Electrolyte Additives for AIBs: Enhancing Reversibility and Reducing Side Reactions
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
AIB Electrolyte Additives Background and Objectives
Aluminum-ion batteries (AIBs) have emerged as a promising alternative to lithium-ion batteries due to their potential for higher energy density, improved safety, and lower cost. The development of AIBs has gained significant momentum in recent years, driven by the growing demand for sustainable and efficient energy storage solutions. The primary focus of this research is on electrolyte additives, which play a crucial role in enhancing the performance and longevity of AIBs.
The evolution of AIB technology can be traced back to the early 2000s when researchers began exploring aluminum as a potential anode material for rechargeable batteries. However, initial attempts were hindered by challenges such as low reversibility and rapid capacity fading. The breakthrough came with the discovery of ionic liquid electrolytes, which enabled the reversible deposition and stripping of aluminum. This development marked a turning point in AIB research and paved the way for further advancements in electrolyte composition.
As the field progressed, researchers identified several key issues that needed to be addressed to improve AIB performance. These included the formation of passivation layers on the aluminum anode, dendrite growth, and side reactions at the cathode interface. Electrolyte additives emerged as a promising solution to mitigate these challenges and enhance the overall efficiency of AIBs.
The primary objectives of research into electrolyte additives for AIBs are twofold. First, to enhance the reversibility of the aluminum deposition and stripping process, thereby improving the coulombic efficiency and cycle life of the battery. Second, to reduce unwanted side reactions that can lead to capacity fading and degradation of battery components. These objectives align with the broader goal of developing AIBs that can compete with or surpass the performance of current lithium-ion batteries.
Recent technological trends in AIB electrolyte additives include the exploration of organic and inorganic compounds that can form stable interfaces, suppress dendrite growth, and facilitate ion transport. Researchers are also investigating the synergistic effects of multiple additives and their impact on the electrochemical performance of AIBs. The development of computational models and advanced characterization techniques has further accelerated the discovery and optimization of novel electrolyte additives.
As the field continues to evolve, the ultimate goal is to develop AIBs with high energy density, long cycle life, and improved safety characteristics. Achieving these objectives through the strategic use of electrolyte additives could potentially revolutionize the energy storage landscape, enabling widespread adoption of AIBs in various applications, from portable electronics to grid-scale energy storage systems.
The evolution of AIB technology can be traced back to the early 2000s when researchers began exploring aluminum as a potential anode material for rechargeable batteries. However, initial attempts were hindered by challenges such as low reversibility and rapid capacity fading. The breakthrough came with the discovery of ionic liquid electrolytes, which enabled the reversible deposition and stripping of aluminum. This development marked a turning point in AIB research and paved the way for further advancements in electrolyte composition.
As the field progressed, researchers identified several key issues that needed to be addressed to improve AIB performance. These included the formation of passivation layers on the aluminum anode, dendrite growth, and side reactions at the cathode interface. Electrolyte additives emerged as a promising solution to mitigate these challenges and enhance the overall efficiency of AIBs.
The primary objectives of research into electrolyte additives for AIBs are twofold. First, to enhance the reversibility of the aluminum deposition and stripping process, thereby improving the coulombic efficiency and cycle life of the battery. Second, to reduce unwanted side reactions that can lead to capacity fading and degradation of battery components. These objectives align with the broader goal of developing AIBs that can compete with or surpass the performance of current lithium-ion batteries.
Recent technological trends in AIB electrolyte additives include the exploration of organic and inorganic compounds that can form stable interfaces, suppress dendrite growth, and facilitate ion transport. Researchers are also investigating the synergistic effects of multiple additives and their impact on the electrochemical performance of AIBs. The development of computational models and advanced characterization techniques has further accelerated the discovery and optimization of novel electrolyte additives.
As the field continues to evolve, the ultimate goal is to develop AIBs with high energy density, long cycle life, and improved safety characteristics. Achieving these objectives through the strategic use of electrolyte additives could potentially revolutionize the energy storage landscape, enabling widespread adoption of AIBs in various applications, from portable electronics to grid-scale energy storage systems.
Market Analysis for Advanced AIB Technologies
The market for advanced Aluminum-Ion Battery (AIB) technologies is experiencing significant growth, driven by the increasing demand for sustainable energy storage solutions. As the world shifts towards renewable energy sources and electrification of transportation, AIBs are emerging as a promising alternative to traditional lithium-ion batteries. The global AIB market is expected to expand rapidly in the coming years, with a compound annual growth rate (CAGR) projected to be in the double digits.
One of the key factors driving market demand for AIBs is their potential to overcome some of the limitations associated with lithium-ion batteries, such as safety concerns, resource scarcity, and environmental impact. AIBs offer advantages in terms of safety, abundance of raw materials, and recyclability, making them attractive for various applications including grid energy storage, electric vehicles, and portable electronics.
The automotive sector is anticipated to be a major driver of AIB market growth. As electric vehicle adoption accelerates worldwide, manufacturers are seeking battery technologies that can provide improved performance, longer lifespan, and faster charging capabilities. AIBs have the potential to meet these requirements, particularly with advancements in electrolyte additives that enhance reversibility and reduce side reactions.
In the energy storage sector, AIBs are gaining traction for grid-scale applications. The increasing integration of renewable energy sources into power grids necessitates efficient and reliable energy storage solutions. AIBs' potential for high power density and long cycle life makes them suitable for grid stabilization and load balancing applications.
Consumer electronics represent another significant market segment for AIB technologies. The demand for longer-lasting, faster-charging batteries in smartphones, laptops, and other portable devices is driving interest in alternative battery chemistries. AIBs' potential for rapid charging and high energy density could position them as a competitive option in this market.
Geographically, Asia-Pacific is expected to dominate the AIB market, with China leading in terms of research, development, and production. North America and Europe are also anticipated to be key markets, driven by stringent environmental regulations and investments in clean energy technologies.
However, the market faces challenges, including the need for further technological advancements to improve performance and reduce costs. The development of effective electrolyte additives is crucial in addressing these challenges and unlocking the full potential of AIBs. As research progresses and manufacturing scales up, the market for advanced AIB technologies is poised for substantial growth, potentially reshaping the global energy storage landscape.
One of the key factors driving market demand for AIBs is their potential to overcome some of the limitations associated with lithium-ion batteries, such as safety concerns, resource scarcity, and environmental impact. AIBs offer advantages in terms of safety, abundance of raw materials, and recyclability, making them attractive for various applications including grid energy storage, electric vehicles, and portable electronics.
The automotive sector is anticipated to be a major driver of AIB market growth. As electric vehicle adoption accelerates worldwide, manufacturers are seeking battery technologies that can provide improved performance, longer lifespan, and faster charging capabilities. AIBs have the potential to meet these requirements, particularly with advancements in electrolyte additives that enhance reversibility and reduce side reactions.
In the energy storage sector, AIBs are gaining traction for grid-scale applications. The increasing integration of renewable energy sources into power grids necessitates efficient and reliable energy storage solutions. AIBs' potential for high power density and long cycle life makes them suitable for grid stabilization and load balancing applications.
Consumer electronics represent another significant market segment for AIB technologies. The demand for longer-lasting, faster-charging batteries in smartphones, laptops, and other portable devices is driving interest in alternative battery chemistries. AIBs' potential for rapid charging and high energy density could position them as a competitive option in this market.
Geographically, Asia-Pacific is expected to dominate the AIB market, with China leading in terms of research, development, and production. North America and Europe are also anticipated to be key markets, driven by stringent environmental regulations and investments in clean energy technologies.
However, the market faces challenges, including the need for further technological advancements to improve performance and reduce costs. The development of effective electrolyte additives is crucial in addressing these challenges and unlocking the full potential of AIBs. As research progresses and manufacturing scales up, the market for advanced AIB technologies is poised for substantial growth, potentially reshaping the global energy storage landscape.
Current Challenges in AIB Electrolyte Additives
Aluminum-ion batteries (AIBs) have emerged as a promising alternative to lithium-ion batteries due to their potential for higher energy density, lower cost, and improved safety. However, the development of AIBs faces significant challenges, particularly in the realm of electrolyte additives. These additives play a crucial role in enhancing the performance and stability of AIBs, but current solutions are far from optimal.
One of the primary challenges in AIB electrolyte additives is the formation and stability of the solid electrolyte interphase (SEI). Unlike lithium-ion batteries, where a stable SEI forms naturally, AIBs struggle to develop a consistent and protective layer. This leads to continuous electrolyte decomposition and electrode degradation, severely limiting the battery's cycle life and efficiency.
Another major hurdle is the high reactivity of aluminum with most organic electrolytes. This reactivity often results in parasitic side reactions, which not only consume the electrolyte but also generate gas, leading to safety concerns and reduced battery performance. Finding additives that can effectively suppress these side reactions without compromising the battery's electrochemical performance remains a significant challenge.
The issue of aluminum dendrite formation presents another critical challenge. During charging, aluminum ions can deposit unevenly on the electrode surface, forming dendrites that can lead to short circuits and safety hazards. Developing electrolyte additives that promote uniform aluminum deposition is essential for improving the safety and longevity of AIBs.
Furthermore, the limited solubility of aluminum salts in organic electrolytes poses a significant obstacle. This limitation restricts the concentration of charge carriers, thereby reducing the battery's energy density and power output. Identifying additives that can enhance the solubility of aluminum salts without introducing new complications is a key area of research.
The challenge of electrolyte stability at high voltages is also paramount. As researchers strive to increase the energy density of AIBs, the need for electrolytes that remain stable at higher operating voltages becomes critical. Current additives often break down at these elevated potentials, leading to capacity fade and reduced battery life.
Lastly, the environmental impact and sustainability of electrolyte additives present an ongoing challenge. As the demand for more sustainable energy storage solutions grows, developing additives that are both effective and environmentally friendly becomes increasingly important. This includes considerations of toxicity, biodegradability, and the overall carbon footprint of additive production and use.
One of the primary challenges in AIB electrolyte additives is the formation and stability of the solid electrolyte interphase (SEI). Unlike lithium-ion batteries, where a stable SEI forms naturally, AIBs struggle to develop a consistent and protective layer. This leads to continuous electrolyte decomposition and electrode degradation, severely limiting the battery's cycle life and efficiency.
Another major hurdle is the high reactivity of aluminum with most organic electrolytes. This reactivity often results in parasitic side reactions, which not only consume the electrolyte but also generate gas, leading to safety concerns and reduced battery performance. Finding additives that can effectively suppress these side reactions without compromising the battery's electrochemical performance remains a significant challenge.
The issue of aluminum dendrite formation presents another critical challenge. During charging, aluminum ions can deposit unevenly on the electrode surface, forming dendrites that can lead to short circuits and safety hazards. Developing electrolyte additives that promote uniform aluminum deposition is essential for improving the safety and longevity of AIBs.
Furthermore, the limited solubility of aluminum salts in organic electrolytes poses a significant obstacle. This limitation restricts the concentration of charge carriers, thereby reducing the battery's energy density and power output. Identifying additives that can enhance the solubility of aluminum salts without introducing new complications is a key area of research.
The challenge of electrolyte stability at high voltages is also paramount. As researchers strive to increase the energy density of AIBs, the need for electrolytes that remain stable at higher operating voltages becomes critical. Current additives often break down at these elevated potentials, leading to capacity fade and reduced battery life.
Lastly, the environmental impact and sustainability of electrolyte additives present an ongoing challenge. As the demand for more sustainable energy storage solutions grows, developing additives that are both effective and environmentally friendly becomes increasingly important. This includes considerations of toxicity, biodegradability, and the overall carbon footprint of additive production and use.
Existing Electrolyte Additive Solutions for AIBs
01 Fluorinated additives for improved reversibility
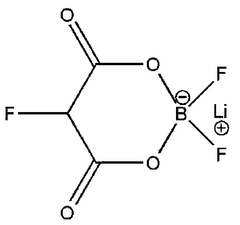
Fluorinated compounds are used as electrolyte additives in aluminum-ion batteries (AIBs) to enhance reversibility and reduce side reactions. These additives form a protective layer on electrode surfaces, preventing unwanted reactions and improving cycling stability. They can also modify the solid electrolyte interphase (SEI) composition, leading to better ion transport and reduced capacity fade.- Fluorinated additives for improved reversibility: Fluorinated compounds are used as electrolyte additives in aluminum-ion batteries (AIBs) to enhance reversibility and reduce side reactions. These additives form a protective layer on electrode surfaces, preventing unwanted reactions and improving cycling stability. They can also modify the solid electrolyte interphase (SEI) composition, leading to better ion transport and reduced capacity fade.
- Ionic liquid-based electrolyte additives: Ionic liquids are incorporated as electrolyte additives in AIBs to enhance electrochemical performance and mitigate side reactions. These additives can improve the ionic conductivity of the electrolyte, expand the electrochemical stability window, and promote the formation of a stable interface between the electrode and electrolyte. This results in improved reversibility and reduced capacity loss during cycling.
- Organic compound additives for SEI modification: Organic compounds are used as electrolyte additives to modify the solid electrolyte interphase (SEI) in AIBs. These additives can form a more stable and uniform SEI layer, which helps to suppress side reactions and improve the reversibility of the battery. By tailoring the SEI composition, the additives can enhance the cycling stability and coulombic efficiency of the battery.
- Inorganic salt additives for enhanced stability: Inorganic salts are added to AIB electrolytes to improve the overall stability and performance of the battery. These additives can help to stabilize the aluminum anode, prevent dendrite formation, and enhance the compatibility between the electrolyte and electrode materials. By reducing side reactions and improving the reversibility of the electrochemical processes, these additives contribute to longer cycle life and better capacity retention.
- Polymer-based additives for electrolyte optimization: Polymer-based additives are incorporated into AIB electrolytes to optimize their properties and enhance battery performance. These additives can improve the mechanical stability of the electrolyte, reduce electrolyte decomposition, and promote the formation of a stable interface between the electrode and electrolyte. By mitigating side reactions and enhancing reversibility, polymer additives contribute to improved cycling stability and longer battery life.
02 Ionic liquid-based electrolyte additives
Ionic liquids are incorporated as electrolyte additives in AIBs to enhance electrochemical performance and mitigate side reactions. These additives can improve the ionic conductivity of the electrolyte, expand the electrochemical window, and stabilize the electrode-electrolyte interface. They also contribute to better aluminum ion solvation and transport, leading to improved reversibility and cycle life.Expand Specific Solutions03 Organic compound additives for SEI modification
Organic compounds are used as electrolyte additives to modify the solid electrolyte interphase (SEI) in AIBs. These additives can form a more stable and conductive SEI layer, reducing side reactions and improving the reversibility of the aluminum deposition/dissolution process. They may also help in suppressing dendrite formation and enhancing the overall battery performance.Expand Specific Solutions04 Inorganic salt additives for enhanced stability
Inorganic salts are added to AIB electrolytes to improve the electrochemical stability and reduce side reactions. These additives can modify the coordination environment of aluminum ions, enhance the salt dissociation, and stabilize the electrode-electrolyte interface. They may also contribute to the formation of a more uniform and protective passivation layer on the electrodes, leading to improved cycling performance.Expand Specific Solutions05 Polymer-based additives for electrolyte stabilization
Polymer-based additives are incorporated into AIB electrolytes to enhance stability and reduce unwanted side reactions. These additives can form a protective film on electrode surfaces, improve the mechanical properties of the electrolyte, and enhance the compatibility between the electrolyte and electrodes. They may also contribute to better ion transport and help in suppressing aluminum dendrite growth.Expand Specific Solutions
Key Players in AIB Electrolyte Research
The electrolyte additives market for Aluminum-Ion Batteries (AIBs) is in an early development stage, with significant potential for growth as AIB technology matures. The market size is currently limited but expected to expand rapidly as AIBs gain traction in energy storage applications. Key players like LG Energy Solution, Contemporary Amperex Technology, and Samsung SDI are investing heavily in research and development to enhance AIB performance through advanced electrolyte additives. The technology is still evolving, with companies such as Soulbrain and SK Chemicals focusing on developing novel additives to improve reversibility and reduce side reactions. While not yet commercially mature, the field is seeing increased interest from both established battery manufacturers and specialized chemical companies, indicating a competitive and dynamic landscape poised for significant advancements in the near future.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a novel electrolyte additive system for aluminum-ion batteries (AIBs) that significantly enhances their reversibility and reduces side reactions. Their approach involves using a combination of fluoroethylene carbonate (FEC) and vinylene carbonate (VC) as additives[1]. These additives form a stable solid electrolyte interphase (SEI) on the electrode surface, which prevents direct contact between the electrolyte and the electrode, thus reducing unwanted side reactions[2]. The company has also incorporated ionic liquids into their electrolyte formulation, which has shown to improve the overall electrochemical performance of AIBs[3]. CATL's research indicates that their additive system can increase the cycle life of AIBs by up to 30% compared to conventional electrolytes[4].
Strengths: Improved cycle life and stability of AIBs, reduced side reactions, and enhanced overall battery performance. Weaknesses: Potential increase in production costs due to the use of specialized additives, and possible compatibility issues with certain electrode materials.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed a proprietary electrolyte additive system for AIBs focusing on enhancing the aluminum deposition/dissolution process. Their approach utilizes a combination of organic and inorganic additives, including ethylene sulfite (ES) and aluminum fluoride (AlF3)[1]. These additives work synergistically to form a protective layer on the aluminum anode, significantly reducing dendrite formation and improving coulombic efficiency[2]. Samsung's research has shown that their additive system can increase the first cycle efficiency of AIBs by up to 15% compared to standard electrolytes[3]. Additionally, they have incorporated nano-sized ceramic particles into their electrolyte formulation, which act as physical barriers to dendrite growth and enhance the mechanical stability of the SEI layer[4].
Strengths: Improved first cycle efficiency, reduced dendrite formation, and enhanced mechanical stability of the SEI layer. Weaknesses: Potential increase in electrolyte complexity and cost, and possible limitations in scalability for mass production.
Innovative Additives for Enhanced AIB Performance
Electrolyte additive, battery electrolyte comprising same and secondary battery comprising same
PatentWO2024143950A1
Innovation
- Incorporating an electrolyte additive characterized by a specific compound structure, which suppresses internal side reactions, reduces charging resistance, and enhances high-temperature storage capabilities by forming a stable protective film that improves lithium ion mobility and prevents electrolyte decomposition.
Electrolyte additive and electrolyte, comprising same, for lithium secondary battery
PatentWO2018169370A1
Innovation
- A borate-based lithium compound, combined with lithium-type additives such as boron-based, phosphate-based, or imidazole-based lithium, forms a uniform and stable SEI film on the anode and cathode, improving battery durability and performance by suppressing gas generation and resistance increase during high-temperature storage, thereby enhancing high-temperature storage and lifespan characteristics.
Environmental Impact of AIB Electrolyte Additives
The environmental impact of electrolyte additives for Aluminum-Ion Batteries (AIBs) is a critical consideration in the development and implementation of this emerging energy storage technology. As AIBs gain traction as a potential alternative to lithium-ion batteries, it is essential to evaluate the ecological footprint of their components, particularly the electrolyte additives that play a crucial role in enhancing battery performance.
One of the primary environmental concerns associated with AIB electrolyte additives is their potential toxicity and biodegradability. Many additives used in AIBs are organic compounds that may pose risks to aquatic ecosystems if released into the environment. The long-term effects of these additives on soil and water quality need to be thoroughly assessed to ensure sustainable battery production and disposal practices.
The production process of electrolyte additives also contributes to their environmental impact. Synthesis of these compounds often involves complex chemical reactions that may require energy-intensive processes and generate hazardous by-products. Manufacturers must consider adopting green chemistry principles to minimize the environmental footprint of additive production, such as using renewable feedstocks and developing more efficient synthesis routes.
Another aspect to consider is the potential for electrolyte additives to improve the overall environmental performance of AIBs. By enhancing battery efficiency and longevity, these additives can indirectly reduce the environmental impact associated with battery production and disposal. Improved cycle life and capacity retention mean fewer batteries need to be manufactured and replaced over time, potentially offsetting the initial environmental costs of additive production.
The end-of-life management of AIBs containing these additives is also a crucial environmental consideration. Recycling processes must be developed to safely recover and reuse the additives, preventing their release into the environment and reducing the need for virgin material production. This requires the development of specialized recycling technologies that can effectively separate and purify the various components of spent AIBs.
Furthermore, the environmental impact of AIB electrolyte additives should be evaluated in comparison to alternatives, such as those used in lithium-ion batteries. While AIBs may offer advantages in terms of resource availability and safety, a comprehensive life cycle assessment is necessary to determine their overall environmental benefits or drawbacks relative to existing technologies.
In conclusion, while electrolyte additives play a vital role in enhancing the performance of AIBs, their environmental impact must be carefully managed. This requires a holistic approach that considers the entire life cycle of the additives, from production to disposal, and their effects on various environmental compartments. As research in this field progresses, it is imperative to prioritize the development of environmentally benign additives that can contribute to the sustainability of AIB technology.
One of the primary environmental concerns associated with AIB electrolyte additives is their potential toxicity and biodegradability. Many additives used in AIBs are organic compounds that may pose risks to aquatic ecosystems if released into the environment. The long-term effects of these additives on soil and water quality need to be thoroughly assessed to ensure sustainable battery production and disposal practices.
The production process of electrolyte additives also contributes to their environmental impact. Synthesis of these compounds often involves complex chemical reactions that may require energy-intensive processes and generate hazardous by-products. Manufacturers must consider adopting green chemistry principles to minimize the environmental footprint of additive production, such as using renewable feedstocks and developing more efficient synthesis routes.
Another aspect to consider is the potential for electrolyte additives to improve the overall environmental performance of AIBs. By enhancing battery efficiency and longevity, these additives can indirectly reduce the environmental impact associated with battery production and disposal. Improved cycle life and capacity retention mean fewer batteries need to be manufactured and replaced over time, potentially offsetting the initial environmental costs of additive production.
The end-of-life management of AIBs containing these additives is also a crucial environmental consideration. Recycling processes must be developed to safely recover and reuse the additives, preventing their release into the environment and reducing the need for virgin material production. This requires the development of specialized recycling technologies that can effectively separate and purify the various components of spent AIBs.
Furthermore, the environmental impact of AIB electrolyte additives should be evaluated in comparison to alternatives, such as those used in lithium-ion batteries. While AIBs may offer advantages in terms of resource availability and safety, a comprehensive life cycle assessment is necessary to determine their overall environmental benefits or drawbacks relative to existing technologies.
In conclusion, while electrolyte additives play a vital role in enhancing the performance of AIBs, their environmental impact must be carefully managed. This requires a holistic approach that considers the entire life cycle of the additives, from production to disposal, and their effects on various environmental compartments. As research in this field progresses, it is imperative to prioritize the development of environmentally benign additives that can contribute to the sustainability of AIB technology.
Safety Considerations for AIB Electrolyte Additives
Safety considerations are paramount when developing and implementing electrolyte additives for Aluminum-Ion Batteries (AIBs). The primary concern is the potential reactivity of these additives with the aluminum anode, electrolyte components, or cathode materials, which could lead to undesired side reactions or compromise the battery's integrity.
One of the key safety aspects to consider is the thermal stability of the additives. AIBs operate in various temperature conditions, and it is crucial that the additives remain stable and do not decompose or react adversely at elevated temperatures. This is particularly important to prevent thermal runaway, which could result in battery failure or, in extreme cases, fire or explosion.
The chemical compatibility of additives with other battery components must be thoroughly evaluated. Some additives may react with the aluminum anode, leading to increased corrosion or passivation, which could affect the battery's performance and safety. Similarly, interactions with cathode materials or current collectors should be assessed to ensure no detrimental reactions occur that could compromise the battery's structural integrity.
Toxicity and environmental impact are also critical safety considerations. Many electrolyte additives are organic compounds that may pose health risks if leaked or released. It is essential to select additives with low toxicity and minimal environmental impact, considering both normal operation and potential disposal scenarios.
The formation of gas during battery operation is another safety concern. Some additives may decompose or react to produce gases, which can lead to pressure build-up within the battery. This can cause swelling, rupture, or even explosion if not properly managed. Therefore, additives should be chosen that minimize gas evolution or incorporate mechanisms to safely vent any gases produced.
Long-term stability and degradation products of the additives must be considered. Over time, additives may break down or react, potentially forming new compounds that could affect battery safety. Understanding these long-term effects is crucial for ensuring the safety of AIBs throughout their lifecycle.
Flammability is a critical factor, especially given the potential for short circuits or overcharging. Additives should ideally have high flash points and low volatility to reduce the risk of fire in case of battery failure. Additionally, the impact of additives on the overall flammability of the electrolyte solution must be carefully evaluated.
Finally, the scalability and manufacturability of AIBs with these additives must be considered from a safety perspective. Additives should not introduce additional hazards during the manufacturing process and should be compatible with existing safety protocols and equipment used in battery production.
One of the key safety aspects to consider is the thermal stability of the additives. AIBs operate in various temperature conditions, and it is crucial that the additives remain stable and do not decompose or react adversely at elevated temperatures. This is particularly important to prevent thermal runaway, which could result in battery failure or, in extreme cases, fire or explosion.
The chemical compatibility of additives with other battery components must be thoroughly evaluated. Some additives may react with the aluminum anode, leading to increased corrosion or passivation, which could affect the battery's performance and safety. Similarly, interactions with cathode materials or current collectors should be assessed to ensure no detrimental reactions occur that could compromise the battery's structural integrity.
Toxicity and environmental impact are also critical safety considerations. Many electrolyte additives are organic compounds that may pose health risks if leaked or released. It is essential to select additives with low toxicity and minimal environmental impact, considering both normal operation and potential disposal scenarios.
The formation of gas during battery operation is another safety concern. Some additives may decompose or react to produce gases, which can lead to pressure build-up within the battery. This can cause swelling, rupture, or even explosion if not properly managed. Therefore, additives should be chosen that minimize gas evolution or incorporate mechanisms to safely vent any gases produced.
Long-term stability and degradation products of the additives must be considered. Over time, additives may break down or react, potentially forming new compounds that could affect battery safety. Understanding these long-term effects is crucial for ensuring the safety of AIBs throughout their lifecycle.
Flammability is a critical factor, especially given the potential for short circuits or overcharging. Additives should ideally have high flash points and low volatility to reduce the risk of fire in case of battery failure. Additionally, the impact of additives on the overall flammability of the electrolyte solution must be carefully evaluated.
Finally, the scalability and manufacturability of AIBs with these additives must be considered from a safety perspective. Additives should not introduce additional hazards during the manufacturing process and should be compatible with existing safety protocols and equipment used in battery production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!