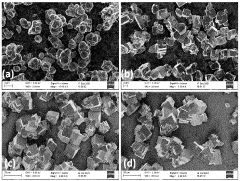
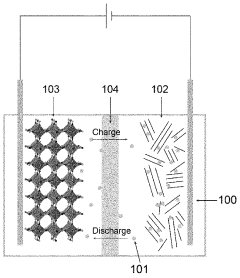
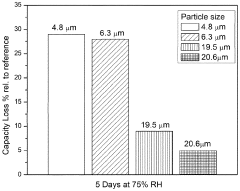
Residual Iron And Copper Control In Prussian White Synthesis Scale-Up
Prussian White Synthesis Background and Objectives
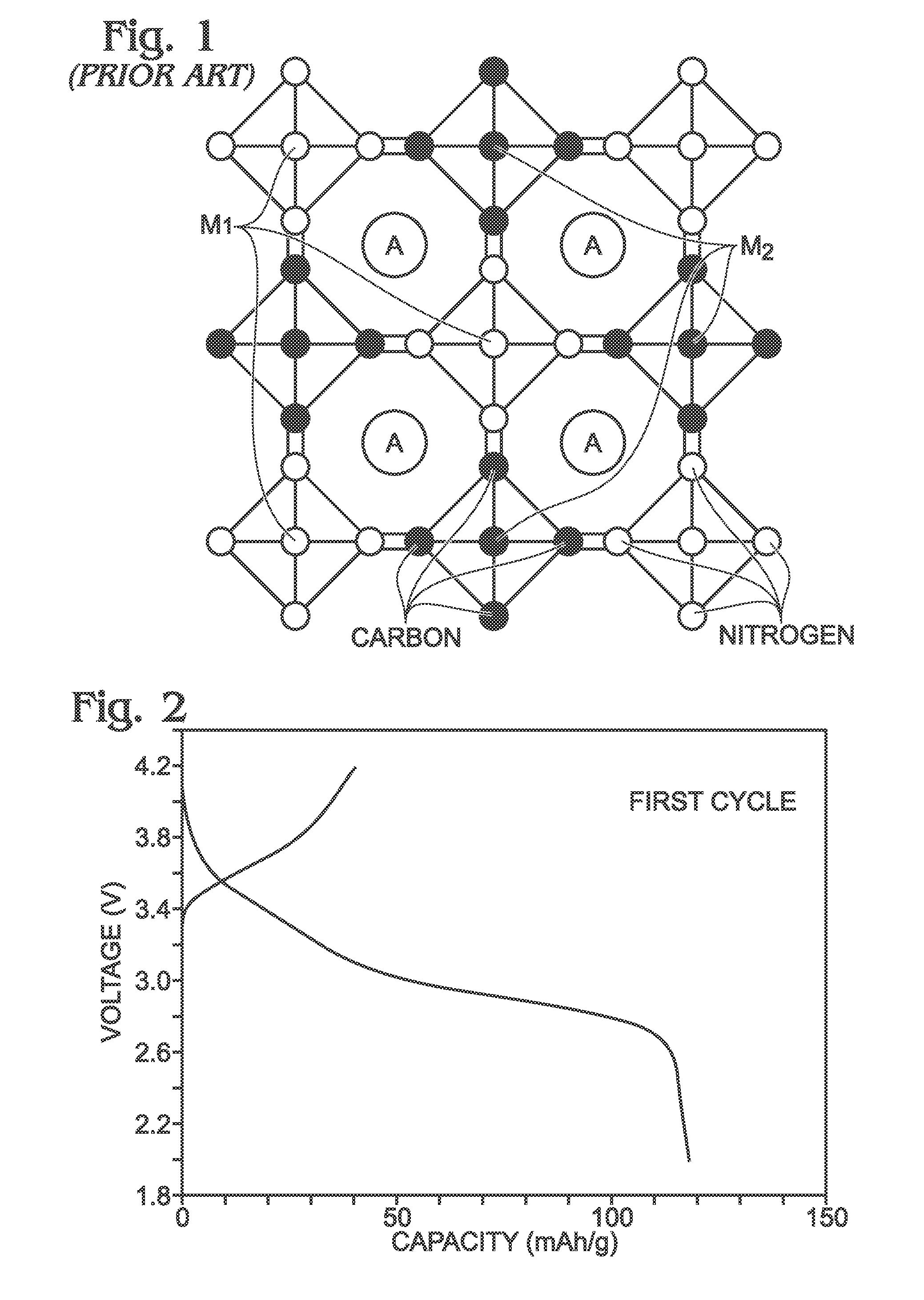
Prussian White, chemically known as potassium iron(II) hexacyanoferrate(II), represents a critical material in the development of advanced energy storage systems, particularly in sodium-ion batteries. The synthesis of this compound has evolved significantly over the past two decades, transitioning from laboratory-scale production to industrial manufacturing processes. Initially developed in the early 2000s as a potential cathode material, Prussian White has gained substantial attention due to its unique crystal structure that facilitates rapid ion insertion and extraction, making it particularly suitable for high-power applications.
The evolution of Prussian White synthesis techniques has been marked by several key milestones. Early methods focused on simple precipitation reactions in aqueous solutions, while more recent approaches have incorporated advanced techniques such as controlled atmosphere synthesis, microwave-assisted methods, and continuous flow processes. These developments have been driven by the increasing demand for higher purity materials with consistent electrochemical performance.
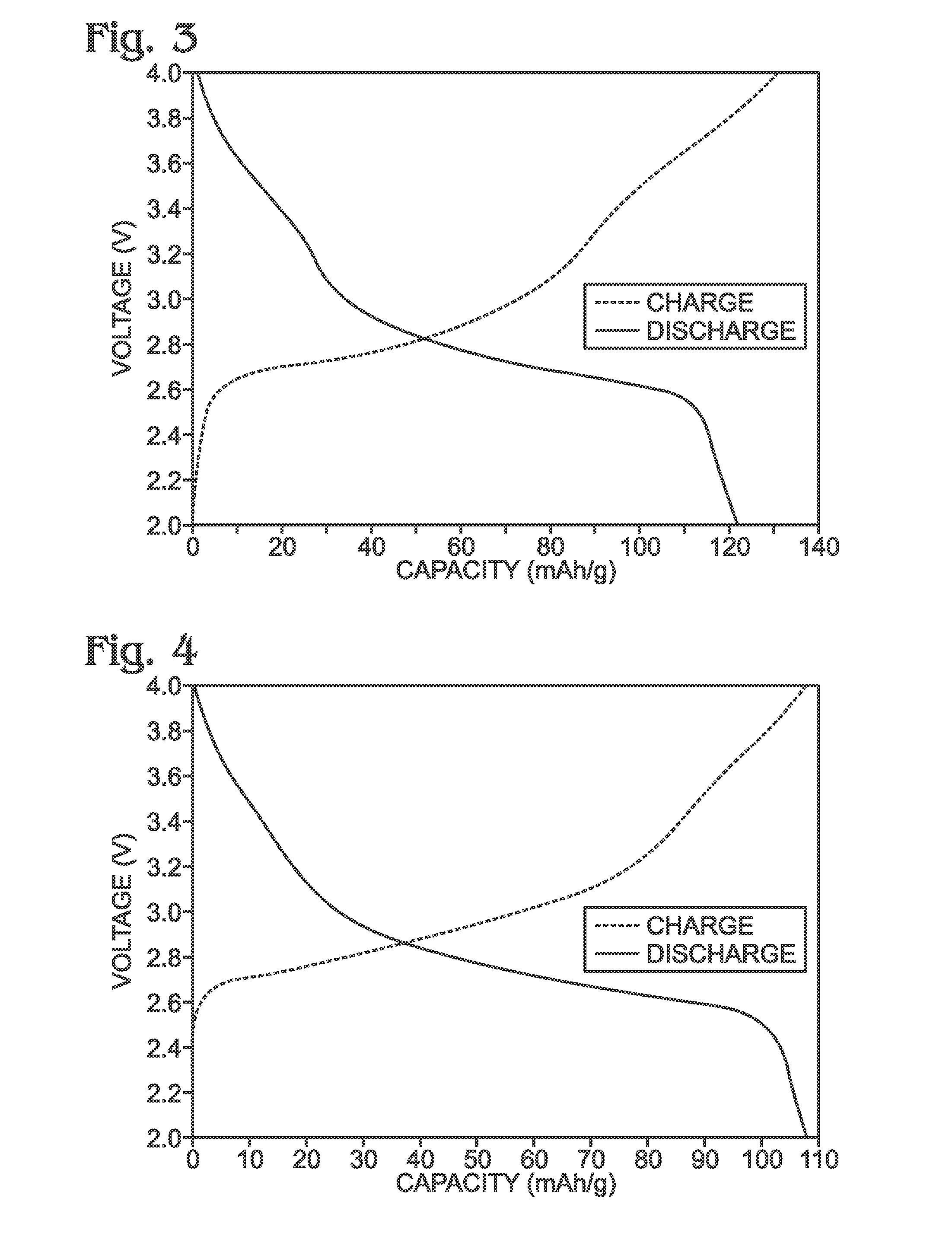
A significant challenge in the scale-up of Prussian White synthesis is the control of residual iron and copper impurities. These metallic contaminants can significantly impact the electrochemical performance, cycle life, and safety characteristics of the final battery systems. Traditional synthesis methods often result in variable levels of these impurities, leading to inconsistent product quality and performance.
The primary technical objective of this research is to develop robust methodologies for controlling residual iron and copper during large-scale Prussian White synthesis. This includes investigating the fundamental mechanisms of impurity incorporation, developing effective purification strategies, and establishing reliable quality control protocols. The ultimate goal is to achieve consistent production of high-purity Prussian White with impurity levels below 50 ppm for both iron and copper.
Secondary objectives include optimizing the synthesis parameters to enhance yield and energy efficiency, reducing production costs through process intensification, and developing in-line monitoring techniques for real-time quality assessment. Additionally, the research aims to establish a comprehensive understanding of how synthesis conditions affect the structural and electrochemical properties of the final product.
The technological trajectory suggests that mastering impurity control in Prussian White synthesis will be crucial for enabling its widespread adoption in commercial energy storage applications. As the demand for high-performance, cost-effective battery materials continues to grow, addressing these fundamental synthesis challenges becomes increasingly important for positioning this technology in the competitive energy storage landscape.
Market Analysis for High-Purity Prussian White Materials
The global market for high-purity Prussian White materials has been experiencing significant growth, driven primarily by the expanding energy storage sector. Prussian White, a key component in sodium-ion batteries, offers a compelling alternative to traditional lithium-ion technology, particularly in grid-scale storage applications where cost considerations outweigh energy density requirements.
Market research indicates that the demand for high-purity Prussian White materials is projected to grow at a compound annual growth rate of 24% through 2030, reaching a market valuation exceeding $2 billion. This growth trajectory is supported by increasing investments in renewable energy infrastructure and the corresponding need for efficient, cost-effective energy storage solutions.
The Asia-Pacific region currently dominates the market landscape, accounting for approximately 65% of global demand. China, in particular, has emerged as both the largest producer and consumer of Prussian White materials, with several major battery manufacturers actively scaling up production capacities. European markets are showing accelerated adoption rates, driven by stringent carbon reduction policies and substantial investments in green energy initiatives.
Critical to market acceptance is the purity level of Prussian White materials. Industry specifications increasingly demand residual iron and copper levels below 50 ppm, as higher contamination significantly impacts battery performance metrics including cycle life, capacity retention, and safety characteristics. Premium pricing tiers have emerged for ultra-high-purity materials (below 10 ppm contaminants), commanding up to 40% price premiums in specialized applications.
Market segmentation reveals distinct customer requirements across different application domains. Grid-scale energy storage projects prioritize cost-effectiveness and long-term stability, while emerging electric mobility applications demand higher performance specifications with stringent purity requirements. Industrial energy management systems represent a growing market segment with intermediate purity requirements.
Supply chain analysis reveals potential bottlenecks in scaling production while maintaining high purity levels. Current manufacturing processes struggle to consistently achieve sub-20 ppm contamination levels in industrial-scale production, creating market opportunities for innovative purification technologies. Companies demonstrating reliable scale-up capabilities while maintaining high purity standards are positioned to capture premium market segments.
Consumer electronics represents an emerging application area with stringent requirements for material consistency and safety performance. While currently a smaller market segment, it offers higher margins and could serve as a proving ground for advanced purification technologies before deployment in larger-scale applications.
Metal Impurity Challenges in Industrial-Scale Synthesis
The scale-up of Prussian White synthesis from laboratory to industrial production introduces significant challenges related to metal impurity control, particularly residual iron and copper. These impurities can severely compromise the electrochemical performance, stability, and safety of the final product when present even at parts-per-million levels. Traditional laboratory synthesis methods often rely on high-purity reagents and controlled environments that become economically unfeasible at industrial scale.
In industrial settings, metal contamination sources are diverse and often difficult to isolate. Raw material quality variations represent a primary concern, as commercial-grade precursors typically contain trace metal impurities that accumulate during the synthesis process. Equipment-derived contamination also becomes prominent during scale-up, with reactor vessels, agitators, and transfer lines introducing metal ions through corrosion, abrasion, or leaching processes.
Water quality emerges as a critical factor in large-scale production, where even trace impurities in process water can significantly impact final product purity. Industrial facilities often utilize water treatment systems that may not achieve the ultra-pure standards maintained in laboratory environments. Additionally, atmospheric contamination becomes relevant in open processing systems, where airborne particulates containing metal species can be introduced during synthesis or drying operations.
The kinetics and thermodynamics of impurity incorporation change dramatically with scale. Larger reaction volumes alter mixing efficiency, heat transfer rates, and concentration gradients, potentially creating localized conditions favorable for impurity incorporation. These scale-dependent phenomena can lead to inconsistent product quality even when following seemingly identical synthesis protocols.
Analytical challenges compound the difficulty of impurity control at industrial scale. Real-time monitoring of trace metal contaminants during production remains technically challenging and cost-prohibitive. Most manufacturers rely on batch sampling and offline analysis, creating significant delays between contamination events and their detection.
Economic constraints further complicate impurity management strategies. The cost-benefit analysis of implementing advanced purification technologies must balance product quality requirements against production economics. High-purity reagents, specialized equipment materials, and sophisticated purification processes significantly increase production costs, potentially rendering the final product commercially unviable.
Regulatory considerations add another dimension to metal impurity control. As energy storage applications face increasingly stringent safety and performance standards, manufacturers must demonstrate consistent control of metal contaminants to meet certification requirements. This regulatory pressure drives the need for robust, scalable purification strategies that can be validated across production batches.
Current Metal Removal Techniques in Prussian White Synthesis
01 Purification methods for removing residual iron and copper
Various purification methods can be employed to remove residual iron and copper during Prussian White synthesis. These include precipitation techniques, filtration processes, and washing steps that effectively separate metal impurities from the final product. Advanced separation techniques such as ion exchange and selective chelation can also be used to target specific metal ions, ensuring high purity Prussian White compounds with minimal contamination.- Precipitation and washing methods for iron control: Various precipitation and washing techniques are employed to control residual iron during Prussian White synthesis. These methods include controlled precipitation conditions, sequential washing steps with specific solvents, and pH-controlled washing procedures. By optimizing these parameters, the residual iron content can be significantly reduced, leading to higher purity Prussian White compounds suitable for battery applications.
- Chelation and complexation for metal impurity removal: Chelating agents and complexing compounds are utilized to bind and remove residual iron and copper impurities during Prussian White synthesis. These agents form stable complexes with metal ions, facilitating their separation from the desired product. Common chelating agents include EDTA, citric acid, and specialized organic ligands that selectively bind to iron and copper ions, allowing for their efficient removal through subsequent filtration or washing steps.
- Controlled redox conditions during synthesis: Maintaining precise redox conditions during the synthesis process is crucial for controlling residual iron and copper levels in Prussian White compounds. By carefully adjusting oxidation-reduction potentials through controlled addition of reducing agents, reaction temperature modulation, and oxygen-free environments, the formation of unwanted iron and copper species can be minimized. This approach ensures that metal ions are incorporated into the desired crystal structure rather than remaining as soluble impurities.
- Advanced purification techniques: Advanced purification methods are employed to achieve ultra-low levels of residual iron and copper in Prussian White materials. These techniques include recrystallization under controlled conditions, selective ion exchange processes, membrane filtration, and chromatographic separation. Such methods can effectively remove trace metal impurities that conventional washing steps cannot eliminate, resulting in high-purity Prussian White suitable for sensitive applications like energy storage and electrochemical devices.
- Precursor selection and reaction pathway optimization: The choice of iron and copper precursors, along with optimized reaction pathways, significantly impacts residual metal content in the final Prussian White product. By selecting high-purity starting materials, controlling reaction stoichiometry, and designing multi-step synthesis routes that minimize side reactions, the incorporation of excess iron and copper can be prevented. Additionally, the use of stabilizing agents during synthesis helps maintain proper crystal growth while preventing the formation of metal-rich domains or adsorbed impurities.
02 Controlled reaction conditions to minimize metal impurities
Precise control of reaction parameters during Prussian White synthesis can significantly reduce residual metal content. These parameters include temperature, pH, reaction time, and reagent concentration ratios. By optimizing these conditions, the formation of unwanted metal complexes can be minimized, leading to lower residual iron and copper levels in the final product. Controlled oxidation-reduction environments also play a crucial role in preventing metal contamination.Expand Specific Solutions03 Chelating agents for metal ion sequestration
Specific chelating agents can be incorporated during or after Prussian White synthesis to sequester excess iron and copper ions. These chelating compounds form stable complexes with metal ions, facilitating their removal from the reaction mixture. Common chelating agents include EDTA, citric acid, and specialized polymeric chelators designed for selective binding of transition metals, resulting in higher purity Prussian White products.Expand Specific Solutions04 Post-synthesis treatment processes
After the initial synthesis of Prussian White, various post-treatment processes can be applied to further reduce residual metal content. These include recrystallization techniques, controlled aging processes, and sequential washing with specific solvents or solutions. Advanced methods such as electrochemical purification and selective precipitation can also be employed to achieve ultra-low levels of iron and copper contamination in the final product.Expand Specific Solutions05 Analytical methods for metal impurity detection and control
Sophisticated analytical techniques are essential for monitoring and controlling residual iron and copper levels during Prussian White synthesis. These include spectroscopic methods such as atomic absorption spectroscopy, inductively coupled plasma mass spectrometry, and X-ray fluorescence analysis. Real-time monitoring systems can be implemented to provide feedback for process adjustments, ensuring consistent quality control and compliance with purity specifications for the final Prussian White product.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Prussian White Production
The Prussian White synthesis scale-up market is in an early growth phase, characterized by increasing demand for advanced battery technologies. The market size is expanding rapidly due to the growing need for sustainable energy storage solutions, particularly in electric vehicles and renewable energy sectors. Technologically, residual iron and copper control remains a critical challenge in scaling up production. Companies like Altris AB have made significant progress in developing industrial-scale production methods for Prussian White framework materials, while Guangdong Bangpu and Hunan Bangpu Recycling Technology are advancing recycling technologies that address metal contamination issues. Research institutions such as Huazhong University of Science & Technology and Central South University are contributing fundamental research, collaborating with industrial players like Höganäs AB and Kobe Steel to improve synthesis processes and metal impurity control techniques.
Guangdong Bangpu Recycling Technology Co., Ltd.
Central South University
Critical Patents for Iron and Copper Removal Technologies
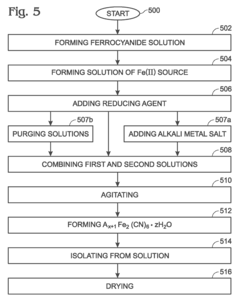
- A method involving the use of reducing agents in a low oxygen environment to maintain Fe(II) species, with solutions purged with inert gases to prevent oxidation, allowing for the synthesis of Na1+xFe2(CN)6 through a straightforward process that avoids hydrothermal processing.
- A sodium iron(II)-hexacyanoferrate(II) material with a particle diameter D50 value between 4 μm and 50 μm and a BET specific surface area between 0.1 m²/g and 10 m²/g is developed, which reduces sodium loss and enhances moisture stability, maintaining high conductivity and capacity over time.
Environmental Impact Assessment of Synthesis Processes
The scale-up of Prussian White synthesis processes presents significant environmental considerations that must be thoroughly assessed. The primary environmental concerns revolve around the management of residual iron and copper ions, which can pose serious ecological risks if released into water systems. These heavy metals are known to bioaccumulate in aquatic organisms and potentially enter the food chain, causing long-term ecosystem damage even at relatively low concentrations.
Current synthesis methods typically generate wastewater containing varying levels of these metal contaminants. Laboratory-scale processes may produce manageable volumes, but industrial-scale production can generate substantial quantities of contaminated effluent requiring specialized treatment. Conventional treatment approaches include chemical precipitation, ion exchange, and advanced oxidation processes, each with different environmental footprints and efficiency profiles.
Energy consumption represents another critical environmental factor in scaled-up synthesis operations. The precise temperature control required for optimal Prussian White formation demands significant energy inputs, particularly when transitioning from laboratory to industrial scales. This increased energy demand directly correlates with higher carbon emissions unless renewable energy sources are integrated into the manufacturing infrastructure.
Life cycle assessment (LCA) studies indicate that the environmental impact of residual metal control strategies varies considerably. Precipitation methods using hydroxides or sulfides create solid waste requiring disposal, while membrane filtration technologies consume less chemicals but demand higher energy inputs. Recent innovations in green chemistry approaches, such as bioremediation using specialized microorganisms or plant-based adsorbents, show promise for reducing the overall environmental footprint.
Regulatory frameworks worldwide are increasingly stringent regarding heavy metal discharge limits. The European Union's Water Framework Directive and the United States EPA guidelines establish strict thresholds for iron and copper in industrial effluents. Compliance with these regulations necessitates comprehensive monitoring systems and may require significant capital investment in advanced treatment technologies during scale-up operations.
Circular economy principles offer promising pathways for minimizing environmental impacts. Recovery and recycling of residual iron and copper from waste streams can transform these potential pollutants into valuable resources. Several pilot programs have demonstrated the technical feasibility of metal recovery systems that can achieve up to 95% reclamation rates, substantially reducing both waste generation and raw material requirements for subsequent synthesis batches.
Quality Control Standards and Testing Protocols
Quality control is paramount in the scale-up of Prussian White synthesis, particularly regarding residual iron and copper control. Establishing rigorous standards and testing protocols ensures consistent product quality and performance across production batches. The industry currently employs several standardized methods for quantifying residual metal content, with inductively coupled plasma mass spectrometry (ICP-MS) and atomic absorption spectroscopy (AAS) being the gold standards for precise metal ion detection at parts-per-billion levels.
For routine quality control in production environments, X-ray fluorescence (XRF) spectroscopy offers rapid, non-destructive analysis of residual metals. Manufacturers typically set acceptance thresholds for residual iron at <50 ppm and copper at <20 ppm, though these specifications vary based on application requirements. More stringent standards apply for high-performance energy storage applications, where even trace metal contamination can significantly impact electrochemical performance.
Standardized sampling protocols are essential for reliable quality assessment. The current best practice involves collecting representative samples at multiple stages of the synthesis process, with particular attention to post-washing phases where residual metal concentrations are most variable. Statistical process control methods, including control charts for tracking residual metal concentrations over time, enable early detection of process drift before specifications are violated.
Testing frequency represents another critical aspect of quality control. Leading manufacturers implement a tiered testing approach: comprehensive metal profile analysis for each new batch of raw materials, in-process testing at critical control points, and final product verification before release. This multi-layered approach balances thoroughness with production efficiency.
Validation protocols for analytical methods must demonstrate specificity, accuracy, precision, linearity, and robustness. Method transfer between R&D and production facilities requires careful standardization to ensure consistent results across different instruments and operators. Interlaboratory proficiency testing programs help maintain analytical quality across the industry.
Documentation standards for residual metal testing include detailed records of instrument calibration, standard preparation, sample handling procedures, and analytical results with measurement uncertainties. Electronic laboratory information management systems (LIMS) facilitate data integrity and traceability throughout the product lifecycle, supporting both quality assurance and regulatory compliance requirements.