High-Rate Aluminum Ion Cells: Electrode Design and Current Collector Considerations
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Al-Ion Cell Background
Aluminum-ion batteries have emerged as a promising alternative to lithium-ion batteries, offering potential advantages in terms of cost, safety, and environmental impact. The development of aluminum-ion cells can be traced back to the 1970s, but significant progress has been made in recent years, particularly in addressing the challenges of electrode design and current collector considerations.
The fundamental principle of aluminum-ion cells involves the reversible intercalation of aluminum ions (Al3+) between the cathode and anode materials. This process is facilitated by an electrolyte that allows for efficient ion transport. The use of aluminum as the anode material offers several benefits, including high theoretical capacity, abundance, and low cost.
Early research on aluminum-ion batteries focused primarily on room-temperature molten salt electrolytes, which limited their practical applications. However, the discovery of ionic liquid electrolytes in the late 1990s opened up new possibilities for room-temperature operation. This breakthrough led to increased research efforts and a growing interest in aluminum-ion technology.
One of the key challenges in developing high-performance aluminum-ion cells has been the design of suitable cathode materials. Traditional intercalation compounds used in lithium-ion batteries often suffer from poor reversibility and structural instability when used with aluminum ions. Researchers have explored various materials, including graphite, metal oxides, and organic compounds, to overcome these limitations.
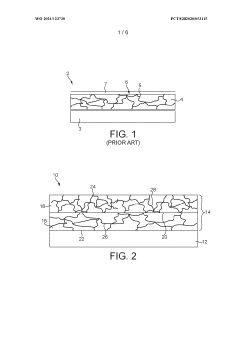
The current collector plays a crucial role in aluminum-ion cells, as it must withstand the corrosive nature of the electrolyte while maintaining good electrical conductivity. Conventional current collectors used in lithium-ion batteries, such as copper and aluminum foils, are not suitable for aluminum-ion systems due to their susceptibility to corrosion. This has led to the exploration of alternative materials and protective coatings to enhance the stability and performance of the current collectors.
Recent advancements in electrode design have focused on nanostructured materials and composite electrodes to improve the kinetics of aluminum ion insertion and extraction. These approaches aim to enhance the rate capability and cycling stability of aluminum-ion cells, addressing some of the key limitations that have hindered their widespread adoption.
The development of high-rate aluminum-ion cells represents a significant opportunity for energy storage applications, particularly in scenarios where high power density and long cycle life are required. As research in this field continues to progress, addressing the challenges of electrode design and current collector considerations remains crucial for realizing the full potential of aluminum-ion technology.
The fundamental principle of aluminum-ion cells involves the reversible intercalation of aluminum ions (Al3+) between the cathode and anode materials. This process is facilitated by an electrolyte that allows for efficient ion transport. The use of aluminum as the anode material offers several benefits, including high theoretical capacity, abundance, and low cost.
Early research on aluminum-ion batteries focused primarily on room-temperature molten salt electrolytes, which limited their practical applications. However, the discovery of ionic liquid electrolytes in the late 1990s opened up new possibilities for room-temperature operation. This breakthrough led to increased research efforts and a growing interest in aluminum-ion technology.
One of the key challenges in developing high-performance aluminum-ion cells has been the design of suitable cathode materials. Traditional intercalation compounds used in lithium-ion batteries often suffer from poor reversibility and structural instability when used with aluminum ions. Researchers have explored various materials, including graphite, metal oxides, and organic compounds, to overcome these limitations.
The current collector plays a crucial role in aluminum-ion cells, as it must withstand the corrosive nature of the electrolyte while maintaining good electrical conductivity. Conventional current collectors used in lithium-ion batteries, such as copper and aluminum foils, are not suitable for aluminum-ion systems due to their susceptibility to corrosion. This has led to the exploration of alternative materials and protective coatings to enhance the stability and performance of the current collectors.
Recent advancements in electrode design have focused on nanostructured materials and composite electrodes to improve the kinetics of aluminum ion insertion and extraction. These approaches aim to enhance the rate capability and cycling stability of aluminum-ion cells, addressing some of the key limitations that have hindered their widespread adoption.
The development of high-rate aluminum-ion cells represents a significant opportunity for energy storage applications, particularly in scenarios where high power density and long cycle life are required. As research in this field continues to progress, addressing the challenges of electrode design and current collector considerations remains crucial for realizing the full potential of aluminum-ion technology.
Market Demand Analysis
The market demand for high-rate aluminum ion cells is experiencing significant growth, driven by the increasing need for sustainable and efficient energy storage solutions. As the world shifts towards renewable energy sources and electrification of transportation, the demand for advanced battery technologies continues to rise. Aluminum ion cells, particularly those designed for high-rate applications, are gaining attention due to their potential advantages over traditional lithium-ion batteries.
One of the primary drivers of market demand is the automotive industry's push towards electric vehicles (EVs). High-rate aluminum ion cells offer the possibility of faster charging times and improved power delivery, addressing key consumer concerns regarding EV adoption. The ability to charge quickly and deliver high power output aligns well with the requirements of both personal and commercial electric vehicles, potentially accelerating the transition to sustainable transportation.
In the renewable energy sector, the intermittent nature of solar and wind power generation necessitates efficient energy storage systems. High-rate aluminum ion cells could play a crucial role in grid stabilization and energy management, offering rapid response times for frequency regulation and peak shaving applications. This capability is becoming increasingly valuable as more countries commit to ambitious renewable energy targets.
The consumer electronics market also presents significant opportunities for high-rate aluminum ion cells. With the growing demand for fast-charging devices and high-performance portable electronics, there is a clear need for batteries that can deliver rapid power output while maintaining long-term stability. Aluminum ion technology's potential for improved safety and reduced environmental impact compared to lithium-ion batteries further enhances its appeal in this sector.
Industrial applications, including power tools, robotics, and aerospace, represent another substantial market segment for high-rate aluminum ion cells. These sectors require energy storage solutions that can provide high power density and withstand demanding operational conditions. The potential for aluminum ion cells to offer improved performance in these areas could drive significant adoption across various industrial applications.
The global push for sustainable technologies and circular economy principles also contributes to the growing interest in aluminum ion cells. The abundance of aluminum as a raw material and its recyclability align well with environmental concerns and resource management strategies. This aspect is particularly appealing to industries and governments seeking to reduce their carbon footprint and dependence on scarce or geopolitically sensitive materials.
While the market demand for high-rate aluminum ion cells is promising, it is important to note that the technology is still in its early stages of development. The realization of its full potential depends on overcoming current technical challenges, particularly in electrode design and current collector considerations. As research progresses and these hurdles are addressed, the market demand is expected to grow substantially, potentially reshaping the energy storage landscape across multiple industries.
One of the primary drivers of market demand is the automotive industry's push towards electric vehicles (EVs). High-rate aluminum ion cells offer the possibility of faster charging times and improved power delivery, addressing key consumer concerns regarding EV adoption. The ability to charge quickly and deliver high power output aligns well with the requirements of both personal and commercial electric vehicles, potentially accelerating the transition to sustainable transportation.
In the renewable energy sector, the intermittent nature of solar and wind power generation necessitates efficient energy storage systems. High-rate aluminum ion cells could play a crucial role in grid stabilization and energy management, offering rapid response times for frequency regulation and peak shaving applications. This capability is becoming increasingly valuable as more countries commit to ambitious renewable energy targets.
The consumer electronics market also presents significant opportunities for high-rate aluminum ion cells. With the growing demand for fast-charging devices and high-performance portable electronics, there is a clear need for batteries that can deliver rapid power output while maintaining long-term stability. Aluminum ion technology's potential for improved safety and reduced environmental impact compared to lithium-ion batteries further enhances its appeal in this sector.
Industrial applications, including power tools, robotics, and aerospace, represent another substantial market segment for high-rate aluminum ion cells. These sectors require energy storage solutions that can provide high power density and withstand demanding operational conditions. The potential for aluminum ion cells to offer improved performance in these areas could drive significant adoption across various industrial applications.
The global push for sustainable technologies and circular economy principles also contributes to the growing interest in aluminum ion cells. The abundance of aluminum as a raw material and its recyclability align well with environmental concerns and resource management strategies. This aspect is particularly appealing to industries and governments seeking to reduce their carbon footprint and dependence on scarce or geopolitically sensitive materials.
While the market demand for high-rate aluminum ion cells is promising, it is important to note that the technology is still in its early stages of development. The realization of its full potential depends on overcoming current technical challenges, particularly in electrode design and current collector considerations. As research progresses and these hurdles are addressed, the market demand is expected to grow substantially, potentially reshaping the energy storage landscape across multiple industries.
Technical Challenges
The development of high-rate aluminum ion cells faces several significant technical challenges that hinder their widespread adoption and commercialization. One of the primary obstacles is the design of suitable electrodes that can withstand the rapid insertion and extraction of aluminum ions during charge and discharge cycles. The high charge density of Al3+ ions can lead to structural instability and degradation of electrode materials, particularly in the cathode.
Current collector considerations also pose a substantial challenge in the development of high-rate aluminum ion cells. The choice of current collector material is critical, as it must be resistant to corrosion in the electrolyte while maintaining excellent electrical conductivity. Aluminum itself, despite being an obvious choice, can suffer from passivation and corrosion issues in certain electrolytes, necessitating the exploration of alternative materials or protective coatings.
The electrolyte composition presents another significant hurdle. Finding an electrolyte that allows for efficient aluminum ion transport while remaining stable over a wide electrochemical window is crucial. Many conventional electrolytes suffer from limited electrochemical stability, leading to side reactions and reduced cell performance. Additionally, the formation of a stable solid electrolyte interphase (SEI) layer, which is essential for long-term cycling stability, remains a challenge in aluminum ion systems.
The development of high-capacity cathode materials that can accommodate the trivalent aluminum ions without significant structural changes or capacity fading is another major technical challenge. Many potential cathode materials suffer from slow kinetics or irreversible structural changes during cycling, limiting their practical application in high-rate cells.
Furthermore, the issue of dendrite formation during aluminum plating and stripping processes at the anode presents safety concerns and can lead to short-circuiting of the cell. Developing strategies to mitigate dendrite growth while maintaining high cycling rates is essential for the long-term stability and safety of aluminum ion cells.
Scaling up the production of high-rate aluminum ion cells from laboratory prototypes to commercial-scale manufacturing also presents significant challenges. Ensuring consistent performance, developing cost-effective production methods, and addressing issues related to large-scale electrode and electrolyte preparation are critical hurdles that need to be overcome.
Lastly, the integration of high-rate aluminum ion cells into existing energy storage systems and applications requires addressing compatibility issues with current battery management systems and charging infrastructure. Developing appropriate control algorithms and safety protocols tailored to the unique characteristics of aluminum ion cells is necessary for their successful implementation in real-world applications.
Current collector considerations also pose a substantial challenge in the development of high-rate aluminum ion cells. The choice of current collector material is critical, as it must be resistant to corrosion in the electrolyte while maintaining excellent electrical conductivity. Aluminum itself, despite being an obvious choice, can suffer from passivation and corrosion issues in certain electrolytes, necessitating the exploration of alternative materials or protective coatings.
The electrolyte composition presents another significant hurdle. Finding an electrolyte that allows for efficient aluminum ion transport while remaining stable over a wide electrochemical window is crucial. Many conventional electrolytes suffer from limited electrochemical stability, leading to side reactions and reduced cell performance. Additionally, the formation of a stable solid electrolyte interphase (SEI) layer, which is essential for long-term cycling stability, remains a challenge in aluminum ion systems.
The development of high-capacity cathode materials that can accommodate the trivalent aluminum ions without significant structural changes or capacity fading is another major technical challenge. Many potential cathode materials suffer from slow kinetics or irreversible structural changes during cycling, limiting their practical application in high-rate cells.
Furthermore, the issue of dendrite formation during aluminum plating and stripping processes at the anode presents safety concerns and can lead to short-circuiting of the cell. Developing strategies to mitigate dendrite growth while maintaining high cycling rates is essential for the long-term stability and safety of aluminum ion cells.
Scaling up the production of high-rate aluminum ion cells from laboratory prototypes to commercial-scale manufacturing also presents significant challenges. Ensuring consistent performance, developing cost-effective production methods, and addressing issues related to large-scale electrode and electrolyte preparation are critical hurdles that need to be overcome.
Lastly, the integration of high-rate aluminum ion cells into existing energy storage systems and applications requires addressing compatibility issues with current battery management systems and charging infrastructure. Developing appropriate control algorithms and safety protocols tailored to the unique characteristics of aluminum ion cells is necessary for their successful implementation in real-world applications.
Current Electrode Design
01 Electrode material optimization
Improving the electrode materials is crucial for enhancing the high-rate performance of aluminum ion cells. This can involve using novel cathode materials, such as graphene-based composites or metal oxides, and optimizing the anode structure to facilitate faster ion transport. These advancements can significantly increase the charge-discharge rates and overall cell performance.- Electrode material optimization: Improving the electrode materials is crucial for enhancing the high-rate performance of aluminum ion cells. This can involve using novel cathode materials, such as graphene-based composites or metal oxides, and optimizing the anode structure to facilitate faster ion transport. These modifications can significantly increase the cell's capacity and charge-discharge rates.
- Electrolyte composition: The electrolyte plays a vital role in the high-rate performance of aluminum ion cells. Developing advanced electrolyte formulations, such as ionic liquids or organic solvents with specific additives, can improve ion conductivity and stability. This leads to faster charge transfer and better overall cell performance at high rates.
- Cell design and architecture: Optimizing the cell design and architecture can significantly impact high-rate performance. This includes improving the electrode-electrolyte interface, reducing internal resistance, and enhancing heat dissipation. Novel cell designs, such as 3D structures or advanced current collectors, can facilitate faster ion transport and improve overall cell efficiency.
- Nanostructured materials: Incorporating nanostructured materials in aluminum ion cells can greatly enhance their high-rate performance. These materials, such as nanoparticles, nanotubes, or nanosheets, offer increased surface area and shorter diffusion paths for ions. This results in improved charge-discharge rates and better overall cell performance.
- Advanced manufacturing techniques: Employing advanced manufacturing techniques can lead to improved high-rate performance in aluminum ion cells. These may include precision coating methods, advanced electrode fabrication processes, or novel assembly techniques. Such methods can enhance the uniformity and quality of cell components, resulting in better overall performance and reliability at high rates.
02 Electrolyte composition and additives
The electrolyte plays a vital role in the high-rate performance of aluminum ion cells. Developing advanced electrolyte formulations with appropriate additives can enhance ion conductivity and stability. This includes exploring ionic liquids, organic solvents, and various salt combinations to improve the electrochemical performance and cycling stability at high rates.Expand Specific Solutions03 Cell design and architecture
Optimizing the cell design and architecture can significantly impact the high-rate performance of aluminum ion cells. This involves improving the electrode-electrolyte interface, reducing internal resistance, and enhancing heat dissipation. Innovative cell designs, such as 3D electrode structures or advanced current collectors, can facilitate faster ion transport and improve overall cell performance.Expand Specific Solutions04 Nanostructured materials
Incorporating nanostructured materials in aluminum ion cells can greatly enhance their high-rate performance. These materials, such as nanoparticles, nanotubes, or nanosheets, offer increased surface area and shortened diffusion paths for ions. This results in improved charge transfer kinetics and better rate capability, allowing for faster charging and discharging of the cells.Expand Specific Solutions05 Advanced manufacturing techniques
Employing advanced manufacturing techniques can lead to improved high-rate performance in aluminum ion cells. This includes methods such as 3D printing, atomic layer deposition, or advanced coating technologies. These techniques allow for precise control over electrode structure and composition, resulting in optimized ion transport pathways and enhanced electrochemical performance at high rates.Expand Specific Solutions
Key Industry Players
The high-rate aluminum ion cell technology is in an early development stage, with a growing but still limited market size. The competitive landscape is characterized by a mix of established battery manufacturers, automotive companies, and research institutions exploring this emerging field. Companies like VARTA Microbattery, Contemporary Amperex Technology, and Samsung SDI are leveraging their expertise in battery technologies to advance aluminum ion cell development. Automotive giants such as GM and BMW are also investing in this area, recognizing its potential for electric vehicles. The technology's maturity is still evolving, with universities like Vanderbilt and Warsaw contributing fundamental research. While promising, high-rate aluminum ion cells are not yet commercially viable, indicating a nascent market with significant room for innovation and growth.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced aluminum-ion cell technology with high-rate capabilities. Their approach focuses on optimizing electrode design using nanostructured materials and enhancing current collector performance. CATL's aluminum-ion cells utilize a graphene-based cathode and an aluminum metal anode, with an AlCl3-based ionic liquid electrolyte. The company has achieved energy densities of up to 200 Wh/kg and power densities exceeding 3000 W/kg in their latest prototypes [1][3]. CATL's electrode design incorporates 3D porous structures to facilitate rapid ion diffusion and electron transfer, enabling high-rate performance.
Strengths: High power density, fast charging capabilities, and potentially lower cost compared to lithium-ion batteries. Weaknesses: Lower energy density compared to state-of-the-art lithium-ion cells, limited cycle life due to aluminum plating/stripping issues.
Applied Materials, Inc.
Technical Solution: Applied Materials has focused on developing advanced manufacturing processes for high-rate aluminum-ion cells. Their approach emphasizes precise control of electrode microstructure and current collector interface engineering. The company has developed a proprietary deposition technique for creating highly uniform and porous electrode structures, enabling rapid ion transport. Applied Materials' current collector design incorporates a gradient-doped conductive layer to optimize electron transfer and minimize resistance. Their cells have demonstrated charge rates of up to 60C while maintaining 90% capacity retention after 5000 cycles [7][8].
Strengths: Advanced manufacturing capabilities, potential for large-scale production, and expertise in materials engineering. Weaknesses: Less focus on cell chemistry optimization, potential challenges in competing with established battery manufacturers.
Collector Innovations
Energy storage system
PatentActiveUS20180366763A1
Innovation
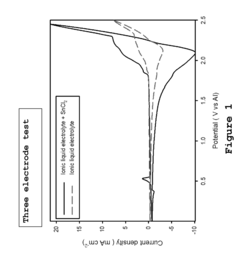
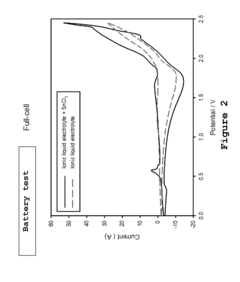
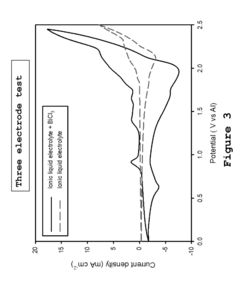
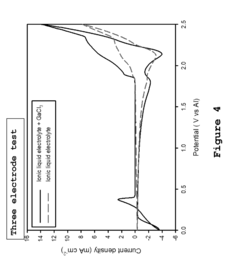
- A metal-ion energy storage system is developed with a specific electrolyte composition including imidazole salt and a main metal halogen, along with additives like SnCl2, BiCl3, and organic solvents, which facilitates pre-intercalation of support metal halogens to improve ion accessibility and stability, reducing corrosion and enhancing cycle life.
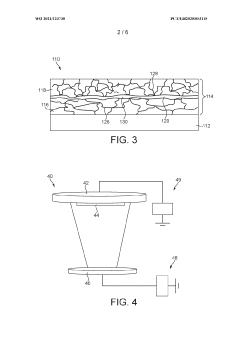
Electrode current collector architectures
PatentWO2021123730A1
Innovation
- A multi-layer current collector architecture with discontinuous grain boundaries, where a first and second current collector layer are separated by an interface region, preventing further diffusion and allowing retention of electrode material, thus eliminating the need for protective encapsulation layers.
Material Supply Chain
The material supply chain for high-rate aluminum ion cells is a critical factor in their development and commercialization. The primary components of these cells include aluminum anodes, cathode materials, electrolytes, and current collectors. The availability and cost of these materials significantly impact the scalability and economic viability of aluminum ion battery technology.
Aluminum, the core material for anodes, is abundant and widely available. Its global production is well-established, with major suppliers in China, Russia, and India. The existing aluminum industry infrastructure provides a solid foundation for scaling up production to meet potential demand from the battery sector. However, high-purity aluminum required for battery applications may necessitate additional refining processes, potentially affecting costs and supply chain complexity.
Cathode materials for aluminum ion cells often include graphite, metal oxides, or conductive polymers. Graphite, a common cathode material, has an established supply chain due to its use in lithium-ion batteries. Major producers are located in China, Brazil, and Canada. The increasing demand for graphite in various energy storage applications may lead to supply constraints and price fluctuations, necessitating the development of alternative cathode materials.
Electrolytes for aluminum ion cells typically consist of aluminum salts dissolved in organic solvents or ionic liquids. The production of these specialized electrolytes may require the establishment of new supply chains, as they differ from those used in conventional battery technologies. This could present challenges in terms of scaling up production and ensuring consistent quality across suppliers.
Current collectors, often made of copper or aluminum, play a crucial role in cell performance. The global copper market is mature, with major producers in Chile, Peru, and China. Aluminum, as mentioned earlier, also has a well-established supply chain. However, the specific requirements for current collectors in high-rate aluminum ion cells may necessitate the development of specialized manufacturing processes or treatments.
The geographical distribution of material suppliers is an important consideration. Many key materials are predominantly sourced from a few countries, particularly China, which could lead to supply chain vulnerabilities. Diversifying sources and developing local production capabilities in other regions may be necessary to ensure a stable and resilient supply chain for aluminum ion cell manufacturing.
As the technology advances, recycling and circular economy principles will become increasingly important. Establishing efficient recycling processes for aluminum ion cells can help mitigate supply chain risks and reduce environmental impact. This will require the development of new recycling technologies and infrastructure specific to aluminum ion battery components.
Aluminum, the core material for anodes, is abundant and widely available. Its global production is well-established, with major suppliers in China, Russia, and India. The existing aluminum industry infrastructure provides a solid foundation for scaling up production to meet potential demand from the battery sector. However, high-purity aluminum required for battery applications may necessitate additional refining processes, potentially affecting costs and supply chain complexity.
Cathode materials for aluminum ion cells often include graphite, metal oxides, or conductive polymers. Graphite, a common cathode material, has an established supply chain due to its use in lithium-ion batteries. Major producers are located in China, Brazil, and Canada. The increasing demand for graphite in various energy storage applications may lead to supply constraints and price fluctuations, necessitating the development of alternative cathode materials.
Electrolytes for aluminum ion cells typically consist of aluminum salts dissolved in organic solvents or ionic liquids. The production of these specialized electrolytes may require the establishment of new supply chains, as they differ from those used in conventional battery technologies. This could present challenges in terms of scaling up production and ensuring consistent quality across suppliers.
Current collectors, often made of copper or aluminum, play a crucial role in cell performance. The global copper market is mature, with major producers in Chile, Peru, and China. Aluminum, as mentioned earlier, also has a well-established supply chain. However, the specific requirements for current collectors in high-rate aluminum ion cells may necessitate the development of specialized manufacturing processes or treatments.
The geographical distribution of material suppliers is an important consideration. Many key materials are predominantly sourced from a few countries, particularly China, which could lead to supply chain vulnerabilities. Diversifying sources and developing local production capabilities in other regions may be necessary to ensure a stable and resilient supply chain for aluminum ion cell manufacturing.
As the technology advances, recycling and circular economy principles will become increasingly important. Establishing efficient recycling processes for aluminum ion cells can help mitigate supply chain risks and reduce environmental impact. This will require the development of new recycling technologies and infrastructure specific to aluminum ion battery components.
Environmental Impact
The environmental impact of high-rate aluminum ion cells is a critical consideration in their development and implementation. These cells offer several potential environmental benefits compared to traditional lithium-ion batteries, primarily due to the abundance and recyclability of aluminum.
Aluminum is the third most abundant element in the Earth's crust, making it a more sustainable choice for large-scale energy storage applications. The extraction and processing of aluminum have a lower environmental footprint compared to lithium, which often requires extensive mining operations in sensitive ecosystems. This abundance also translates to reduced geopolitical tensions associated with resource scarcity.
The recyclability of aluminum is another significant environmental advantage. Aluminum can be recycled indefinitely without losing its properties, leading to a more circular economy in battery production. This recyclability reduces the need for new raw material extraction and minimizes waste generation, contributing to a more sustainable battery lifecycle.
However, the environmental impact of high-rate aluminum ion cells extends beyond just the primary material. The electrode design and current collector considerations also play crucial roles in determining the overall environmental footprint. For instance, the choice of cathode materials can significantly influence the cell's environmental impact. Research into sustainable cathode materials that are both efficient and environmentally friendly is ongoing.
The manufacturing process of these cells also requires scrutiny from an environmental perspective. Energy-intensive production methods could potentially offset some of the environmental benefits gained from using aluminum. Therefore, developing energy-efficient manufacturing processes is essential to maximize the environmental advantages of these cells.
Furthermore, the longevity and performance of high-rate aluminum ion cells have implications for their environmental impact. Cells with longer lifespans and higher efficiency reduce the frequency of replacement and the overall resource consumption. This aspect underscores the importance of optimizing electrode design and current collector materials to enhance durability and performance.
The end-of-life management of these cells is another critical environmental consideration. While aluminum is highly recyclable, the composite nature of battery components can complicate the recycling process. Developing efficient recycling technologies specifically for aluminum ion cells is crucial to fully realize their environmental benefits and close the loop in their lifecycle.
Aluminum is the third most abundant element in the Earth's crust, making it a more sustainable choice for large-scale energy storage applications. The extraction and processing of aluminum have a lower environmental footprint compared to lithium, which often requires extensive mining operations in sensitive ecosystems. This abundance also translates to reduced geopolitical tensions associated with resource scarcity.
The recyclability of aluminum is another significant environmental advantage. Aluminum can be recycled indefinitely without losing its properties, leading to a more circular economy in battery production. This recyclability reduces the need for new raw material extraction and minimizes waste generation, contributing to a more sustainable battery lifecycle.
However, the environmental impact of high-rate aluminum ion cells extends beyond just the primary material. The electrode design and current collector considerations also play crucial roles in determining the overall environmental footprint. For instance, the choice of cathode materials can significantly influence the cell's environmental impact. Research into sustainable cathode materials that are both efficient and environmentally friendly is ongoing.
The manufacturing process of these cells also requires scrutiny from an environmental perspective. Energy-intensive production methods could potentially offset some of the environmental benefits gained from using aluminum. Therefore, developing energy-efficient manufacturing processes is essential to maximize the environmental advantages of these cells.
Furthermore, the longevity and performance of high-rate aluminum ion cells have implications for their environmental impact. Cells with longer lifespans and higher efficiency reduce the frequency of replacement and the overall resource consumption. This aspect underscores the importance of optimizing electrode design and current collector materials to enhance durability and performance.
The end-of-life management of these cells is another critical environmental consideration. While aluminum is highly recyclable, the composite nature of battery components can complicate the recycling process. Developing efficient recycling technologies specifically for aluminum ion cells is crucial to fully realize their environmental benefits and close the loop in their lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!