Prussian White Cathodes For High-Power Sodium-Ion Stationary Storage
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Prussian White Cathode Technology Background and Objectives
Prussian White compounds, a class of open-framework materials with the general formula AxM[Fe(CN)6]y·nH2O (where A represents alkali metal ions, M represents transition metal ions), have emerged as promising cathode materials for sodium-ion batteries (SIBs) over the past decade. The evolution of this technology can be traced back to the early 2010s when researchers began exploring alternatives to lithium-ion batteries due to concerns about lithium resource limitations and cost escalation. Prussian White cathodes represent a significant advancement in this quest, offering a sustainable and economically viable solution for large-scale stationary energy storage applications.
The technological trajectory of Prussian White cathodes has been characterized by continuous improvements in structural stability, electrochemical performance, and manufacturing processes. Initially, these materials suffered from poor cycling stability and low energy density due to structural water and hexacyanoferrate vacancies. However, systematic research efforts have led to substantial enhancements in their performance metrics, making them increasingly attractive for grid-scale energy storage systems.
Recent developments have focused on optimizing the composition and structure of Prussian White cathodes to achieve higher energy density, improved rate capability, and extended cycle life. The incorporation of various transition metals (such as manganese, iron, nickel, and copper) and precise control of sodium content have been key strategies in tailoring the electrochemical properties of these materials. Additionally, advanced synthesis methods have been developed to minimize lattice defects and control water content, addressing the historical limitations of Prussian White compounds.
The primary technical objective in this field is to develop Prussian White cathode materials that can deliver high power density (>500 W/kg), excellent cycling stability (>5000 cycles), and competitive energy density (>80 Wh/kg) at a significantly lower cost compared to lithium-ion technologies. These performance targets are essential for meeting the demanding requirements of stationary storage applications, particularly for grid stabilization, renewable energy integration, and peak shaving.
Another critical objective is to establish scalable and environmentally friendly manufacturing processes that can support the mass production of Prussian White cathodes using earth-abundant elements. This aligns with the broader goal of creating sustainable energy storage solutions that minimize environmental impact throughout their lifecycle.
Looking forward, the technological evolution of Prussian White cathodes is expected to continue along several trajectories, including the development of novel composite structures, surface modification strategies, and electrolyte optimization. These advancements aim to further enhance the performance and durability of sodium-ion batteries, positioning them as a viable alternative to lithium-ion technologies for stationary storage applications where cost and sustainability are paramount considerations.
The technological trajectory of Prussian White cathodes has been characterized by continuous improvements in structural stability, electrochemical performance, and manufacturing processes. Initially, these materials suffered from poor cycling stability and low energy density due to structural water and hexacyanoferrate vacancies. However, systematic research efforts have led to substantial enhancements in their performance metrics, making them increasingly attractive for grid-scale energy storage systems.
Recent developments have focused on optimizing the composition and structure of Prussian White cathodes to achieve higher energy density, improved rate capability, and extended cycle life. The incorporation of various transition metals (such as manganese, iron, nickel, and copper) and precise control of sodium content have been key strategies in tailoring the electrochemical properties of these materials. Additionally, advanced synthesis methods have been developed to minimize lattice defects and control water content, addressing the historical limitations of Prussian White compounds.
The primary technical objective in this field is to develop Prussian White cathode materials that can deliver high power density (>500 W/kg), excellent cycling stability (>5000 cycles), and competitive energy density (>80 Wh/kg) at a significantly lower cost compared to lithium-ion technologies. These performance targets are essential for meeting the demanding requirements of stationary storage applications, particularly for grid stabilization, renewable energy integration, and peak shaving.
Another critical objective is to establish scalable and environmentally friendly manufacturing processes that can support the mass production of Prussian White cathodes using earth-abundant elements. This aligns with the broader goal of creating sustainable energy storage solutions that minimize environmental impact throughout their lifecycle.
Looking forward, the technological evolution of Prussian White cathodes is expected to continue along several trajectories, including the development of novel composite structures, surface modification strategies, and electrolyte optimization. These advancements aim to further enhance the performance and durability of sodium-ion batteries, positioning them as a viable alternative to lithium-ion technologies for stationary storage applications where cost and sustainability are paramount considerations.
Market Analysis for Sodium-Ion Stationary Storage Solutions
The global energy storage market is experiencing unprecedented growth, with stationary storage solutions becoming increasingly vital for grid stability and renewable energy integration. Sodium-ion battery technology, particularly those utilizing Prussian White cathodes, is emerging as a compelling alternative to lithium-ion batteries in this sector. Current market projections indicate the stationary energy storage market will reach $31.2 billion by 2025, with a compound annual growth rate of 34% from 2020.
The demand for sodium-ion stationary storage is driven by several key factors. First, the dramatic cost reduction potential compared to lithium-ion technologies - sodium resources are approximately 1,000 times more abundant than lithium, with significantly lower raw material costs. Second, the growing concern over lithium supply chain vulnerabilities, with over 70% of lithium resources concentrated in South America's "Lithium Triangle." Third, the increasing need for long-duration energy storage solutions to support renewable energy integration, where cost-effectiveness becomes more critical than energy density.
Market segmentation reveals particularly strong potential for sodium-ion technologies in utility-scale applications, grid services, and remote/off-grid installations. These segments prioritize cost, safety, and longevity over the energy density advantages that lithium-ion batteries offer for mobile applications. Prussian White cathode-based systems specifically address these market requirements through their high power capabilities and excellent cycle stability.
Regional analysis shows Asia-Pacific leading the adoption curve, with China investing heavily in sodium-ion technology development. The European market follows closely, driven by stringent sustainability regulations and renewable energy targets. North America presents significant growth potential, particularly as utilities seek cost-effective solutions for grid modernization initiatives.
Customer surveys indicate that utility operators prioritize levelized cost of storage (LCOS) as their primary decision factor, followed by cycle life and safety characteristics. Prussian White cathode-based sodium-ion batteries excel in these specific metrics, positioning them favorably against competing technologies.
Competitive landscape assessment reveals that while lithium-ion technologies currently dominate with 90% market share in new installations, alternative chemistries including sodium-ion are gaining traction. Flow batteries, compressed air, and thermal storage solutions represent other competing technologies, each with distinct advantages for specific use cases.
Market barriers include technology maturity concerns, limited manufacturing scale, and conservative adoption practices in the utility sector. However, the accelerating research focus on Prussian White cathodes, combined with increasing commercial interest, suggests these barriers will diminish over the next 3-5 years as the technology demonstrates its value proposition in real-world deployments.
The demand for sodium-ion stationary storage is driven by several key factors. First, the dramatic cost reduction potential compared to lithium-ion technologies - sodium resources are approximately 1,000 times more abundant than lithium, with significantly lower raw material costs. Second, the growing concern over lithium supply chain vulnerabilities, with over 70% of lithium resources concentrated in South America's "Lithium Triangle." Third, the increasing need for long-duration energy storage solutions to support renewable energy integration, where cost-effectiveness becomes more critical than energy density.
Market segmentation reveals particularly strong potential for sodium-ion technologies in utility-scale applications, grid services, and remote/off-grid installations. These segments prioritize cost, safety, and longevity over the energy density advantages that lithium-ion batteries offer for mobile applications. Prussian White cathode-based systems specifically address these market requirements through their high power capabilities and excellent cycle stability.
Regional analysis shows Asia-Pacific leading the adoption curve, with China investing heavily in sodium-ion technology development. The European market follows closely, driven by stringent sustainability regulations and renewable energy targets. North America presents significant growth potential, particularly as utilities seek cost-effective solutions for grid modernization initiatives.
Customer surveys indicate that utility operators prioritize levelized cost of storage (LCOS) as their primary decision factor, followed by cycle life and safety characteristics. Prussian White cathode-based sodium-ion batteries excel in these specific metrics, positioning them favorably against competing technologies.
Competitive landscape assessment reveals that while lithium-ion technologies currently dominate with 90% market share in new installations, alternative chemistries including sodium-ion are gaining traction. Flow batteries, compressed air, and thermal storage solutions represent other competing technologies, each with distinct advantages for specific use cases.
Market barriers include technology maturity concerns, limited manufacturing scale, and conservative adoption practices in the utility sector. However, the accelerating research focus on Prussian White cathodes, combined with increasing commercial interest, suggests these barriers will diminish over the next 3-5 years as the technology demonstrates its value proposition in real-world deployments.
Technical Challenges and Global Development Status
Prussian White cathode materials for sodium-ion batteries face several significant technical challenges despite their promising theoretical properties. The primary obstacle remains the structural instability during cycling, particularly at high charge/discharge rates. The crystal framework experiences volume changes and lattice distortions that lead to capacity fading over extended cycling periods. This instability is exacerbated by the larger ionic radius of sodium compared to lithium, creating more substantial mechanical stress during ion insertion and extraction processes.
Another critical challenge is the relatively low electronic conductivity of Prussian White compounds, which limits power performance and rate capability. While the open framework structure facilitates fast sodium-ion diffusion, the electron transfer kinetics often become the rate-limiting factor, especially at high current densities required for stationary storage applications.
Water coordination within the crystal structure presents a double-edged sword. While it can enhance ionic conductivity, excessive water content leads to side reactions, structural degradation, and reduced voltage stability. Controlling moisture during synthesis and battery assembly remains technically demanding, requiring specialized manufacturing environments.
Globally, research on Prussian White cathodes has accelerated significantly since 2015, with major development centers emerging in China, Japan, the United States, and several European countries. China currently leads in terms of patent applications and commercial development, with companies like CATL and BYD investing heavily in sodium-ion technology. Their focus has been on scalable synthesis methods and integration with existing lithium-ion manufacturing infrastructure.
Japanese research institutions, particularly those affiliated with Sumitomo Electric and Panasonic, have made notable advances in improving the cycling stability through novel electrolyte formulations and protective surface coatings. These innovations have extended cycle life from hundreds to several thousand cycles in laboratory settings.
In Europe, the ALISTORE European Research Institute has coordinated efforts across multiple countries, focusing on fundamental understanding of sodium storage mechanisms in Prussian White structures. Their work has led to significant improvements in theoretical models predicting long-term performance.
The United States has contributed primarily through academic research at institutions like Stanford University and Argonne National Laboratory, where advanced characterization techniques have revealed degradation mechanisms at atomic scales. This fundamental research has informed more practical engineering solutions.
Despite these global efforts, the technology readiness level (TRL) of Prussian White cathodes remains at 6-7, indicating prototype demonstration in relevant environments but not yet full commercial deployment. The gap between laboratory performance and commercial requirements continues to narrow, with several companies announcing plans for pilot production lines by 2024-2025.
Another critical challenge is the relatively low electronic conductivity of Prussian White compounds, which limits power performance and rate capability. While the open framework structure facilitates fast sodium-ion diffusion, the electron transfer kinetics often become the rate-limiting factor, especially at high current densities required for stationary storage applications.
Water coordination within the crystal structure presents a double-edged sword. While it can enhance ionic conductivity, excessive water content leads to side reactions, structural degradation, and reduced voltage stability. Controlling moisture during synthesis and battery assembly remains technically demanding, requiring specialized manufacturing environments.
Globally, research on Prussian White cathodes has accelerated significantly since 2015, with major development centers emerging in China, Japan, the United States, and several European countries. China currently leads in terms of patent applications and commercial development, with companies like CATL and BYD investing heavily in sodium-ion technology. Their focus has been on scalable synthesis methods and integration with existing lithium-ion manufacturing infrastructure.
Japanese research institutions, particularly those affiliated with Sumitomo Electric and Panasonic, have made notable advances in improving the cycling stability through novel electrolyte formulations and protective surface coatings. These innovations have extended cycle life from hundreds to several thousand cycles in laboratory settings.
In Europe, the ALISTORE European Research Institute has coordinated efforts across multiple countries, focusing on fundamental understanding of sodium storage mechanisms in Prussian White structures. Their work has led to significant improvements in theoretical models predicting long-term performance.
The United States has contributed primarily through academic research at institutions like Stanford University and Argonne National Laboratory, where advanced characterization techniques have revealed degradation mechanisms at atomic scales. This fundamental research has informed more practical engineering solutions.
Despite these global efforts, the technology readiness level (TRL) of Prussian White cathodes remains at 6-7, indicating prototype demonstration in relevant environments but not yet full commercial deployment. The gap between laboratory performance and commercial requirements continues to narrow, with several companies announcing plans for pilot production lines by 2024-2025.
Current Prussian White Cathode Design Approaches
01 Prussian White cathode materials for high-power batteries
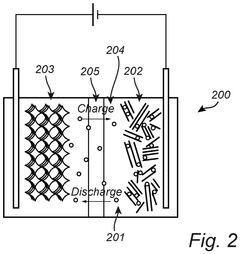
Prussian White compounds are used as cathode materials in high-power batteries due to their excellent electrochemical properties. These materials demonstrate high capacity, good cycling stability, and fast charge-discharge capabilities, making them suitable for high-power applications. The specific crystal structure of Prussian White allows for efficient ion insertion and extraction, contributing to enhanced battery performance.- Prussian white cathode materials for high-power batteries: Prussian white compounds are used as cathode materials in high-power batteries due to their excellent electrochemical properties. These materials feature a unique crystal structure that allows for rapid ion insertion and extraction, making them suitable for high-power applications. The formulation of these cathodes typically involves optimizing the composition to enhance conductivity and stability during charge-discharge cycles.
- Synthesis methods for high-performance Prussian white cathodes: Various synthesis methods are employed to produce high-performance Prussian white cathodes. These include precipitation techniques, hydrothermal methods, and sol-gel processes that control particle size, morphology, and crystallinity. Advanced synthesis approaches focus on creating uniform nanostructures with optimized surface area and reduced defects to enhance electrochemical performance and power output.
- Doping and modification of Prussian white cathodes: Doping and modification strategies are implemented to enhance the performance of Prussian white cathodes. These include incorporating metal ions such as zinc, manganese, or nickel into the crystal structure, surface coating with conductive materials, and creating composite structures. These modifications improve conductivity, structural stability, and cycling performance, resulting in higher power capabilities.
- Electrolyte optimization for Prussian white high-power systems: Electrolyte formulations are specifically designed to complement Prussian white cathodes in high-power applications. These electrolytes typically contain optimized salt concentrations, additives to form stable solid-electrolyte interfaces, and solvents with high ionic conductivity. The electrolyte composition significantly affects the power capability, rate performance, and cycle life of Prussian white-based battery systems.
- Battery system design for Prussian white high-power applications: Complete battery system designs incorporating Prussian white cathodes focus on maximizing power output while maintaining thermal stability. These designs include optimized electrode architectures, current collector configurations, and cell packaging to minimize internal resistance. Advanced battery management systems are implemented to control charge-discharge rates, monitor cell health, and prevent degradation during high-power operation.
02 Synthesis methods for high-performance Prussian White cathodes
Various synthesis methods have been developed to produce high-quality Prussian White cathode materials with improved electrochemical performance. These methods include precipitation techniques, hydrothermal synthesis, and sol-gel processes. By controlling synthesis parameters such as temperature, pH, and reaction time, the morphology, particle size, and crystallinity of Prussian White can be optimized to enhance high-power performance.Expand Specific Solutions03 Modification strategies for Prussian White cathodes
Modification strategies for Prussian White cathodes include doping with transition metals, surface coating, and defect engineering. These modifications aim to improve the structural stability, electronic conductivity, and ion diffusion kinetics of Prussian White materials. Enhanced structural stability prevents degradation during high-power cycling, while improved conductivity and ion diffusion facilitate faster charge-discharge rates.Expand Specific Solutions04 Electrolyte optimization for Prussian White high-power batteries
Electrolyte composition plays a crucial role in the performance of Prussian White cathodes for high-power applications. Optimized electrolytes can enhance ion transport, reduce interfacial resistance, and improve the stability of the electrode-electrolyte interface. Additives in the electrolyte can also form protective films on the cathode surface, preventing side reactions and extending battery life under high-power conditions.Expand Specific Solutions05 Battery system design for Prussian White high-power applications
Battery system design considerations for Prussian White high-power applications include thermal management, electrode architecture, and cell configuration. Effective thermal management prevents overheating during high-power operation, while optimized electrode architecture enhances ion and electron transport. Advanced cell configurations can maximize the utilization of active materials and improve overall system performance for high-power applications.Expand Specific Solutions
Leading Companies and Research Institutions in Na-Ion Storage
The Prussian White cathode technology for sodium-ion stationary storage is in an early growth phase, with market size expanding as energy storage demands increase globally. The technology offers a promising alternative to lithium-ion batteries due to its cost-effectiveness and use of abundant materials. Companies like Altris AB have pioneered commercial production of Prussian White cathodes, while major players including CATL, LG Energy Solution, and various Chinese companies (Guangdong Bangpu, Zhejiang Sodium Innovation Energy) are actively developing this technology. Research institutions such as Wuhan University of Technology, KAIST, and IIT Bombay are advancing the fundamental science. The technology is approaching commercial maturity with pilot projects underway, though challenges in energy density and cycle life remain before widespread adoption.
Contemporary Amperex Technology Co., Ltd.
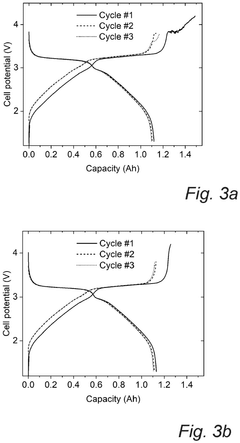
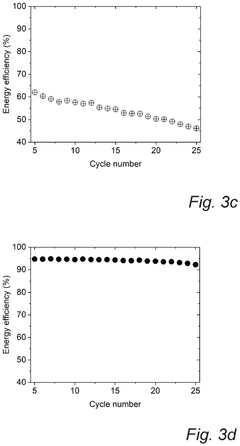
Technical Solution: CATL has developed an advanced Prussian White cathode formulation for sodium-ion batteries featuring a highly ordered Fe-vacancy-free structure. Their technology employs a controlled precipitation method with precise regulation of Na/Fe ratio and reaction kinetics to minimize lattice defects. CATL's approach incorporates carbon coating and surface modification techniques to enhance electronic conductivity and structural stability during cycling. Their Prussian White cathodes deliver energy densities of 160 Wh/kg at the cell level with power densities exceeding 500 W/kg, making them suitable for grid-scale energy storage applications. CATL has also developed a compatible hard carbon anode and optimized electrolyte formulation specifically designed to work with their Prussian White cathodes, creating a complete sodium-ion battery system that can operate efficiently across wide temperature ranges (-20°C to 60°C).
Strengths: Extensive manufacturing infrastructure allows for rapid scaling; integrated supply chain from materials to finished cells; comprehensive battery management systems optimized for sodium-ion chemistry. Weaknesses: Higher production costs compared to their established lithium-ion manufacturing; technology still in early commercialization phase with limited field deployment data.
Altris AB
Technical Solution: Altris AB has developed a proprietary Prussian White cathode material called "Fennac" specifically for sodium-ion batteries. Their technology utilizes a solvent-free manufacturing process that produces sodium-iron-based Prussian White cathodes with high capacity (>160 mAh/g) and excellent cycling stability. The company employs a unique low-temperature synthesis method that eliminates the need for organic solvents, significantly reducing production costs and environmental impact. Altris's Fennac material features optimized crystal structure with minimal lattice defects and controlled water content, which addresses the common issues of capacity fading in Prussian White compounds. Their manufacturing process also ensures uniform particle size distribution (1-5 μm), enhancing electrode packing density and rate capability for high-power applications.
Strengths: Solvent-free production reduces costs by approximately 30% compared to conventional methods; environmentally friendly manufacturing process; material shows excellent stability over 2000+ cycles at high rates. Weaknesses: Limited production scale compared to larger battery manufacturers; relatively new to market with less established supply chain infrastructure.
Key Patents and Breakthroughs in Prussian White Technology
A method for manufacturing a sodium or potassium ion battery cell
PatentPendingEP4451355A1
Innovation
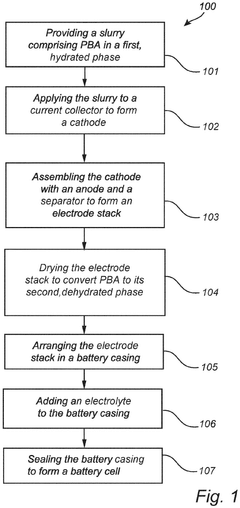
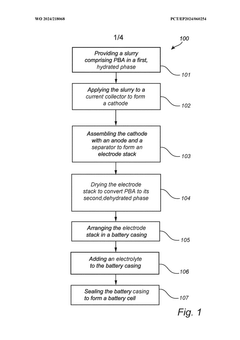
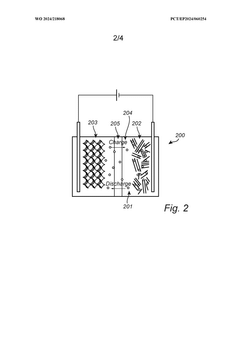
- A method involving a slurry of Prussian Blue analogue in a hydrated phase applied to a current collector, followed by drying under controlled conditions to achieve a dehydrated phase, with assembly and sealing performed in an atmosphere with a dew point temperature between -40°C to -80°C to prevent rehydration, allowing for efficient conversion and maintaining the material in a stable dehydrated state.
A method for manufacturing a sodium or potassium ion battery cell
PatentWO2024218068A1
Innovation
- A method involving a slurry of Prussian Blue analogue in a hydrated phase applied to a current collector, followed by drying under controlled conditions to achieve a dehydrated phase, with assembly and sealing performed in an atmosphere with a dew point temperature between -40°C to -80°C to prevent moisture reabsorption, allowing for efficient large-scale production.
Cost-Performance Analysis of Prussian White vs. Alternatives
The economic viability of Prussian White (PW) cathodes for sodium-ion batteries (SIBs) presents a compelling case when compared to alternative energy storage technologies. Initial cost analysis reveals that PW-based SIBs can be manufactured at approximately $70-90/kWh, significantly lower than the current lithium-ion battery costs of $130-150/kWh. This cost advantage stems primarily from the abundant and widely distributed sodium resources, which are approximately 1000 times more plentiful than lithium in the earth's crust.
Performance metrics indicate that PW cathodes deliver energy densities of 300-350 Wh/kg at the material level, which translates to 120-160 Wh/kg at the cell level. While this falls short of high-end lithium-ion batteries (180-240 Wh/kg), it remains competitive for stationary storage applications where volumetric constraints are less critical than cost considerations.
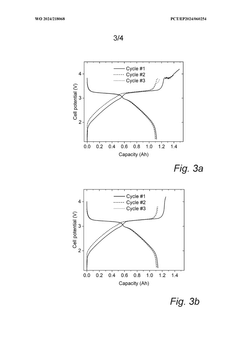
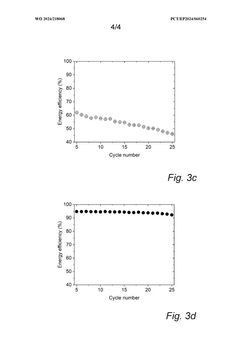
Cycle life analysis demonstrates that optimized PW cathodes can achieve 3000-5000 cycles at 80% depth of discharge, comparable to LiFePO4 batteries but at a substantially lower cost point. This longevity translates to a levelized cost of storage (LCOS) of approximately $0.05-0.07/kWh-cycle, positioning PW-SIBs favorably against both lithium-ion ($0.07-0.10/kWh-cycle) and lead-acid ($0.15-0.20/kWh-cycle) alternatives for grid storage applications.
Manufacturing scalability presents another advantage for PW cathodes, as they can be produced using existing lithium-ion battery production infrastructure with minimal modifications. The water-based synthesis routes for PW materials further reduce production costs and environmental impact compared to the organic solvent-dependent processes required for many lithium-ion cathode materials.
Raw material supply chain analysis reveals that PW cathodes primarily utilize iron, carbon, nitrogen, and sodium—all abundant elements with stable supply chains and minimal geopolitical constraints. This contrasts sharply with lithium-ion technologies dependent on cobalt, nickel, and lithium, which face supply constraints and significant price volatility.
Safety performance metrics favor PW-SIBs, which demonstrate superior thermal stability compared to conventional lithium-ion chemistries. Thermal runaway tests show ignition temperatures above 300°C for PW cells versus 150-200°C for typical NMC lithium-ion cells, reducing cooling system requirements and associated costs for large-scale installations.
The environmental footprint assessment indicates that PW-SIB production generates approximately 30-40% lower carbon emissions compared to equivalent lithium-ion batteries, primarily due to simplified manufacturing processes and reduced energy-intensive purification steps required for sodium compounds versus lithium.
Performance metrics indicate that PW cathodes deliver energy densities of 300-350 Wh/kg at the material level, which translates to 120-160 Wh/kg at the cell level. While this falls short of high-end lithium-ion batteries (180-240 Wh/kg), it remains competitive for stationary storage applications where volumetric constraints are less critical than cost considerations.
Cycle life analysis demonstrates that optimized PW cathodes can achieve 3000-5000 cycles at 80% depth of discharge, comparable to LiFePO4 batteries but at a substantially lower cost point. This longevity translates to a levelized cost of storage (LCOS) of approximately $0.05-0.07/kWh-cycle, positioning PW-SIBs favorably against both lithium-ion ($0.07-0.10/kWh-cycle) and lead-acid ($0.15-0.20/kWh-cycle) alternatives for grid storage applications.
Manufacturing scalability presents another advantage for PW cathodes, as they can be produced using existing lithium-ion battery production infrastructure with minimal modifications. The water-based synthesis routes for PW materials further reduce production costs and environmental impact compared to the organic solvent-dependent processes required for many lithium-ion cathode materials.
Raw material supply chain analysis reveals that PW cathodes primarily utilize iron, carbon, nitrogen, and sodium—all abundant elements with stable supply chains and minimal geopolitical constraints. This contrasts sharply with lithium-ion technologies dependent on cobalt, nickel, and lithium, which face supply constraints and significant price volatility.
Safety performance metrics favor PW-SIBs, which demonstrate superior thermal stability compared to conventional lithium-ion chemistries. Thermal runaway tests show ignition temperatures above 300°C for PW cells versus 150-200°C for typical NMC lithium-ion cells, reducing cooling system requirements and associated costs for large-scale installations.
The environmental footprint assessment indicates that PW-SIB production generates approximately 30-40% lower carbon emissions compared to equivalent lithium-ion batteries, primarily due to simplified manufacturing processes and reduced energy-intensive purification steps required for sodium compounds versus lithium.
Environmental Impact and Sustainability Considerations
The environmental impact of Prussian White cathodes for sodium-ion batteries represents a significant advantage over traditional lithium-ion technologies. These cathodes utilize abundant materials like sodium, iron, and carbon, reducing dependence on critical raw materials such as lithium, cobalt, and nickel that face supply constraints and ethical mining concerns. The extraction processes for sodium compounds generally require less energy and water compared to lithium extraction, particularly avoiding the intensive brine evaporation methods used in lithium mining that can deplete water resources in arid regions.
Manufacturing Prussian White cathodes involves relatively low-temperature synthesis routes, consuming less energy than the high-temperature calcination processes required for conventional lithium cathode materials. This translates to a reduced carbon footprint during production. Additionally, the aqueous processing capabilities of these materials eliminate the need for toxic NMP (N-Methyl-2-pyrrolidone) solvents commonly used in lithium-ion battery manufacturing, decreasing harmful emissions and improving workplace safety.
From a life-cycle perspective, sodium-ion stationary storage systems using Prussian White cathodes demonstrate promising sustainability metrics. The absence of critical materials facilitates easier recycling processes, with potential for higher material recovery rates. Current research indicates that recycling protocols for these batteries can be less energy-intensive and generate fewer hazardous byproducts compared to lithium-ion recycling methods.
The long-term environmental benefits extend to grid-scale applications, where these batteries support renewable energy integration. Their high power capabilities enable efficient energy storage from intermittent renewable sources, potentially reducing reliance on fossil fuel peaker plants. This application directly contributes to carbon emission reductions in the electricity sector, amplifying the environmental benefits beyond the battery's own footprint.
Regulatory frameworks increasingly favor technologies with lower environmental impacts. Prussian White cathode-based storage systems are well-positioned to meet stringent future environmental regulations, including potential extended producer responsibility requirements and carbon taxation schemes. Their favorable sustainability profile may translate to competitive advantages as environmental compliance costs rise for less sustainable alternatives.
Despite these advantages, ongoing research must address remaining environmental challenges, including optimizing electrolyte formulations to reduce fluorinated compounds and developing water-based processing for all battery components. Comprehensive life cycle assessments comparing these systems to alternative storage technologies will be essential for quantifying their full environmental benefits and identifying areas for further improvement.
Manufacturing Prussian White cathodes involves relatively low-temperature synthesis routes, consuming less energy than the high-temperature calcination processes required for conventional lithium cathode materials. This translates to a reduced carbon footprint during production. Additionally, the aqueous processing capabilities of these materials eliminate the need for toxic NMP (N-Methyl-2-pyrrolidone) solvents commonly used in lithium-ion battery manufacturing, decreasing harmful emissions and improving workplace safety.
From a life-cycle perspective, sodium-ion stationary storage systems using Prussian White cathodes demonstrate promising sustainability metrics. The absence of critical materials facilitates easier recycling processes, with potential for higher material recovery rates. Current research indicates that recycling protocols for these batteries can be less energy-intensive and generate fewer hazardous byproducts compared to lithium-ion recycling methods.
The long-term environmental benefits extend to grid-scale applications, where these batteries support renewable energy integration. Their high power capabilities enable efficient energy storage from intermittent renewable sources, potentially reducing reliance on fossil fuel peaker plants. This application directly contributes to carbon emission reductions in the electricity sector, amplifying the environmental benefits beyond the battery's own footprint.
Regulatory frameworks increasingly favor technologies with lower environmental impacts. Prussian White cathode-based storage systems are well-positioned to meet stringent future environmental regulations, including potential extended producer responsibility requirements and carbon taxation schemes. Their favorable sustainability profile may translate to competitive advantages as environmental compliance costs rise for less sustainable alternatives.
Despite these advantages, ongoing research must address remaining environmental challenges, including optimizing electrolyte formulations to reduce fluorinated compounds and developing water-based processing for all battery components. Comprehensive life cycle assessments comparing these systems to alternative storage technologies will be essential for quantifying their full environmental benefits and identifying areas for further improvement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!