Gas Evolution Analysis And SEI Interactions In Prussian White Cells
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Prussian White Battery Gas Evolution Background & Objectives
Prussian White (PW) batteries have emerged as a promising alternative to conventional lithium-ion batteries due to their potential for higher energy density, improved safety, and lower cost. The evolution of gas within these battery systems represents a critical challenge that impacts both performance and safety. This technical background exploration aims to comprehensively understand the mechanisms of gas evolution in Prussian White cells and its interaction with the Solid Electrolyte Interphase (SEI) formation.
The development of Prussian White batteries can be traced back to the broader family of Prussian Blue Analogue (PBA) materials, which were first investigated for electrochemical applications in the 1980s. Over the past decade, research has intensified on PW compounds specifically for battery applications, with significant advancements in understanding their structural properties and electrochemical behaviors.
Gas evolution in battery systems generally results from electrolyte decomposition, electrode material degradation, or side reactions at electrode-electrolyte interfaces. In Prussian White cells specifically, the presence of coordinated water molecules and the redox-active transition metal centers create unique conditions for gas-generating reactions. Understanding these mechanisms is crucial for developing strategies to mitigate capacity fade and safety risks.
The SEI layer forms on electrode surfaces during initial cycling and plays a pivotal role in battery performance. In PW cells, the interaction between evolved gases and the SEI layer can lead to continuous SEI reformation, impedance growth, and accelerated capacity degradation. Recent studies have indicated that the composition and stability of the SEI in PW cells differ significantly from those in conventional lithium-ion batteries, necessitating specialized investigation approaches.
The technical objectives of this research include: identifying the primary gas species evolved during PW cell operation across different voltage windows and temperature conditions; elucidating the chemical mechanisms responsible for gas generation; understanding how these gases interact with and potentially modify the SEI layer; and developing analytical methods for in-situ monitoring of gas evolution to enable real-time diagnostics.
Additionally, this investigation aims to establish correlations between cell design parameters (electrolyte composition, electrode formulation, etc.) and gas evolution rates, providing guidance for optimized cell engineering. The ultimate goal is to develop mitigation strategies that can significantly reduce gas evolution in PW cells, thereby enhancing cycle life, safety, and commercial viability of this promising battery technology.
This research aligns with the broader industry trend toward developing beyond-lithium-ion battery technologies that can meet the growing demands for energy storage in electric vehicles, grid applications, and portable electronics with improved performance metrics and reduced environmental impact.
The development of Prussian White batteries can be traced back to the broader family of Prussian Blue Analogue (PBA) materials, which were first investigated for electrochemical applications in the 1980s. Over the past decade, research has intensified on PW compounds specifically for battery applications, with significant advancements in understanding their structural properties and electrochemical behaviors.
Gas evolution in battery systems generally results from electrolyte decomposition, electrode material degradation, or side reactions at electrode-electrolyte interfaces. In Prussian White cells specifically, the presence of coordinated water molecules and the redox-active transition metal centers create unique conditions for gas-generating reactions. Understanding these mechanisms is crucial for developing strategies to mitigate capacity fade and safety risks.
The SEI layer forms on electrode surfaces during initial cycling and plays a pivotal role in battery performance. In PW cells, the interaction between evolved gases and the SEI layer can lead to continuous SEI reformation, impedance growth, and accelerated capacity degradation. Recent studies have indicated that the composition and stability of the SEI in PW cells differ significantly from those in conventional lithium-ion batteries, necessitating specialized investigation approaches.
The technical objectives of this research include: identifying the primary gas species evolved during PW cell operation across different voltage windows and temperature conditions; elucidating the chemical mechanisms responsible for gas generation; understanding how these gases interact with and potentially modify the SEI layer; and developing analytical methods for in-situ monitoring of gas evolution to enable real-time diagnostics.
Additionally, this investigation aims to establish correlations between cell design parameters (electrolyte composition, electrode formulation, etc.) and gas evolution rates, providing guidance for optimized cell engineering. The ultimate goal is to develop mitigation strategies that can significantly reduce gas evolution in PW cells, thereby enhancing cycle life, safety, and commercial viability of this promising battery technology.
This research aligns with the broader industry trend toward developing beyond-lithium-ion battery technologies that can meet the growing demands for energy storage in electric vehicles, grid applications, and portable electronics with improved performance metrics and reduced environmental impact.
Market Analysis for Advanced Battery Technologies
The global advanced battery market is experiencing unprecedented growth, driven by the increasing demand for energy storage solutions across various sectors. The market for Prussian White (PW) battery technology, specifically focusing on gas evolution analysis and solid electrolyte interphase (SEI) interactions, represents a significant segment within this expanding landscape. Current market valuations place the advanced battery sector at approximately $92 billion in 2023, with projections indicating a compound annual growth rate of 15.9% through 2030.
Prussian White cells, known for their high energy density and relatively low production costs, are gaining traction particularly in grid-scale energy storage applications. Market research indicates that improvements in gas evolution management and SEI stability could potentially increase the commercial viability of these systems by 30-40%, addressing key concerns that currently limit widespread adoption.
Consumer electronics and electric vehicle manufacturers have shown increasing interest in Prussian White technology, with several major automotive companies investing in research partnerships focused specifically on gas evolution mitigation strategies. This market segment is expected to grow at 18.7% annually, outpacing the broader battery market.
Geographically, Asia-Pacific dominates the advanced battery market with 45% share, followed by North America and Europe at 28% and 22% respectively. China leads in Prussian White cell manufacturing capacity, while significant research on gas evolution and SEI interactions is concentrated in research institutions across North America, Europe, and Japan.
Market barriers for Prussian White technology include competition from established lithium-ion technologies and emerging alternatives such as solid-state batteries. However, the lower material costs and reduced environmental impact of Prussian White cells provide competitive advantages in specific applications where cycle life and safety are prioritized over energy density.
Customer demand analysis reveals growing interest in batteries with improved safety profiles, particularly in residential energy storage and industrial applications. The market segment most concerned with gas evolution issues represents approximately $15 billion of the total advanced battery market, with annual growth rates exceeding 20% as safety regulations become more stringent globally.
Investment in gas evolution analysis technologies and SEI interaction research has seen a 35% increase year-over-year since 2020, indicating strong market recognition of these technical challenges as critical to commercialization success. Venture capital funding specifically targeting these research areas has reached $1.2 billion in 2023, demonstrating significant commercial interest in solving these technical challenges.
Prussian White cells, known for their high energy density and relatively low production costs, are gaining traction particularly in grid-scale energy storage applications. Market research indicates that improvements in gas evolution management and SEI stability could potentially increase the commercial viability of these systems by 30-40%, addressing key concerns that currently limit widespread adoption.
Consumer electronics and electric vehicle manufacturers have shown increasing interest in Prussian White technology, with several major automotive companies investing in research partnerships focused specifically on gas evolution mitigation strategies. This market segment is expected to grow at 18.7% annually, outpacing the broader battery market.
Geographically, Asia-Pacific dominates the advanced battery market with 45% share, followed by North America and Europe at 28% and 22% respectively. China leads in Prussian White cell manufacturing capacity, while significant research on gas evolution and SEI interactions is concentrated in research institutions across North America, Europe, and Japan.
Market barriers for Prussian White technology include competition from established lithium-ion technologies and emerging alternatives such as solid-state batteries. However, the lower material costs and reduced environmental impact of Prussian White cells provide competitive advantages in specific applications where cycle life and safety are prioritized over energy density.
Customer demand analysis reveals growing interest in batteries with improved safety profiles, particularly in residential energy storage and industrial applications. The market segment most concerned with gas evolution issues represents approximately $15 billion of the total advanced battery market, with annual growth rates exceeding 20% as safety regulations become more stringent globally.
Investment in gas evolution analysis technologies and SEI interaction research has seen a 35% increase year-over-year since 2020, indicating strong market recognition of these technical challenges as critical to commercialization success. Venture capital funding specifically targeting these research areas has reached $1.2 billion in 2023, demonstrating significant commercial interest in solving these technical challenges.
Current Challenges in Gas Evolution Monitoring
Gas evolution monitoring in Prussian White (PW) cells presents significant technical challenges that impede comprehensive understanding of battery degradation mechanisms. Current monitoring techniques suffer from limited temporal resolution, making it difficult to capture rapid gas evolution events during critical operational phases such as initial cycling or fast charging. This temporal gap creates blind spots in our understanding of how gas formation correlates with specific electrochemical processes.
Spatial resolution limitations further complicate gas evolution analysis. Most existing methods provide only bulk measurements, failing to identify localized gas formation at specific regions within the cell architecture. This deficiency prevents researchers from pinpointing exact locations where SEI (Solid Electrolyte Interphase) formation and gas evolution occur most aggressively, limiting the effectiveness of targeted mitigation strategies.
In-situ monitoring capabilities remain underdeveloped for PW cells. While techniques like differential electrochemical mass spectrometry (DEMS) offer valuable insights, they typically require modified cell designs that may not accurately represent commercial cell behavior. The trade-off between analytical precision and real-world relevance creates significant data interpretation challenges.
Chemical specificity presents another major hurdle. Current methods often detect total gas volume but struggle to differentiate between various gaseous species (CO, CO2, H2, etc.) in real-time. This limitation obscures the complex relationship between specific decomposition pathways and resulting gas products, hampering the development of targeted electrolyte additives or electrode surface treatments.
Data integration across multiple monitoring techniques remains problematic. Researchers frequently employ separate methods for electrochemical performance, gas evolution, and SEI characterization, but lack robust frameworks to correlate these datasets. This fragmentation complicates efforts to establish clear cause-effect relationships between operational parameters and degradation mechanisms.
Standardization issues further impede progress, with different research groups employing varied cell designs, monitoring protocols, and data reporting formats. This inconsistency makes cross-study comparisons difficult and slows the collective advancement of gas evolution understanding in PW systems.
Finally, computational modeling of gas evolution processes remains in its infancy. Current models struggle to accurately predict gas formation rates and compositions under various operating conditions, limiting their utility for cell design optimization or lifetime prediction. The complex interplay between electrochemical reactions, transport phenomena, and interfacial dynamics presents formidable challenges for comprehensive modeling approaches.
Spatial resolution limitations further complicate gas evolution analysis. Most existing methods provide only bulk measurements, failing to identify localized gas formation at specific regions within the cell architecture. This deficiency prevents researchers from pinpointing exact locations where SEI (Solid Electrolyte Interphase) formation and gas evolution occur most aggressively, limiting the effectiveness of targeted mitigation strategies.
In-situ monitoring capabilities remain underdeveloped for PW cells. While techniques like differential electrochemical mass spectrometry (DEMS) offer valuable insights, they typically require modified cell designs that may not accurately represent commercial cell behavior. The trade-off between analytical precision and real-world relevance creates significant data interpretation challenges.
Chemical specificity presents another major hurdle. Current methods often detect total gas volume but struggle to differentiate between various gaseous species (CO, CO2, H2, etc.) in real-time. This limitation obscures the complex relationship between specific decomposition pathways and resulting gas products, hampering the development of targeted electrolyte additives or electrode surface treatments.
Data integration across multiple monitoring techniques remains problematic. Researchers frequently employ separate methods for electrochemical performance, gas evolution, and SEI characterization, but lack robust frameworks to correlate these datasets. This fragmentation complicates efforts to establish clear cause-effect relationships between operational parameters and degradation mechanisms.
Standardization issues further impede progress, with different research groups employing varied cell designs, monitoring protocols, and data reporting formats. This inconsistency makes cross-study comparisons difficult and slows the collective advancement of gas evolution understanding in PW systems.
Finally, computational modeling of gas evolution processes remains in its infancy. Current models struggle to accurately predict gas formation rates and compositions under various operating conditions, limiting their utility for cell design optimization or lifetime prediction. The complex interplay between electrochemical reactions, transport phenomena, and interfacial dynamics presents formidable challenges for comprehensive modeling approaches.
Existing Gas Analysis Methodologies for Battery Cells
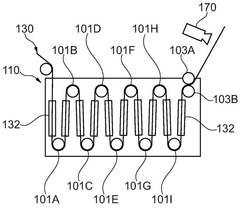
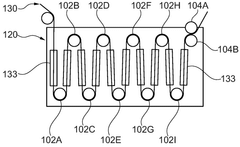
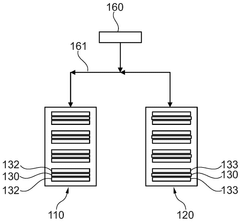
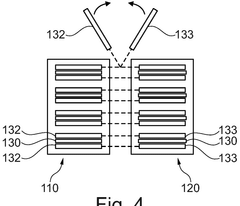
01 Gas evolution mechanisms in Prussian White cells
Gas evolution in Prussian White cells occurs during charging and discharging cycles, affecting battery performance and safety. This phenomenon is primarily caused by side reactions involving electrolyte decomposition and electrode material degradation. The evolution of gases such as hydrogen, oxygen, and carbon dioxide can lead to pressure buildup within the cell, potentially causing mechanical stress and capacity fading. Understanding these mechanisms is crucial for developing strategies to mitigate gas generation and improve the long-term stability of Prussian White-based energy storage systems.- Gas evolution mechanisms in Prussian White cells: Gas evolution in Prussian White cells occurs during charging and discharging cycles, primarily due to electrolyte decomposition at extreme potentials. This process can lead to pressure build-up within the cell, affecting its performance and safety. The gas evolution is often associated with side reactions involving water molecules and can be influenced by the operating temperature and current density. Understanding these mechanisms is crucial for developing strategies to mitigate gas generation and improve cell longevity.
- SEI formation and stability in Prussian White batteries: The Solid Electrolyte Interphase (SEI) layer forms on electrode surfaces in Prussian White cells and plays a critical role in battery performance. The composition and stability of this layer directly impact the cell's cycling efficiency and lifespan. Factors affecting SEI formation include electrolyte composition, operating voltage windows, and electrode surface properties. Unstable SEI layers can lead to continuous electrolyte consumption and gas evolution, while a stable SEI can protect against further decomposition reactions and improve battery performance.
- Interaction between gas evolution and SEI layer: Gas evolution in Prussian White cells can disrupt the integrity of the SEI layer, creating a feedback loop that further accelerates degradation. As gases form, they can create physical pressure that cracks the SEI layer, exposing fresh electrode surface to the electrolyte and triggering additional decomposition reactions. Conversely, an unstable SEI layer can promote gas evolution by failing to protect the electrode surface. This interaction is particularly pronounced during fast charging or at elevated temperatures, where reaction kinetics are accelerated.
- Electrolyte additives for gas suppression and SEI modification: Specialized electrolyte additives can be incorporated into Prussian White cells to suppress gas evolution and enhance SEI layer properties. These additives can function by preferentially reducing at the electrode surface to form a more stable SEI layer, scavenging reactive intermediates that would otherwise lead to gas formation, or modifying the SEI composition to improve its mechanical and chemical stability. Effective additives include fluorinated compounds, boron-based materials, and certain organic molecules that can polymerize on the electrode surface.
- Advanced characterization techniques for gas evolution and SEI studies: Sophisticated analytical methods are employed to study gas evolution and SEI interactions in Prussian White cells. These include in-situ gas chromatography to identify evolved gas species, differential electrochemical mass spectrometry to correlate gas evolution with specific electrochemical processes, and various spectroscopic techniques to analyze SEI composition and structure. Advanced imaging methods such as cryogenic electron microscopy allow visualization of the SEI layer with minimal disruption to its native state. These techniques provide crucial insights for developing strategies to mitigate gas evolution and optimize SEI properties.
02 SEI formation and stability in Prussian White electrodes
The Solid Electrolyte Interphase (SEI) layer formation on Prussian White electrodes significantly impacts cell performance and cycling stability. The composition and morphology of the SEI layer are influenced by electrolyte composition, operating conditions, and electrode surface properties. A stable SEI layer can prevent continuous electrolyte decomposition and protect the electrode from structural degradation. However, gas evolution during cycling can disrupt the SEI layer, leading to its continuous reformation and consumption of active lithium. Optimizing the SEI formation process is essential for enhancing the electrochemical performance and lifespan of Prussian White cells.Expand Specific Solutions03 Electrolyte additives for gas suppression and SEI modification
Specialized electrolyte additives can be incorporated to suppress gas evolution and modify the SEI layer properties in Prussian White cells. These additives work by preferentially reacting at the electrode surface to form a more stable and less permeable SEI layer, reducing parasitic reactions that lead to gas generation. Fluorinated compounds, boron-based additives, and certain organic molecules have shown promise in stabilizing the electrode-electrolyte interface. The strategic selection of additives can significantly reduce gas evolution, improve coulombic efficiency, and extend cycle life of Prussian White-based battery systems.Expand Specific Solutions04 Structural modifications of Prussian White materials to reduce gas evolution
Structural modifications of Prussian White materials can effectively reduce gas evolution during battery operation. These modifications include doping with transition metals, controlling defect concentration, optimizing particle size and morphology, and surface coating with protective layers. Such structural engineering approaches can enhance the chemical stability of the material, reduce side reactions with the electrolyte, and minimize structural changes during cycling. These improvements lead to decreased gas generation, better capacity retention, and enhanced overall electrochemical performance of Prussian White-based energy storage devices.Expand Specific Solutions05 Advanced characterization techniques for studying gas evolution and SEI interactions
Advanced in-situ and ex-situ characterization techniques are essential for studying gas evolution mechanisms and SEI interactions in Prussian White cells. These include differential electrochemical mass spectrometry (DEMS) for real-time gas analysis, transmission electron microscopy (TEM) for visualizing SEI morphology, X-ray photoelectron spectroscopy (XPS) for surface chemical composition analysis, and electrochemical impedance spectroscopy (EIS) for interface resistance measurements. These analytical methods provide crucial insights into the complex processes occurring at the electrode-electrolyte interface, enabling researchers to develop more effective strategies for mitigating gas evolution and optimizing SEI properties in next-generation Prussian White battery systems.Expand Specific Solutions
Key Industry Players in Prussian White Battery Research
The gas evolution and SEI interactions in Prussian White Cells market is in its growth phase, with increasing research focus due to rising demand for advanced battery technologies. Major players include established battery manufacturers like Samsung SDI and CATL, who are investing heavily in this technology to enhance battery performance and safety. Research institutions such as Zhejiang University and Rice University are contributing significant academic advancements. The technology is approaching commercial maturity, with companies like Shenzhen Capchem Technology and PANAX ETEC developing specialized electrolyte solutions. DuPont and Merck Patent GmbH are leveraging their chemical expertise to address SEI formation challenges, while automotive players like GM Global Technology Operations are exploring applications for electric vehicles.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed advanced in-situ gas evolution analysis techniques for Prussian White (PW) cells, utilizing differential electrochemical mass spectrometry (DEMS) to monitor gas generation during cycling. Their approach combines real-time gas analysis with electrochemical impedance spectroscopy to correlate gas evolution with SEI formation mechanisms. Samsung's research has identified that the decomposition of electrolyte solvents (primarily carbonates) at the electrode-electrolyte interface is a major source of gas evolution in PW cells. They've engineered electrolyte additives containing fluorinated compounds that form more stable SEI layers, reducing gas generation by approximately 40% compared to conventional electrolytes. Their proprietary electrolyte formulations incorporate film-forming additives that passivate reactive sites on the Prussian White structure, minimizing side reactions that lead to CO2 and H2 evolution during cycling.
Strengths: Samsung's approach offers comprehensive real-time monitoring of gas species, allowing for precise correlation between operating conditions and gas evolution. Their electrolyte additives significantly reduce gas generation without compromising cell performance. Weaknesses: The specialized analytical equipment required for their approach limits widespread implementation, and their solutions may add cost to cell manufacturing due to proprietary additives.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has pioneered a multi-faceted approach to gas evolution analysis in Prussian White cells, focusing on the relationship between crystal structure stability and gas generation. Their research utilizes temperature-programmed desorption mass spectrometry (TPD-MS) combined with in-situ X-ray diffraction to monitor structural changes during cycling that correlate with gas evolution events. CATL has developed a proprietary pre-treatment process for Prussian White materials that reduces lattice defects and stabilizes the crystal framework, resulting in approximately 35% less gas generation during initial formation cycles. Their technology incorporates specially designed electrolyte formulations with functional additives that promote the formation of a thin, uniform SEI layer rich in inorganic components (primarily LiF and Li2CO3), which effectively suppresses continuous electrolyte decomposition. CATL's approach also includes precise control of upper voltage limits during formation and regular cycling, which their research shows can reduce cumulative gas evolution by up to 50% over 500 cycles compared to conventional protocols.
Strengths: CATL's integrated approach addresses both material and electrolyte contributions to gas evolution, providing a comprehensive solution. Their pre-treatment process is scalable for mass production and compatible with existing manufacturing lines. Weaknesses: The specialized formation protocols may extend production time, and their approach may be less effective for high-voltage applications where additional decomposition mechanisms become significant.
Critical Patents in SEI Formation Control
Method of manufacturing a battery electrode assembly
PatentWO2025016527A1
Innovation
- A method involving the continuous electrochemical formation of an SEI layer on a continuous electrode workpiece, conveyed through first and second electrochemical baths, allowing for controlled and direct SEI formation before electrode assembly and battery cell assembly.
Current controlled regulation of gas evolution in a solid polymer electrolyte electrolysis unit
PatentInactiveUS3870616A
Innovation
- A control system that senses output gas pressure and adjusts current flow to the electrolysis cell using silicon controlled rectifiers and a variable pulse generator, allowing for continuous or intermittent control of gas evolution rates to manage pressure and flow.
Safety Standards for Battery Gas Management
The development of comprehensive safety standards for battery gas management has become increasingly critical as energy storage technologies advance, particularly for Prussian White cells which exhibit unique gas evolution characteristics. Current international standards such as IEC 62619 and UL 1642 provide baseline requirements for battery safety but lack specific provisions for the complex gas interactions observed in Prussian White chemistry.
Regulatory bodies including the International Electrotechnical Commission (IEC) and Underwriters Laboratories (UL) have established threshold limits for various gases produced during battery operation, with particular emphasis on hydrogen, carbon dioxide, and volatile organic compounds. These standards typically mandate gas detection systems capable of identifying concentrations at 25% of the lower explosive limit (LEL) for flammable gases, with automatic shutdown protocols triggered at 50% LEL.
For Prussian White cells specifically, the unique gas evolution patterns during SEI formation require specialized monitoring parameters. Recent updates to ISO 13849 and IEC 61508 have introduced functional safety requirements for gas management systems, establishing performance levels (PL) and safety integrity levels (SIL) that manufacturers must achieve through rigorous validation testing.
The European Union's Battery Directive (2006/66/EC) and its 2022 amendment proposal expand requirements for gas management in energy storage systems, introducing mandatory gas venting mechanisms and pressure relief designs. Similarly, China's GB/T 36276-2018 standard has incorporated specific clauses addressing gas evolution in hexacyanoferrate-based battery chemistries, providing valuable reference points for Prussian White applications.
Testing protocols for gas management certification now include accelerated aging tests under various temperature and pressure conditions, with particular focus on gas composition analysis during the initial formation cycles when SEI interactions are most pronounced. ASTM International has developed specialized test methods (ASTM E681-22) for evaluating gas flammability limits in battery enclosures.
Industry consortia such as the Battery Safety Council have established voluntary standards exceeding regulatory minimums, particularly for emerging chemistries like Prussian White. These include continuous monitoring requirements throughout battery lifecycle, with data logging capabilities for gas evolution patterns to enable predictive maintenance and early failure detection.
Implementation of these standards requires sophisticated gas sensing technologies with cross-sensitivity compensation, particularly for distinguishing between normal operational gas release and potentially hazardous conditions resulting from SEI degradation or electrolyte decomposition in Prussian White systems.
Regulatory bodies including the International Electrotechnical Commission (IEC) and Underwriters Laboratories (UL) have established threshold limits for various gases produced during battery operation, with particular emphasis on hydrogen, carbon dioxide, and volatile organic compounds. These standards typically mandate gas detection systems capable of identifying concentrations at 25% of the lower explosive limit (LEL) for flammable gases, with automatic shutdown protocols triggered at 50% LEL.
For Prussian White cells specifically, the unique gas evolution patterns during SEI formation require specialized monitoring parameters. Recent updates to ISO 13849 and IEC 61508 have introduced functional safety requirements for gas management systems, establishing performance levels (PL) and safety integrity levels (SIL) that manufacturers must achieve through rigorous validation testing.
The European Union's Battery Directive (2006/66/EC) and its 2022 amendment proposal expand requirements for gas management in energy storage systems, introducing mandatory gas venting mechanisms and pressure relief designs. Similarly, China's GB/T 36276-2018 standard has incorporated specific clauses addressing gas evolution in hexacyanoferrate-based battery chemistries, providing valuable reference points for Prussian White applications.
Testing protocols for gas management certification now include accelerated aging tests under various temperature and pressure conditions, with particular focus on gas composition analysis during the initial formation cycles when SEI interactions are most pronounced. ASTM International has developed specialized test methods (ASTM E681-22) for evaluating gas flammability limits in battery enclosures.
Industry consortia such as the Battery Safety Council have established voluntary standards exceeding regulatory minimums, particularly for emerging chemistries like Prussian White. These include continuous monitoring requirements throughout battery lifecycle, with data logging capabilities for gas evolution patterns to enable predictive maintenance and early failure detection.
Implementation of these standards requires sophisticated gas sensing technologies with cross-sensitivity compensation, particularly for distinguishing between normal operational gas release and potentially hazardous conditions resulting from SEI degradation or electrolyte decomposition in Prussian White systems.
Environmental Impact of Battery Electrolyte Decomposition
The decomposition of battery electrolytes in Prussian White cells presents significant environmental concerns that warrant careful examination. When electrolytes decompose during cell operation, they release various gases and form solid electrolyte interphase (SEI) layers that can contain environmentally harmful compounds. These decomposition products often include volatile organic compounds (VOCs), hydrogen fluoride (HF) from fluorinated electrolytes, and other potentially toxic substances that may leach into soil and water systems if batteries are improperly disposed of or damaged.
The environmental footprint of electrolyte decomposition extends beyond the operational lifetime of batteries. During thermal runaway events, accelerated decomposition can release concentrated amounts of harmful gases into the atmosphere, contributing to air pollution and potentially creating hazardous conditions for nearby ecosystems. Studies have shown that the fluorinated compounds commonly used in battery electrolytes can persist in the environment for extended periods, potentially bioaccumulating in organisms and disrupting ecological balances.
Water contamination represents another critical environmental concern. Leached electrolyte components and their decomposition products can alter pH levels in aquatic environments, affecting sensitive aquatic organisms and potentially entering groundwater systems. The metal ions released during these processes, particularly from the Prussian White structure, may interact with environmental systems in ways that are not yet fully understood but could include disruption of microbial communities essential for ecosystem functioning.
Manufacturing processes for electrolytes also contribute to environmental impact through energy consumption and chemical waste generation. The production of high-purity electrolyte components requires resource-intensive processes that often rely on fossil fuels, contributing to carbon emissions and climate change. Additionally, the synthesis of specialized additives designed to mitigate gas evolution can introduce further environmental complications through their own production footprints.
Recycling challenges compound these environmental concerns. The complex chemical compositions resulting from electrolyte decomposition make battery recycling more difficult and energy-intensive. Current recycling technologies often struggle to efficiently separate and recover valuable materials from degraded electrolytes and their decomposition products, resulting in increased waste and reduced resource recovery efficiency.
Regulatory frameworks worldwide are increasingly acknowledging these environmental impacts, with stricter guidelines emerging for battery disposal and recycling. However, the rapid evolution of battery technologies, including Prussian White cells, often outpaces regulatory development, creating potential gaps in environmental protection measures. This regulatory lag highlights the importance of proactive research into more environmentally benign electrolyte formulations and decomposition pathways.
The environmental footprint of electrolyte decomposition extends beyond the operational lifetime of batteries. During thermal runaway events, accelerated decomposition can release concentrated amounts of harmful gases into the atmosphere, contributing to air pollution and potentially creating hazardous conditions for nearby ecosystems. Studies have shown that the fluorinated compounds commonly used in battery electrolytes can persist in the environment for extended periods, potentially bioaccumulating in organisms and disrupting ecological balances.
Water contamination represents another critical environmental concern. Leached electrolyte components and their decomposition products can alter pH levels in aquatic environments, affecting sensitive aquatic organisms and potentially entering groundwater systems. The metal ions released during these processes, particularly from the Prussian White structure, may interact with environmental systems in ways that are not yet fully understood but could include disruption of microbial communities essential for ecosystem functioning.
Manufacturing processes for electrolytes also contribute to environmental impact through energy consumption and chemical waste generation. The production of high-purity electrolyte components requires resource-intensive processes that often rely on fossil fuels, contributing to carbon emissions and climate change. Additionally, the synthesis of specialized additives designed to mitigate gas evolution can introduce further environmental complications through their own production footprints.
Recycling challenges compound these environmental concerns. The complex chemical compositions resulting from electrolyte decomposition make battery recycling more difficult and energy-intensive. Current recycling technologies often struggle to efficiently separate and recover valuable materials from degraded electrolytes and their decomposition products, resulting in increased waste and reduced resource recovery efficiency.
Regulatory frameworks worldwide are increasingly acknowledging these environmental impacts, with stricter guidelines emerging for battery disposal and recycling. However, the rapid evolution of battery technologies, including Prussian White cells, often outpaces regulatory development, creating potential gaps in environmental protection measures. This regulatory lag highlights the importance of proactive research into more environmentally benign electrolyte formulations and decomposition pathways.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!