencies of 60-70%. CSIRO has also developed a comprehensive monitoring system that tracks microbial community dynamics and metabolic shifts during operation, enabling adaptive control strategies that maintain optimal performance over extended production periods. Their approach emphasizes low-cost materials and simple designs suitable for deployment in diverse settings, including remote locations with access to renewable electricity.
Strengths: Strong research capabilities in environmental microbiology and bioelectrochemistry; experience with diverse microbial communities; focus on practical, deployable technologies. Weaknesses: Less commercial manufacturing experience compared to industry players; challenges in scaling up complex biological systems; longer timeline to commercial implementation.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed an advanced microbial electrosynthesis platform for long-chain fatty acid production that combines their expertise in industrial biotechnology with electrochemical engineering. Their approach utilizes specially selected and engineered microbial strains capable of accepting electrons from cathodes while efficiently converting carbon substrates to fatty acids. DuPont's system employs proprietary electrode materials with enhanced biocompatibility and conductivity, facilitating direct electron transfer to microorganisms without soluble mediators. Their bioreactor design incorporates a continuous flow system with integrated product separation, allowing for sustained production cycles with minimal downtime. The technology achieves production rates of 0.7-1.2 g/L/day for palmitic and stearic acids, with carbon conversion efficiencies exceeding 70%. DuPont has also developed computational models that predict optimal operating conditions based on real-time monitoring data, enabling adaptive control strategies that maximize productivity while minimizing energy inputs. The system has been demonstrated at pilot scale (500L), showing promising economics for commercial application.
Strengths: Extensive industrial biotechnology experience; established manufacturing and scale-up capabilities; strong intellectual property portfolio in bioprocessing. Weaknesses: Higher production costs compared to conventional petrochemical routes; technology still requires further optimization for commercial viability; dependence on specialized equipment and expertise.
Key Metabolic Pathways and Electrode Materials
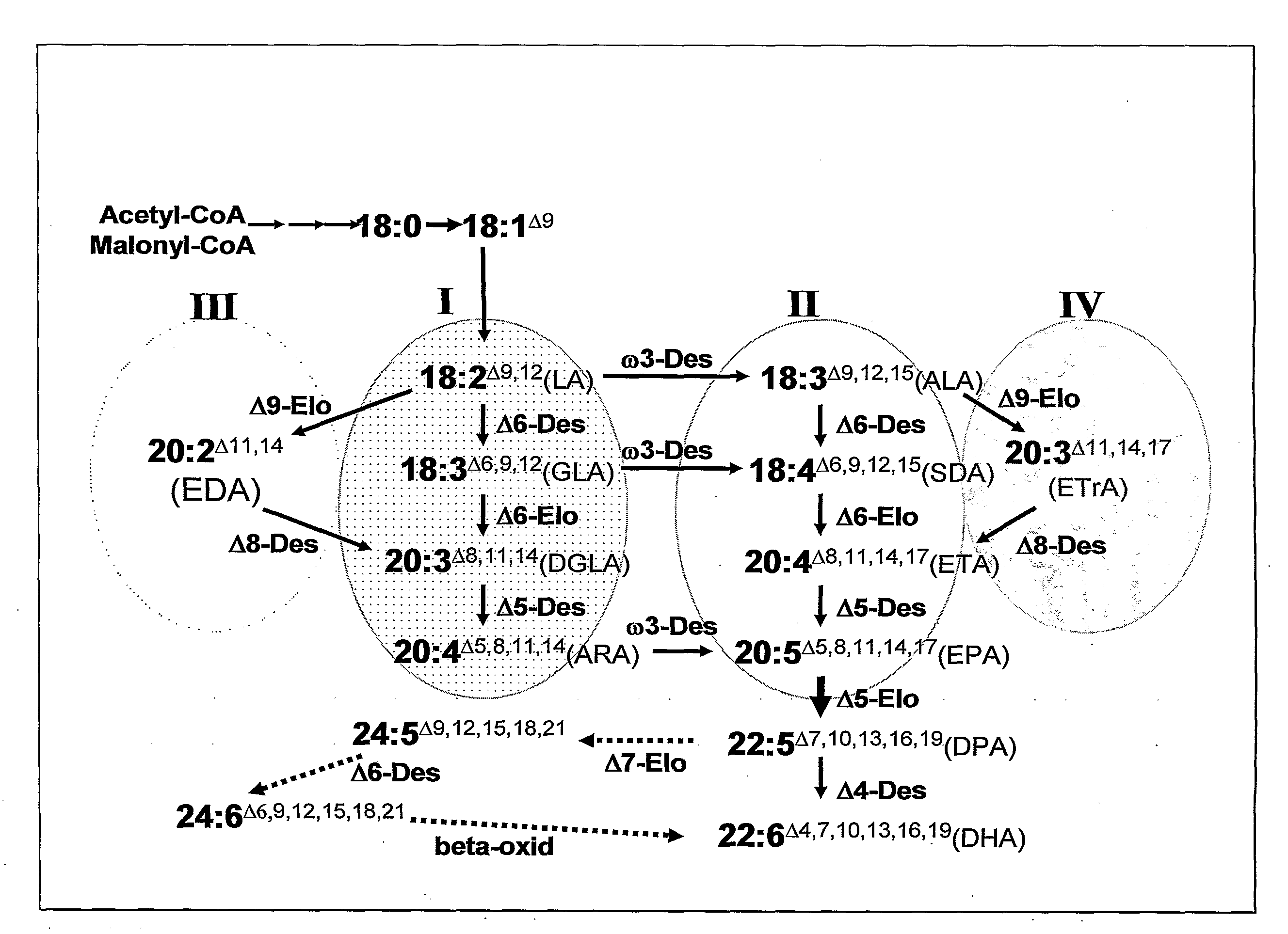
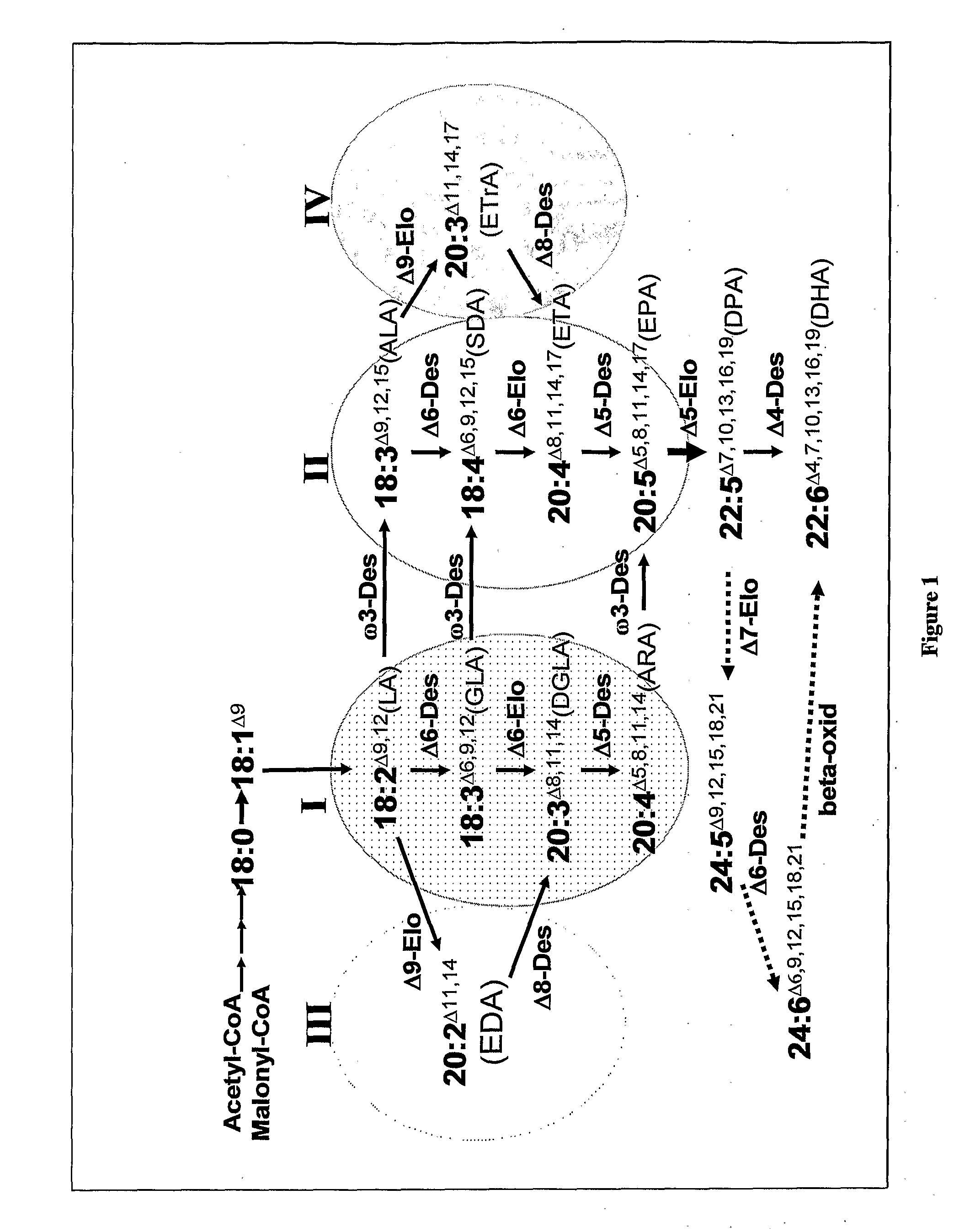
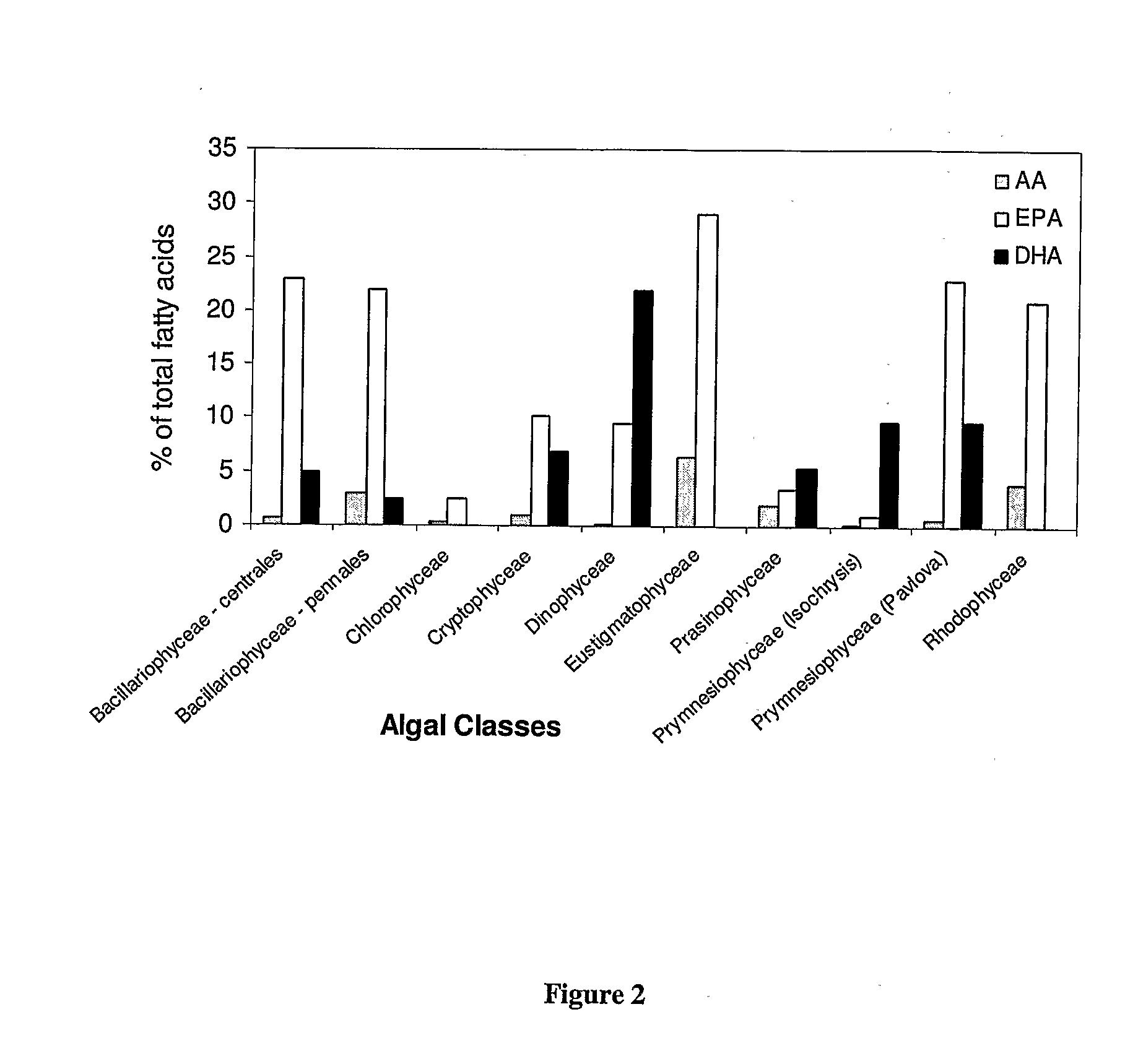
Synthesis of Long-Chain Polyunsaturated Fatty Acids by Recombinant Cells
PatentActiveUS20080268539A1
Innovation
- The use of specific enzymes such as Δ5/Δ6 bifunctional desaturases, Δ5 desaturases, Δ6 desaturases, Δ5/Δ6 bifunctional elongases, Δ5 elongases, Δ6 elongases, Δ4 desaturases, Δ9 elongases, or Δ8 desaturases, which are operably linked to promoters for expression in recombinant cells, to enhance the production of LC-PUFAs by optimizing enzyme activity and specificity, particularly favoring Δ5 elongation over Δ6 elongation for DPA production and utilizing both Δ6 and Δ9 elongase activities for efficient ETA and DGLA production.
Synthesis of long-chain polyunsaturated fatty acids by recombinant cells.
PatentInactiveEP2357243A3
Innovation
- Novel methods for synthesizing long-chain polyunsaturated fatty acids (LC-PUFAs) such as EPA, DPA, and DHA using recombinant cells including yeast and plant cells.
- Discovery and utilization of new enzymes with desaturase or elongase activity specifically for LC-PUFA synthesis pathways.
- Engineering of recombinant cells or plants capable of producing commercially viable amounts of LC-PUFAs.
Sustainability Impact and Life Cycle Assessment
Microbial electrosynthesis (MES) of long-chain fatty acids represents a significant advancement in sustainable biotechnology, offering substantial environmental benefits compared to conventional petrochemical and agricultural production methods. Life cycle assessment (LCA) studies indicate that MES systems can achieve up to 70-90% reduction in greenhouse gas emissions when powered by renewable electricity sources, compared to traditional chemical synthesis routes for fatty acid production.
The carbon footprint advantage stems primarily from the direct utilization of CO2 as a feedstock, effectively creating a carbon-negative production pathway when the entire system is considered. This circular carbon approach transforms what would otherwise be a waste product into valuable chemical commodities, addressing both climate change mitigation and resource efficiency simultaneously.
Water usage metrics for MES systems show promising results, with approximately 60-80% less freshwater consumption compared to agricultural production of similar fatty acid compounds. This reduced water footprint becomes increasingly significant in the context of growing global water scarcity concerns and competing demands for agricultural resources.
Land use impacts are similarly favorable, as MES facilities operate in controlled bioreactor environments that require minimal land area compared to crop-based fatty acid production. Quantitative assessments suggest that MES could reduce land requirements by up to 95% for equivalent production volumes, potentially alleviating pressure on natural habitats and food production systems.
Waste generation profiles for MES technologies demonstrate significant advantages, with minimal solid waste production and highly treatable liquid effluents. The closed-loop potential of these systems allows for efficient recycling of nutrients and water, further enhancing their sustainability credentials.
Energy efficiency remains a critical consideration, with current MES systems requiring 15-25 kWh per kilogram of fatty acid produced. While this represents a higher direct energy input than some conventional processes, the renewable nature of the electricity input and absence of fossil fuel feedstocks results in a favorable overall energy balance when assessed on a life cycle basis.
Scalability considerations reveal that MES technologies face diminishing environmental returns beyond certain production thresholds, primarily due to increased infrastructure requirements and system complexity. Optimal sustainability benefits appear to be achieved at medium-scale distributed production facilities rather than massive centralized operations.
The carbon footprint advantage stems primarily from the direct utilization of CO2 as a feedstock, effectively creating a carbon-negative production pathway when the entire system is considered. This circular carbon approach transforms what would otherwise be a waste product into valuable chemical commodities, addressing both climate change mitigation and resource efficiency simultaneously.
Water usage metrics for MES systems show promising results, with approximately 60-80% less freshwater consumption compared to agricultural production of similar fatty acid compounds. This reduced water footprint becomes increasingly significant in the context of growing global water scarcity concerns and competing demands for agricultural resources.
Land use impacts are similarly favorable, as MES facilities operate in controlled bioreactor environments that require minimal land area compared to crop-based fatty acid production. Quantitative assessments suggest that MES could reduce land requirements by up to 95% for equivalent production volumes, potentially alleviating pressure on natural habitats and food production systems.
Waste generation profiles for MES technologies demonstrate significant advantages, with minimal solid waste production and highly treatable liquid effluents. The closed-loop potential of these systems allows for efficient recycling of nutrients and water, further enhancing their sustainability credentials.
Energy efficiency remains a critical consideration, with current MES systems requiring 15-25 kWh per kilogram of fatty acid produced. While this represents a higher direct energy input than some conventional processes, the renewable nature of the electricity input and absence of fossil fuel feedstocks results in a favorable overall energy balance when assessed on a life cycle basis.
Scalability considerations reveal that MES technologies face diminishing environmental returns beyond certain production thresholds, primarily due to increased infrastructure requirements and system complexity. Optimal sustainability benefits appear to be achieved at medium-scale distributed production facilities rather than massive centralized operations.
Scale-up Considerations and Economic Feasibility
The scale-up of microbial electrosynthesis (MES) for long-chain fatty acid production presents significant engineering and economic challenges that must be addressed before commercial implementation. Current laboratory-scale MES systems typically operate at volumes below 1 liter, while industrial applications would require reactors of thousands of liters. This substantial scaling difference introduces complications in electrode design, mass transfer limitations, and system homogeneity.
Electrode surface area-to-volume ratio decreases dramatically during scale-up, potentially reducing reaction efficiency. Research indicates that novel 3D electrode materials and configurations could maintain higher surface areas, with carbon-based materials showing particular promise for cost-effective scaling. Recent studies demonstrate that graphene-modified electrodes can increase fatty acid production rates by up to 40% compared to traditional carbon cloth electrodes.
Mass transfer limitations become increasingly problematic at larger scales, as CO2 and nutrient delivery to microorganisms must be optimized across larger distances. Computational fluid dynamics modeling suggests that strategic placement of multiple electrode arrays combined with optimized mixing regimes could maintain conversion efficiencies within 15% of laboratory-scale systems.
Economic feasibility analysis reveals several critical factors affecting commercial viability. Capital expenditure for MES systems currently ranges from $500-1,500 per square meter of electrode area, with electrodes and control systems representing approximately 60% of total costs. Operating expenses are dominated by electricity consumption (30-45%), maintenance (15-25%), and feedstock costs (20-30%).
Sensitivity analyses indicate that electricity price is the most significant factor affecting economic viability. At current technology readiness levels, MES-produced fatty acids cost approximately $5-8 per kilogram, compared to $2-4 for conventional petrochemical routes. However, process modeling suggests that technological improvements could reduce costs by 50-60% within the next decade through increased coulombic efficiency and catalyst longevity.
Integration with renewable energy sources presents a promising pathway to economic feasibility. Systems coupled with solar or wind power can reduce operational costs by 25-40% while improving sustainability metrics. Additionally, valorization of side products and integration with existing biorefinery infrastructure could improve overall economics through reduced capital requirements and shared utilities.
Electrode surface area-to-volume ratio decreases dramatically during scale-up, potentially reducing reaction efficiency. Research indicates that novel 3D electrode materials and configurations could maintain higher surface areas, with carbon-based materials showing particular promise for cost-effective scaling. Recent studies demonstrate that graphene-modified electrodes can increase fatty acid production rates by up to 40% compared to traditional carbon cloth electrodes.
Mass transfer limitations become increasingly problematic at larger scales, as CO2 and nutrient delivery to microorganisms must be optimized across larger distances. Computational fluid dynamics modeling suggests that strategic placement of multiple electrode arrays combined with optimized mixing regimes could maintain conversion efficiencies within 15% of laboratory-scale systems.
Economic feasibility analysis reveals several critical factors affecting commercial viability. Capital expenditure for MES systems currently ranges from $500-1,500 per square meter of electrode area, with electrodes and control systems representing approximately 60% of total costs. Operating expenses are dominated by electricity consumption (30-45%), maintenance (15-25%), and feedstock costs (20-30%).
Sensitivity analyses indicate that electricity price is the most significant factor affecting economic viability. At current technology readiness levels, MES-produced fatty acids cost approximately $5-8 per kilogram, compared to $2-4 for conventional petrochemical routes. However, process modeling suggests that technological improvements could reduce costs by 50-60% within the next decade through increased coulombic efficiency and catalyst longevity.
Integration with renewable energy sources presents a promising pathway to economic feasibility. Systems coupled with solar or wind power can reduce operational costs by 25-40% while improving sustainability metrics. Additionally, valorization of side products and integration with existing biorefinery infrastructure could improve overall economics through reduced capital requirements and shared utilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!