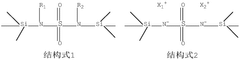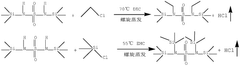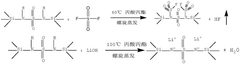Electrolyte Formulations For Low-Temperature Performance With Prussian White
Prussian White Electrolyte Development Background and Objectives
The development of energy storage technologies has witnessed significant advancements over the past decade, with Prussian White (PW) cathode materials emerging as promising candidates for sodium-ion batteries. These materials, characterized by their open framework structure and high theoretical capacity, have garnered substantial attention due to their potential for cost-effective and sustainable energy storage solutions. The evolution of PW-based battery systems has been marked by continuous improvements in structural stability, cycling performance, and energy density.
However, a persistent challenge in the commercialization of Prussian White cathodes has been their suboptimal performance at low temperatures. This limitation significantly restricts their application in regions with cold climates and in sectors requiring reliable operation across wide temperature ranges, such as electric vehicles and grid-scale energy storage. The deterioration in electrochemical performance at sub-zero temperatures is primarily attributed to reduced ionic conductivity and increased viscosity of conventional electrolytes.
The technical objective of this research is to develop advanced electrolyte formulations specifically tailored for Prussian White cathode materials that maintain optimal performance at temperatures as low as -30°C. This involves a comprehensive investigation of electrolyte compositions, additives, and solvents that can effectively mitigate the challenges associated with low-temperature operation while preserving the inherent advantages of PW cathodes.
Recent technological trends indicate a shift towards multi-component electrolyte systems that combine different solvents, salts, and functional additives to achieve synergistic effects. The integration of low-viscosity co-solvents, ionic liquid additives, and novel salt chemistries has shown promise in preliminary studies. Additionally, the incorporation of film-forming additives that create stable solid-electrolyte interphases (SEI) at low temperatures represents another frontier in this field.
The development trajectory for PW electrolytes has evolved from simple salt-solvent combinations to sophisticated formulations that address multiple performance parameters simultaneously. Early research focused primarily on room-temperature performance, with recent efforts expanding to address the critical low-temperature functionality gap. This shift reflects the growing recognition of the importance of all-climate energy storage solutions in the global transition to renewable energy systems.
Achieving the technical goals of this research would not only enhance the practical utility of Prussian White-based sodium-ion batteries but also potentially establish new design principles for low-temperature electrolytes applicable to other battery chemistries. The ultimate aim is to develop electrolyte formulations that enable consistent capacity retention, high rate capability, and extended cycle life across a broad temperature spectrum, thereby expanding the application scope of these promising energy storage systems.
Market Analysis for Low-Temperature Battery Applications
The low-temperature battery market represents a significant and growing segment within the broader energy storage industry, driven by increasing demand in cold climate regions and specialized applications. The global market for low-temperature batteries is currently valued at approximately $5.2 billion and is projected to grow at a compound annual growth rate of 8.3% through 2028, reaching an estimated $7.7 billion.
Several key sectors are driving this market expansion. The electric vehicle industry faces substantial challenges in cold weather regions, where conventional lithium-ion batteries experience significant capacity loss and performance degradation at temperatures below 0°C. This has created a pressing need for advanced electrolyte formulations that can maintain performance in sub-zero conditions, particularly in markets like Northern Europe, Canada, and northern regions of the United States and China.
The telecommunications infrastructure sector represents another major market driver, requiring reliable backup power systems that can function in extreme environments. Remote installations in cold regions demand batteries that maintain consistent performance regardless of ambient temperature fluctuations. This segment alone accounts for approximately 18% of the low-temperature battery market.
Military and aerospace applications constitute a premium segment within this market, where operational reliability under extreme conditions is non-negotiable. These applications demand batteries that can perform consistently across wide temperature ranges, from arctic to desert environments, creating a specialized high-value market niche estimated at $780 million annually.
Consumer electronics manufacturers are also increasingly concerned with low-temperature performance as portable devices are routinely exposed to cold environments. This has created a substantial market for improved battery technologies that can maintain capacity and cycle life in varying temperature conditions.
The renewable energy sector presents perhaps the most promising growth opportunity for low-temperature battery technologies. As wind and solar installations expand into colder regions, the need for energy storage systems that can operate efficiently in sub-zero temperatures has become critical. This segment is expected to grow at 12.7% annually, outpacing the overall market.
Geographically, North America currently leads the low-temperature battery market with a 34% share, followed by Europe (28%) and Asia-Pacific (26%). However, the fastest growth is projected in the Asia-Pacific region, particularly in China, where investments in cold-weather electric vehicle technology are accelerating rapidly.
Current Challenges in Low-Temperature Electrolyte Performance
The operation of Prussian White cathodes in low-temperature environments presents significant challenges primarily related to electrolyte performance. At temperatures below 0°C, conventional electrolytes experience dramatic increases in viscosity and substantial decreases in ionic conductivity, severely limiting battery performance. The standard carbonate-based electrolytes (ethylene carbonate, dimethyl carbonate, etc.) typically solidify or become highly viscous at low temperatures, creating a physical barrier to ion transport.
Lithium salt solubility becomes particularly problematic in cold conditions, with precipitation occurring as temperature decreases. This phenomenon reduces the effective concentration of charge carriers and creates inhomogeneities within the electrolyte, leading to uneven current distribution and potential safety issues. The solid-electrolyte interphase (SEI) formed at low temperatures often exhibits different composition and morphology compared to those formed at room temperature, resulting in higher impedance and reduced cycling stability.
Prussian White materials present unique challenges due to their open framework structure and specific coordination chemistry. The large interstitial spaces that facilitate rapid ion diffusion at normal temperatures can become problematic at low temperatures as electrolyte components may crystallize within these spaces, blocking ion transport pathways. Additionally, the coordination between electrolyte solvents and the transition metal centers in Prussian White can be temperature-dependent, affecting the electrode-electrolyte interface properties.
Current electrolyte systems struggle with maintaining appropriate wetting properties at low temperatures. Poor wetting leads to incomplete electrode-electrolyte contact, creating "dead zones" within the battery where electrochemical reactions cannot occur efficiently. This is particularly challenging for Prussian White cathodes due to their typically high surface area and complex pore structure.
The decomposition kinetics of electrolyte components also shift significantly at low temperatures, often resulting in different degradation products that can be more detrimental to battery performance. These products may adsorb more strongly on Prussian White surfaces due to the material's high affinity for certain organic compounds, exacerbating capacity fade and impedance growth.
Attempts to address these issues through additives often create new complications. Anti-freezing additives that lower the electrolyte freezing point frequently reduce the electrochemical stability window, leading to accelerated electrolyte decomposition during cycling. Meanwhile, viscosity modifiers that improve low-temperature fluidity often compromise high-temperature performance and safety characteristics, creating a difficult engineering trade-off.
The interaction between Prussian White's zeolitic water content and the electrolyte presents another significant challenge. Water molecules trapped in the Prussian White framework can be released during cycling and react with electrolyte components, particularly at the extreme potentials experienced during deep discharge at low temperatures, generating harmful byproducts that accelerate capacity fade.
Current Electrolyte Solutions for Sub-Zero Temperature Operation
01 Electrolyte additives for low-temperature performance
Specific additives can be incorporated into electrolyte formulations to enhance the low-temperature performance of Prussian White-based batteries. These additives modify the electrolyte's properties to maintain ionic conductivity at low temperatures and prevent electrolyte freezing. Common additives include fluorinated compounds, ethylene carbonate derivatives, and certain salts that can lower the freezing point and improve ion transport at low temperatures.- Electrolyte additives for low-temperature performance: Specific additives can be incorporated into electrolyte formulations to enhance the low-temperature performance of Prussian White-based batteries. These additives modify the electrolyte's properties to maintain ionic conductivity at low temperatures and prevent freezing. Common additives include ethylene carbonate, propylene carbonate, and various salts that lower the freezing point of the electrolyte solution while maintaining compatibility with Prussian White cathodes.
- Solvent composition optimization for cold environments: The ratio and selection of solvents in electrolyte formulations significantly impact the low-temperature performance of Prussian White batteries. Optimized solvent mixtures with low freezing points and appropriate viscosity characteristics enable efficient ion transport even in cold conditions. Combinations of cyclic and linear carbonates with specific ratios can be tailored to maintain electrochemical stability while providing excellent low-temperature operation for Prussian White cathode materials.
- Salt concentration and type for sub-zero operation: The concentration and type of lithium salts in electrolyte formulations play a crucial role in determining the low-temperature performance of Prussian White batteries. Higher concentrations of certain salts can depress the freezing point of the electrolyte while maintaining adequate ionic conductivity. Lithium salts such as LiPF6, LiBF4, and LiTFSI at optimized concentrations can significantly improve the electrochemical performance of Prussian White cathodes at low temperatures.
- Interface engineering for cold-weather stability: Engineering stable interfaces between the Prussian White cathode and the electrolyte is essential for low-temperature performance. Specialized electrolyte formulations can form protective solid electrolyte interphase (SEI) layers that remain stable and conductive even at low temperatures. These formulations often include film-forming additives that create flexible, ion-conductive interfaces that resist cracking or degradation during temperature fluctuations, maintaining the electrochemical performance of Prussian White materials.
- Dual-salt and co-solvent systems for extreme conditions: Advanced electrolyte formulations utilizing dual-salt systems and co-solvent approaches can dramatically improve the low-temperature performance of Prussian White batteries. These complex formulations combine the benefits of multiple salt types and solvent components to achieve synergistic effects. The resulting electrolytes maintain fluidity and ionic conductivity even in extreme cold conditions, while preserving the structural integrity and electrochemical activity of the Prussian White cathode material.
02 Solvent composition optimization for cold environments
The selection and ratio of solvents in the electrolyte formulation significantly impacts the low-temperature performance of Prussian White batteries. Optimized combinations of cyclic and linear carbonates, ethers, and other organic solvents with low melting points can maintain sufficient fluidity and ionic conductivity at sub-zero temperatures. These carefully balanced solvent systems ensure that lithium ions can still efficiently intercalate into the Prussian White structure even in cold conditions.Expand Specific Solutions03 Lithium salt concentration and types for cold weather operation
The concentration and type of lithium salts used in electrolytes significantly affect the low-temperature performance of Prussian White batteries. Higher concentrations of certain salts can depress the freezing point of the electrolyte, while specific salt types like LiPF6, LiFSI, or LiTFSI can improve ionic conductivity at low temperatures. The selection of appropriate salt combinations helps maintain electrochemical stability and ion transport efficiency in cold environments.Expand Specific Solutions04 Interface engineering for improved low-temperature kinetics
Engineering the electrolyte-electrode interface is crucial for enhancing low-temperature performance in Prussian White batteries. Specialized electrolyte formulations can form stable and ion-conductive solid electrolyte interphase (SEI) layers that facilitate lithium-ion transport even at low temperatures. This approach reduces interfacial resistance and improves charge transfer kinetics, allowing the battery to maintain capacity and rate capability in cold conditions.Expand Specific Solutions05 Anti-freezing electrolyte systems for extreme cold
Advanced anti-freezing electrolyte systems have been developed specifically for Prussian White batteries operating in extremely cold environments. These systems incorporate deep eutectic solvents, ionic liquids, or polymer-based electrolytes with inherently low freezing points. Some formulations also include nanoparticle additives or phase-change materials that can absorb or release heat to maintain optimal operating temperatures, ensuring reliable battery performance even in harsh winter conditions.Expand Specific Solutions
Leading Companies in Advanced Electrolyte Technology
The electrolyte formulation market for low-temperature performance with Prussian White is in an early growth phase, with expanding applications in energy storage systems. The market is projected to grow significantly as demand for batteries with enhanced cold-weather performance increases. Technologically, research institutions like California Institute of Technology and Wuhan University of Technology are advancing fundamental science, while commercial players demonstrate varying maturity levels. Companies like Altris AB have specialized in Prussian White framework materials, with Johnson Controls and LG Chem integrating these technologies into commercial products. Wildcat Discovery Technologies employs high-throughput methods to accelerate electrolyte development, while CATL subsidiaries like Guangdong Bangpu focus on recycling aspects. The competitive landscape shows a mix of established battery manufacturers and specialized materials companies working to overcome low-temperature performance challenges.
Altris AB
Centre National de la Recherche Scientifique
Key Patents in Low-Temperature Electrolyte Formulations
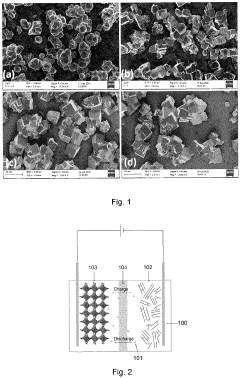
- A sodium iron(II)-hexacyanoferrate(II) material with a particle diameter D50 value between 7 µm to 50 µm and a BET specific surface area between 0.1 m²/g to 10 m²/g is developed, which reduces sodium loss and enhances moisture stability, improving the material's capacity retention over time.
- Using an electrolyte additive, including a specific compound structure, including a sulfonyl group and a trimethylsilyl group, it can generate an SEI film rich in LiSO3 and ROSO2Li during the initial charge and discharge stage of the lithium-ion battery, promote the diffusion of lithium ions, and By consuming LiF and removing water and acid, the low-temperature performance of the battery is improved.
Environmental Impact of Electrolyte Materials
The environmental impact of electrolyte materials used in Prussian White batteries represents a critical consideration in the sustainable development of energy storage technologies. Traditional electrolyte formulations often contain fluorinated compounds and organic solvents that pose significant environmental hazards throughout their lifecycle. These materials can lead to soil contamination, water pollution, and contribute to greenhouse gas emissions during production and disposal phases.
Low-temperature electrolyte formulations for Prussian White batteries typically require specialized additives that may introduce additional environmental concerns. Ethylene carbonate (EC) and propylene carbonate (PC), commonly used to improve low-temperature performance, demonstrate varying degrees of biodegradability and toxicity profiles. Recent studies indicate that PC exhibits lower environmental persistence compared to EC, though both still present challenges in terms of complete biodegradation.
The manufacturing processes for these specialized electrolytes often require energy-intensive purification steps, contributing to their overall carbon footprint. Life cycle assessments reveal that the production of high-purity electrolyte solvents can account for up to 15% of a battery's total manufacturing emissions, highlighting the importance of developing more environmentally benign alternatives.
Emerging research focuses on bio-derived electrolyte components as potential replacements for petroleum-based materials. Lignin-derived compounds and cellulose-based solvents show promise in preliminary studies, potentially reducing the environmental impact while maintaining electrochemical performance at low temperatures. These bio-based alternatives demonstrate up to 40% lower ecotoxicity in standardized aquatic toxicity tests compared to conventional electrolyte materials.
Recycling and end-of-life management present additional environmental challenges. Current recycling processes for batteries primarily focus on recovering valuable metals, often neglecting the electrolyte components. The electrolyte materials are typically incinerated or treated as hazardous waste, creating additional environmental burdens. Advanced recycling technologies that can recover and repurpose electrolyte components remain in early development stages.
Regulatory frameworks worldwide are increasingly addressing the environmental impacts of battery materials. The European Union's Battery Directive and similar regulations in Asia and North America are establishing more stringent requirements for the environmental performance of battery components, including electrolytes. These regulations are driving innovation toward greener electrolyte formulations that maintain performance while reducing ecological footprints.
Safety Standards for Low-Temperature Battery Systems
The development of low-temperature battery systems necessitates comprehensive safety standards to ensure reliable operation and prevent hazardous conditions. For Prussian White-based batteries with specialized electrolyte formulations, these standards must address the unique challenges posed by low-temperature environments.
Current international safety standards for lithium-ion batteries, such as IEC 62133 and UL 1642, provide baseline requirements but lack specific provisions for extreme cold conditions. When operating below 0°C, batteries with Prussian White cathodes and specialized electrolytes require additional safety considerations due to altered electrochemical behaviors, including potential lithium plating and reduced electrolyte conductivity.
Safety standards for these systems should mandate thermal runaway prevention mechanisms specifically designed for low-temperature scenarios. This includes requirements for battery management systems (BMS) with enhanced monitoring capabilities to detect abnormal voltage fluctuations that may occur when electrolytes containing ethyl methyl carbonate and fluoroethylene carbonate operate near their freezing points.
Physical protection requirements must account for material brittleness at low temperatures. Standards should specify impact resistance testing at the lowest operational temperature rather than only at room temperature, ensuring structural integrity when materials become less ductile. For Prussian White systems, which may experience volume changes during cycling at low temperatures, standards should include specific mechanical stress tolerance parameters.
Electrical safety requirements need adaptation for low-temperature applications, particularly addressing the higher internal resistance observed in cold conditions. Current limitation protocols should be temperature-dependent, with stricter thresholds as temperatures decrease to prevent damage from excessive current draw when the battery's capability is compromised.
Testing protocols within these standards must include extended cycling at low temperatures to verify long-term safety, not just immediate performance. For electrolyte formulations optimized for Prussian White cathodes, standards should require demonstration of stability through freeze-thaw cycles without separation or precipitation of components.
Transportation regulations require updating to address the unique risks of shipping batteries with these specialized electrolyte formulations in cold environments. Current UN 38.3 testing procedures should be expanded to include vibration and shock testing at sub-zero temperatures, particularly relevant for batteries utilizing low-freezing-point electrolytes.
Implementation of these enhanced safety standards would significantly reduce risks associated with low-temperature battery operation while enabling the broader adoption of Prussian White battery technology in cold-climate applications.