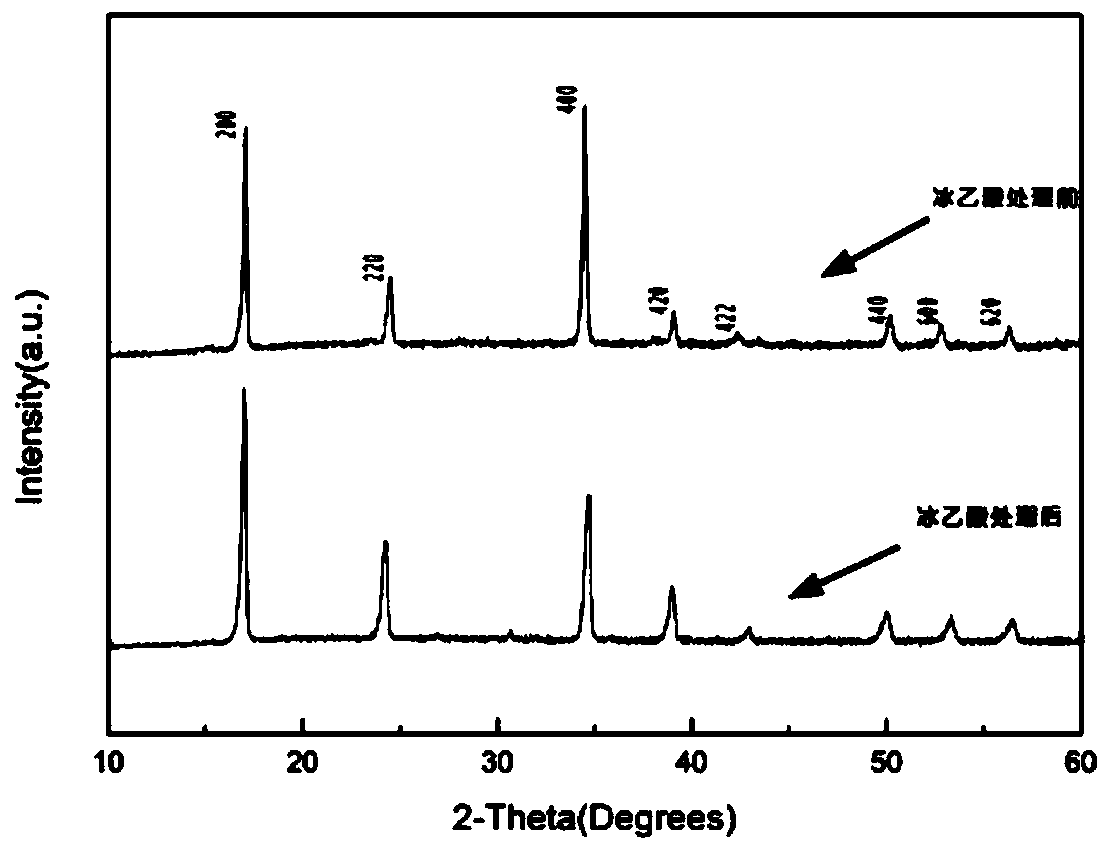
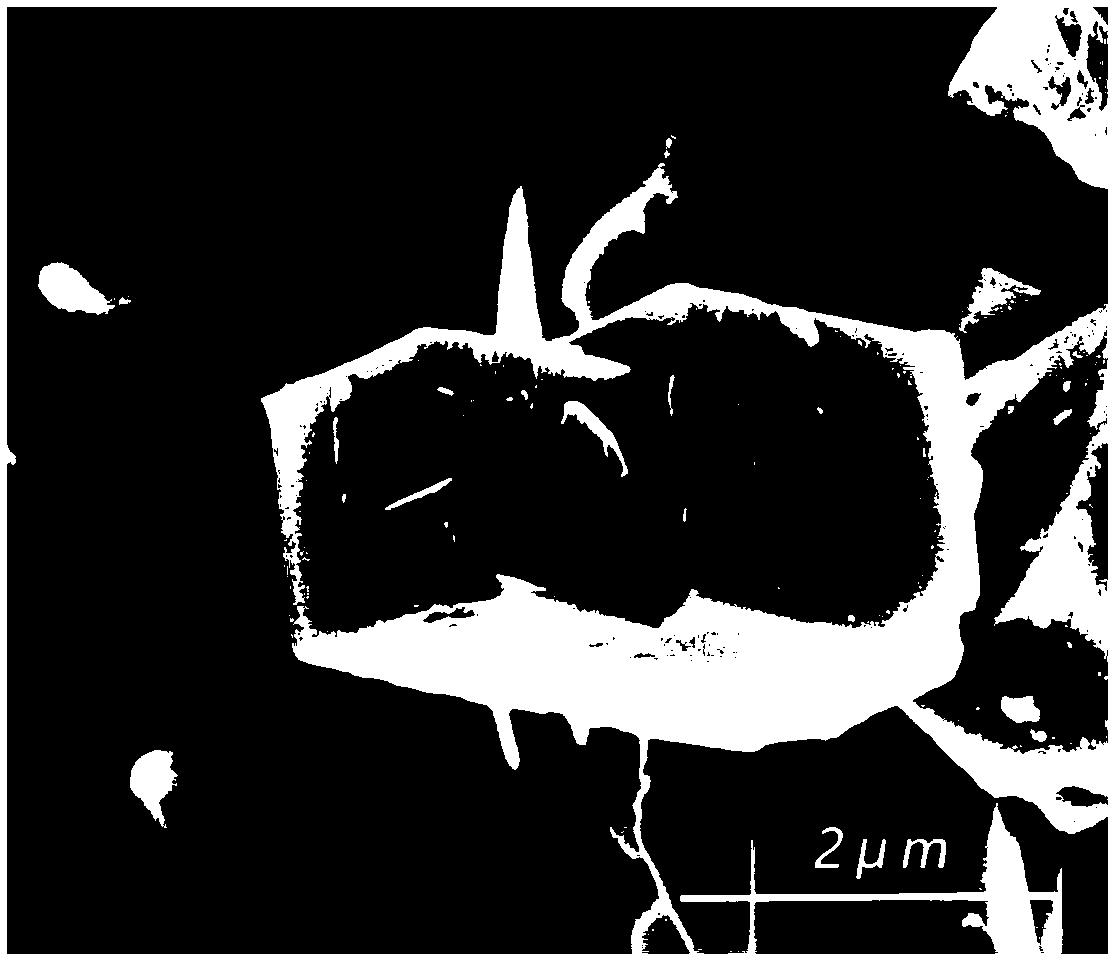
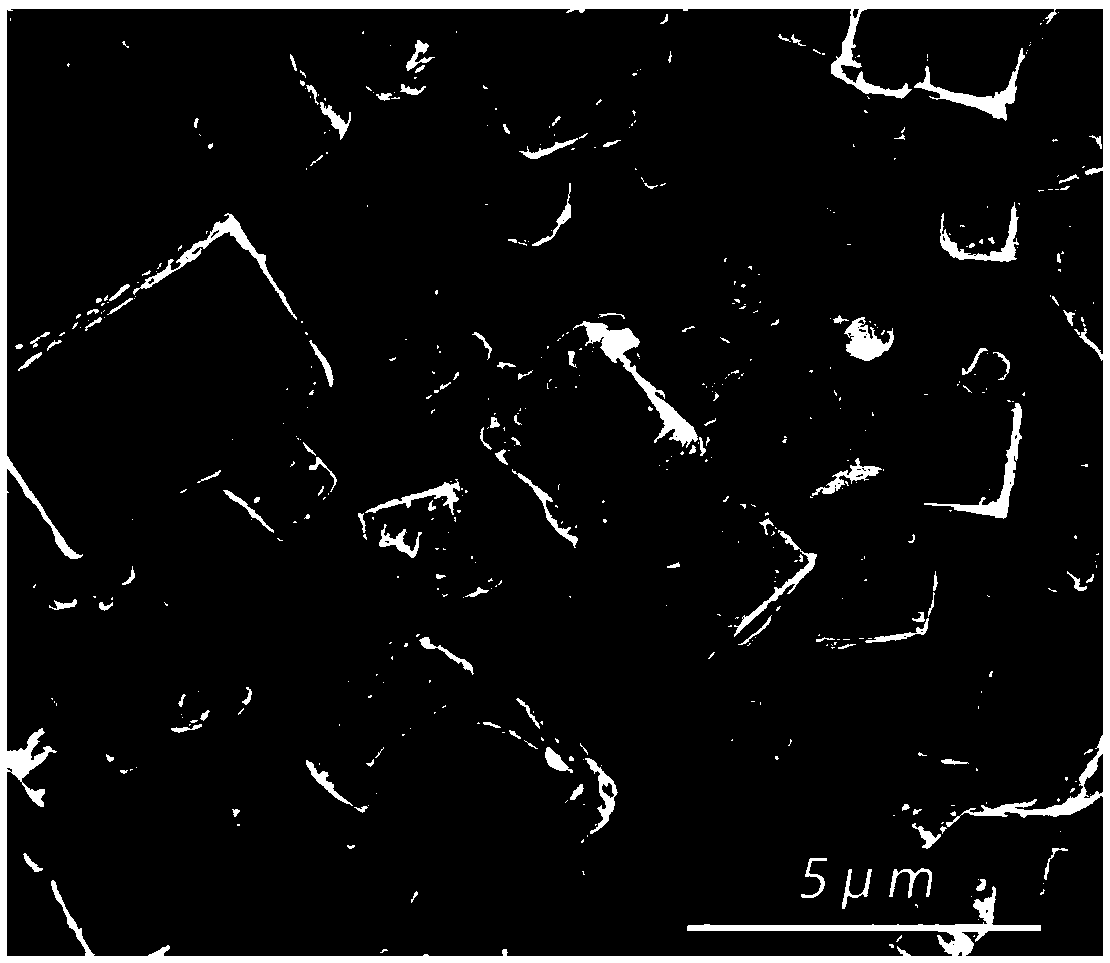
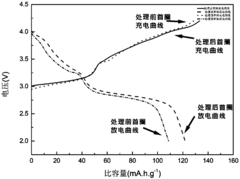
Sodium Vacancy Engineering In Prussian White For Rate Capability
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Vacancy Engineering Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The development of SIBs has gained significant momentum over the past decade, driven by concerns about the limited availability and increasing costs of lithium resources. Among various cathode materials for SIBs, Prussian white compounds have attracted considerable attention due to their open framework structure, which facilitates rapid sodium-ion insertion/extraction.
Sodium vacancy engineering in Prussian white represents a critical frontier in advancing the rate capability of sodium-ion batteries. Historically, the development of Prussian blue analogues (PBAs) began in the early 18th century as pigments, but their application in energy storage systems only gained traction in the late 20th century. The evolution of these materials has accelerated dramatically in recent years, with researchers focusing on optimizing their electrochemical performance through structural modifications.
The primary objective of sodium vacancy engineering is to enhance the rate capability of Prussian white cathodes by creating and controlling sodium vacancies within the crystal structure. These engineered vacancies serve as additional diffusion pathways for sodium ions, potentially reducing the energy barriers for ion migration and improving the kinetics of electrochemical reactions. This approach aims to address one of the fundamental limitations of sodium-ion batteries: their relatively slower charge-discharge rates compared to lithium-ion counterparts.
Current technical goals in this field include developing systematic methods for creating controlled sodium vacancies, understanding the relationship between vacancy concentration and electrochemical performance, and establishing design principles for optimizing the spatial distribution of vacancies. Researchers are also working toward improving the structural stability of vacancy-engineered Prussian white to ensure long-term cycling performance.
The technological trajectory suggests a growing emphasis on atomic-level precision in engineering these materials. Advanced characterization techniques, including in-situ X-ray diffraction and neutron scattering, are increasingly being employed to monitor structural changes during battery operation. Computational methods, particularly density functional theory calculations, have become essential tools for predicting the effects of vacancy engineering on sodium-ion diffusion and electronic conductivity.
As the field progresses, the integration of sodium vacancy engineering with other optimization strategies, such as particle size reduction, carbon coating, and heteroatom doping, is expected to yield synergistic improvements in the overall performance of Prussian white cathodes for next-generation sodium-ion batteries.
Sodium vacancy engineering in Prussian white represents a critical frontier in advancing the rate capability of sodium-ion batteries. Historically, the development of Prussian blue analogues (PBAs) began in the early 18th century as pigments, but their application in energy storage systems only gained traction in the late 20th century. The evolution of these materials has accelerated dramatically in recent years, with researchers focusing on optimizing their electrochemical performance through structural modifications.
The primary objective of sodium vacancy engineering is to enhance the rate capability of Prussian white cathodes by creating and controlling sodium vacancies within the crystal structure. These engineered vacancies serve as additional diffusion pathways for sodium ions, potentially reducing the energy barriers for ion migration and improving the kinetics of electrochemical reactions. This approach aims to address one of the fundamental limitations of sodium-ion batteries: their relatively slower charge-discharge rates compared to lithium-ion counterparts.
Current technical goals in this field include developing systematic methods for creating controlled sodium vacancies, understanding the relationship between vacancy concentration and electrochemical performance, and establishing design principles for optimizing the spatial distribution of vacancies. Researchers are also working toward improving the structural stability of vacancy-engineered Prussian white to ensure long-term cycling performance.
The technological trajectory suggests a growing emphasis on atomic-level precision in engineering these materials. Advanced characterization techniques, including in-situ X-ray diffraction and neutron scattering, are increasingly being employed to monitor structural changes during battery operation. Computational methods, particularly density functional theory calculations, have become essential tools for predicting the effects of vacancy engineering on sodium-ion diffusion and electronic conductivity.
As the field progresses, the integration of sodium vacancy engineering with other optimization strategies, such as particle size reduction, carbon coating, and heteroatom doping, is expected to yield synergistic improvements in the overall performance of Prussian white cathodes for next-generation sodium-ion batteries.
Market Analysis for High-Rate Sodium-Ion Batteries
The global market for sodium-ion batteries is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. As lithium resources face supply constraints and price volatility, sodium-ion technology emerges as a compelling alternative due to sodium's natural abundance and wide geographical distribution. The market for high-rate sodium-ion batteries specifically is projected to expand rapidly as applications requiring fast charging and high power delivery gain prominence.
Industrial sectors including grid energy storage, electric vehicles (particularly in urban mobility solutions), and consumer electronics represent the primary market segments for high-rate sodium-ion batteries. The grid storage sector shows particular promise as renewable energy integration accelerates worldwide, creating demand for cost-effective storage solutions that can respond rapidly to fluctuating supply and demand patterns.
Electric vehicle manufacturers are increasingly exploring sodium-ion technology as a complementary solution to lithium-ion batteries, especially for applications where cost considerations outweigh energy density requirements. The ability of engineered Prussian White cathodes to deliver improved rate capability addresses a critical market need in this sector, potentially enabling affordable electric mobility solutions with practical charging times.
Regional market analysis reveals that Asia-Pacific currently dominates the sodium-ion battery landscape, with China leading research, development, and commercialization efforts. European markets show strong growth potential driven by stringent environmental regulations and substantial investments in green technology. North American markets are gradually increasing adoption, particularly in grid storage applications.
Market barriers include the technology's current performance limitations compared to established lithium-ion batteries, manufacturing scalability challenges, and limited commercial deployment history. However, innovations in electrode materials, particularly sodium vacancy engineering in Prussian White structures, are directly addressing the critical rate capability limitations that have historically constrained market penetration.
Economic analysis indicates that high-rate sodium-ion batteries utilizing engineered Prussian White cathodes could achieve a 30-40% cost reduction compared to lithium-ion alternatives in specific applications. This cost advantage, combined with performance improvements through sodium vacancy engineering, positions the technology favorably in price-sensitive market segments.
Consumer and industry surveys indicate growing awareness of sodium-ion technology benefits, with particular interest in applications where cost-effectiveness and sustainability outweigh absolute energy density requirements. Market forecasts suggest that high-rate sodium-ion batteries could capture a significant share of the stationary storage market within the next five years, with gradual penetration into mobility applications as the technology matures.
Industrial sectors including grid energy storage, electric vehicles (particularly in urban mobility solutions), and consumer electronics represent the primary market segments for high-rate sodium-ion batteries. The grid storage sector shows particular promise as renewable energy integration accelerates worldwide, creating demand for cost-effective storage solutions that can respond rapidly to fluctuating supply and demand patterns.
Electric vehicle manufacturers are increasingly exploring sodium-ion technology as a complementary solution to lithium-ion batteries, especially for applications where cost considerations outweigh energy density requirements. The ability of engineered Prussian White cathodes to deliver improved rate capability addresses a critical market need in this sector, potentially enabling affordable electric mobility solutions with practical charging times.
Regional market analysis reveals that Asia-Pacific currently dominates the sodium-ion battery landscape, with China leading research, development, and commercialization efforts. European markets show strong growth potential driven by stringent environmental regulations and substantial investments in green technology. North American markets are gradually increasing adoption, particularly in grid storage applications.
Market barriers include the technology's current performance limitations compared to established lithium-ion batteries, manufacturing scalability challenges, and limited commercial deployment history. However, innovations in electrode materials, particularly sodium vacancy engineering in Prussian White structures, are directly addressing the critical rate capability limitations that have historically constrained market penetration.
Economic analysis indicates that high-rate sodium-ion batteries utilizing engineered Prussian White cathodes could achieve a 30-40% cost reduction compared to lithium-ion alternatives in specific applications. This cost advantage, combined with performance improvements through sodium vacancy engineering, positions the technology favorably in price-sensitive market segments.
Consumer and industry surveys indicate growing awareness of sodium-ion technology benefits, with particular interest in applications where cost-effectiveness and sustainability outweigh absolute energy density requirements. Market forecasts suggest that high-rate sodium-ion batteries could capture a significant share of the stationary storage market within the next five years, with gradual penetration into mobility applications as the technology matures.
Current Challenges in Prussian White Cathode Materials
Despite the promising potential of Prussian White (PW) cathode materials for sodium-ion batteries, several significant challenges currently impede their widespread commercial adoption. The primary issue facing PW cathodes is their inherently low electronic conductivity, which severely limits electron transport during charge-discharge cycles. This fundamental limitation restricts the rate capability of PW-based batteries, particularly at high current densities where rapid electron movement is essential.
Structural instability presents another major challenge, as PW materials often undergo significant volume changes during sodium insertion and extraction processes. These volumetric fluctuations can lead to mechanical stress within the cathode structure, resulting in particle cracking, pulverization, and eventual capacity fading over extended cycling. The structural degradation is particularly pronounced at high sodium content states.
Water coordination in the PW framework constitutes a complex challenge with dual implications. While zeolitic water molecules are necessary for maintaining structural integrity, coordinated water can simultaneously facilitate unwanted side reactions at the electrode-electrolyte interface. Finding the optimal water content remains an unresolved challenge in PW engineering.
The presence of vacancies and defects in the crystal structure, though potentially beneficial for ion diffusion when properly engineered, often occurs randomly during synthesis. This uncontrolled defect formation leads to inconsistent electrochemical performance across different batches of materials. Controlling vacancy distribution represents a significant technical hurdle.
Sodium diffusion kinetics within the PW framework is another critical limitation. The diffusion pathways can become restricted due to structural distortions or blockages from transition metal ions, particularly at high states of charge. This kinetic bottleneck directly impacts the rate capability of PW cathodes.
Manufacturing scalability presents additional challenges, as current synthesis methods often yield materials with inconsistent composition, morphology, and performance. The precise control of sodium vacancy engineering at industrial scales remains particularly difficult to achieve with current production technologies.
Electrolyte compatibility issues further complicate PW implementation, as the interaction between PW cathodes and conventional electrolytes can lead to undesirable side reactions, especially at high voltages. These reactions contribute to capacity loss and reduced cycling stability, particularly when attempting to maximize energy density through wider voltage windows.
Structural instability presents another major challenge, as PW materials often undergo significant volume changes during sodium insertion and extraction processes. These volumetric fluctuations can lead to mechanical stress within the cathode structure, resulting in particle cracking, pulverization, and eventual capacity fading over extended cycling. The structural degradation is particularly pronounced at high sodium content states.
Water coordination in the PW framework constitutes a complex challenge with dual implications. While zeolitic water molecules are necessary for maintaining structural integrity, coordinated water can simultaneously facilitate unwanted side reactions at the electrode-electrolyte interface. Finding the optimal water content remains an unresolved challenge in PW engineering.
The presence of vacancies and defects in the crystal structure, though potentially beneficial for ion diffusion when properly engineered, often occurs randomly during synthesis. This uncontrolled defect formation leads to inconsistent electrochemical performance across different batches of materials. Controlling vacancy distribution represents a significant technical hurdle.
Sodium diffusion kinetics within the PW framework is another critical limitation. The diffusion pathways can become restricted due to structural distortions or blockages from transition metal ions, particularly at high states of charge. This kinetic bottleneck directly impacts the rate capability of PW cathodes.
Manufacturing scalability presents additional challenges, as current synthesis methods often yield materials with inconsistent composition, morphology, and performance. The precise control of sodium vacancy engineering at industrial scales remains particularly difficult to achieve with current production technologies.
Electrolyte compatibility issues further complicate PW implementation, as the interaction between PW cathodes and conventional electrolytes can lead to undesirable side reactions, especially at high voltages. These reactions contribute to capacity loss and reduced cycling stability, particularly when attempting to maximize energy density through wider voltage windows.
Current Approaches to Enhance Rate Capability
01 Composition modifications for improved rate capability
Various compositional modifications can enhance the rate capability of Prussian White materials. These include doping with specific elements, adjusting the ratio of transition metals, and incorporating conductive additives. Such modifications can optimize the crystal structure, increase electronic conductivity, and facilitate ion diffusion pathways, resulting in improved charge-discharge rates and cycling stability at high current densities.- Composition optimization for enhanced rate capability: Optimizing the composition of Prussian White materials can significantly enhance rate capability. This includes controlling the ratio of transition metals, incorporating specific dopants, and adjusting the stoichiometry of the crystal structure. These modifications can improve electron transfer kinetics and ion diffusion pathways, leading to better high-rate performance in energy storage applications.
- Nanostructure engineering for improved rate performance: Engineering the nanostructure of Prussian White materials can dramatically improve rate capability. Approaches include creating hollow structures, developing hierarchical architectures, reducing particle size, and controlling crystal facet exposure. These structural modifications reduce ion diffusion distances and provide more active sites, enabling faster charge/discharge rates while maintaining capacity.
- Carbon composite strategies for enhanced conductivity: Forming composites between Prussian White and various carbon materials significantly enhances electronic conductivity and rate capability. Carbon materials such as graphene, carbon nanotubes, carbon fibers, and porous carbon frameworks create conductive networks that facilitate electron transport. These composites also help maintain structural integrity during rapid charging and discharging cycles.
- Defect engineering and vacancy control: Deliberately introducing and controlling defects and vacancies in Prussian White structures can enhance rate capability. Strategic manipulation of cation/anion vacancies, interstitial defects, and coordination environments affects ion diffusion channels and redox kinetics. These approaches can be achieved through controlled synthesis conditions, post-synthesis treatments, or selective element substitution.
- Surface modification and interface engineering: Surface modification and interface engineering of Prussian White materials can significantly improve rate capability. Techniques include coating with conductive polymers, creating core-shell structures, surface functionalization, and electrolyte optimization. These modifications enhance charge transfer at interfaces, reduce resistance, and improve stability during high-rate cycling, leading to better overall electrochemical performance.
02 Nanostructuring approaches for enhanced rate performance
Nanostructuring Prussian White materials can significantly improve their rate capability. Techniques include synthesizing nanoparticles, creating hollow structures, and developing hierarchical architectures. These approaches reduce ion diffusion distances, increase active surface area, and provide buffer space for volume changes during cycling, enabling faster charge transfer kinetics and improved high-rate performance.Expand Specific Solutions03 Carbon composite strategies for Prussian White electrodes
Integrating Prussian White with various carbon materials creates composites with enhanced rate capability. Carbon materials such as graphene, carbon nanotubes, and conductive carbon coatings provide electron transport highways, prevent agglomeration of active particles, and maintain structural integrity during cycling. These composites demonstrate superior rate performance due to the synergistic effects between Prussian White and the carbon matrix.Expand Specific Solutions04 Electrolyte optimization for fast-charging Prussian White batteries
The composition and properties of electrolytes significantly impact the rate capability of Prussian White electrodes. Optimized electrolytes with tailored salt concentrations, solvent mixtures, and additives can enhance ion transport, reduce interfacial resistance, and improve the stability of the electrode-electrolyte interface. These factors collectively contribute to better rate performance and cycling stability at high current densities.Expand Specific Solutions05 Advanced electrode engineering for high-rate applications
Innovative electrode engineering approaches can maximize the rate capability of Prussian White materials. These include designing flexible electrodes, developing 3D architectures, optimizing binder systems, and controlling electrode thickness and porosity. Such engineering strategies enhance electron/ion transport pathways, accommodate volume changes, and maintain electrode integrity during rapid charging and discharging processes.Expand Specific Solutions
Leading Research Groups and Industrial Players
Sodium vacancy engineering in Prussian White for rate capability is currently in an emerging growth phase, with increasing market interest driven by the demand for sustainable energy storage solutions. The global market for this technology is expanding, though still relatively niche compared to lithium-ion technologies. Companies like Altris AB have made significant progress in scaling up production from laboratory to industrial levels, while academic institutions such as Wuhan University of Technology and Tsinghua University are advancing fundamental research. CATL and its subsidiary Guangdong Bangpu are leveraging their battery manufacturing expertise to explore commercial applications. The technology shows promising maturity for specific applications requiring high power capabilities and wider operating temperature ranges, though challenges remain in optimizing performance and scaling production for mass market adoption.
Altris AB
Technical Solution: Altris AB has developed an innovative sodium vacancy engineering approach for Prussian White cathode materials, focusing on their proprietary Fennac® technology. This technology creates controlled sodium vacancies within the Prussian White crystal structure through precise synthesis conditions and post-processing treatments. By manipulating the Fe(II)/Fe(III) ratio and introducing strategic defects in the crystal lattice, Altris achieves enhanced Na+ ion diffusion pathways. Their process involves a low-temperature solution-based synthesis method that maintains specific pH levels to control vacancy formation, followed by careful thermal treatment to stabilize these vacancies without compromising structural integrity. The engineered vacancies significantly improve rate capability by reducing diffusion barriers and creating additional channels for sodium ion transport, enabling their cathodes to deliver up to 80% capacity retention at 10C rates.
Strengths: Proprietary solution-based synthesis method allows for precise control of sodium vacancy concentration; environmentally friendly manufacturing process using abundant materials; excellent rate performance without sacrificing capacity. Weaknesses: Potential challenges with long-term cycling stability of engineered vacancies; may require specialized electrolyte formulations to fully leverage the enhanced rate capability.
Tsinghua University
Technical Solution: Tsinghua University has pioneered a sophisticated sodium vacancy engineering approach for Prussian White cathodes through their "controlled defect generation" methodology. Their research team has developed a precise hydrothermal synthesis technique that manipulates reaction temperature, time, and precursor ratios to create optimized sodium vacancy distributions. The process involves introducing specific chelating agents during synthesis that temporarily occupy sodium sites, which are later removed through careful thermal treatment, leaving behind engineered vacancies. Their most significant innovation is the development of a "vacancy stabilization" technique using selected dopants (including Mn, Co, and Ni) at specific lattice positions to prevent vacancy collapse during cycling. This approach has demonstrated remarkable improvements in rate capability, with their engineered materials delivering up to 85% capacity retention at 20C rates. The research team has further enhanced performance by coupling vacancy engineering with particle size optimization and carbon coating techniques, creating a comprehensive solution for high-rate Prussian White cathodes. Their materials show excellent structural stability during high-rate cycling, maintaining performance over 2000+ cycles.
Strengths: Exceptional rate capability through sophisticated vacancy engineering and stabilization techniques; comprehensive understanding of structure-property relationships; excellent cycling stability even at high rates. Weaknesses: Complex synthesis procedures may present challenges for large-scale production; requires high-purity precursors that could increase manufacturing costs.
Key Patents and Breakthroughs in Vacancy Engineering
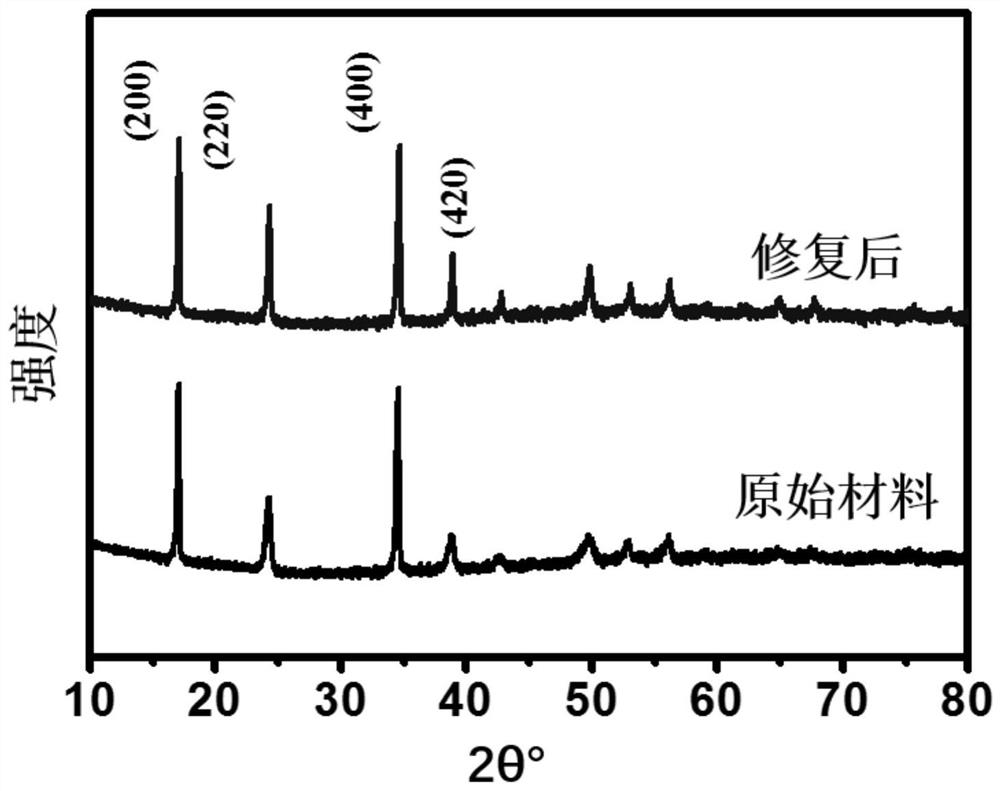
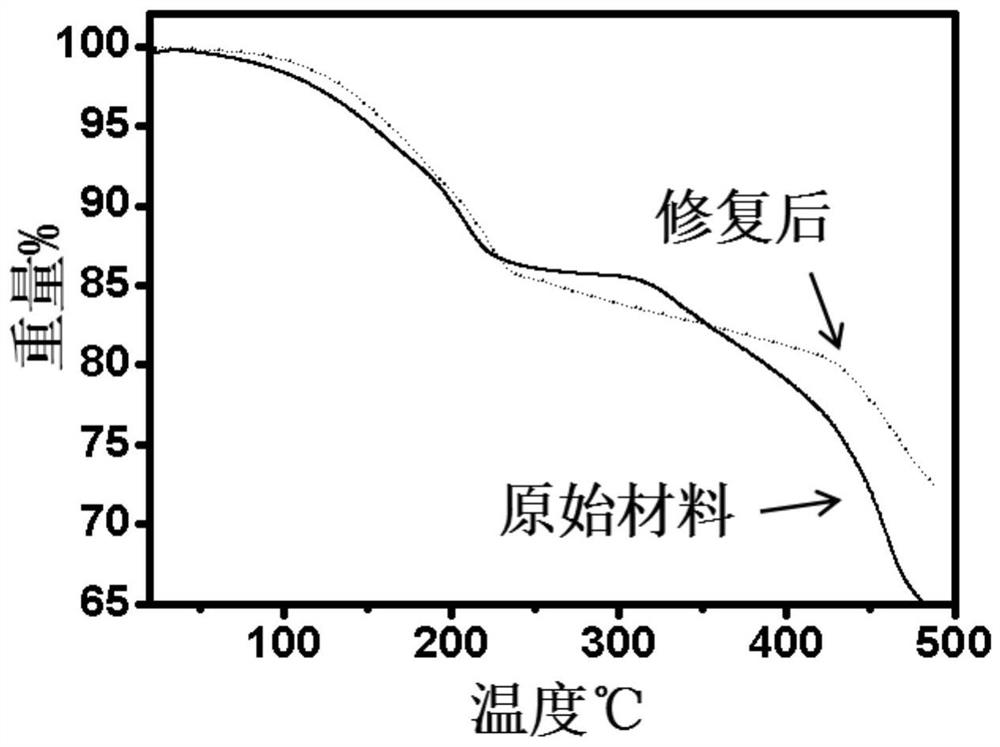
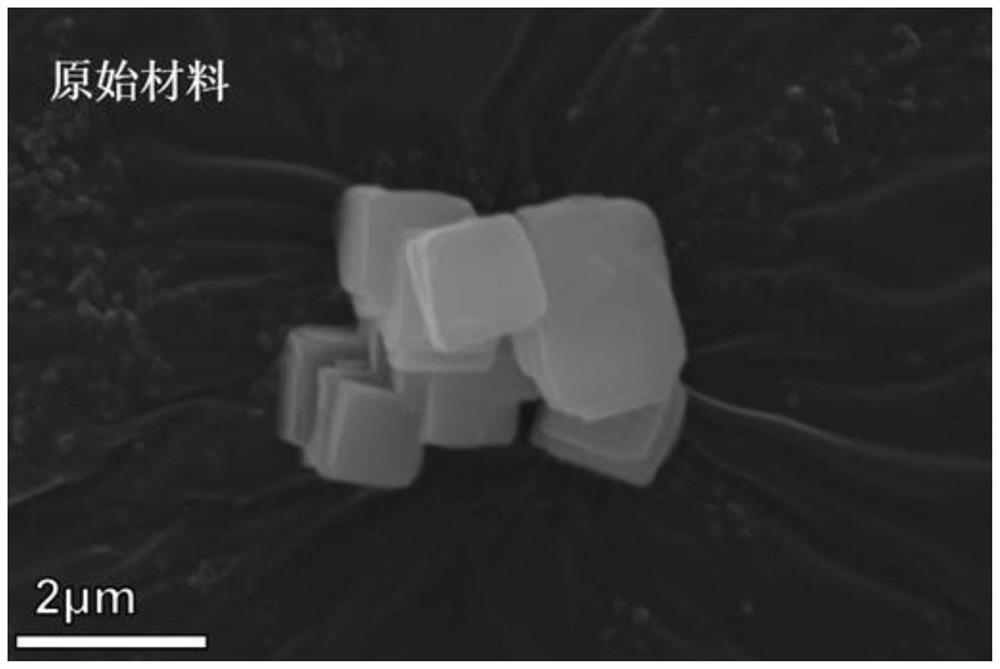
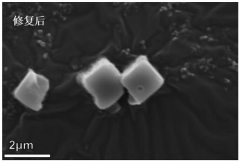
Prussian blue and analogue thereof, and defect repair method and application thereof
PatentActiveCN113353955A
Innovation
- Prussian blue and its analogues are soaked and stirred in a ferrocyanide anion solution. Through replacement with water molecules or hydroxide ions, [Fe(CN)6]4-vacancy defects are repaired and the structural integrity of the material is enhanced.
A method for modifying Prussian blue and its analogues and a sodium ion battery
PatentActiveCN109065883A
Innovation
- Anhydrous organic solvents are used to modify dehydrated Prussian blue and its analogues. Organic molecules fill surface vacancies or enter internal gaps to reduce water absorption, thereby improving electrochemical performance and storage stability.
Sustainability Impact of Sodium-Ion Technology
The adoption of sodium-ion battery technology represents a significant step towards more sustainable energy storage solutions. Unlike lithium-ion batteries, sodium-ion technology relies on sodium, which is approximately 1,000 times more abundant in the Earth's crust than lithium. This abundance translates directly to reduced environmental impact from mining operations, as sodium can be extracted from seawater or common salt deposits with substantially lower ecological disruption.
Sodium vacancy engineering in Prussian White compounds further enhances this sustainability profile by optimizing material efficiency. By strategically creating and controlling sodium vacancies, researchers can improve the rate capability of these batteries without requiring additional rare materials or energy-intensive manufacturing processes. This approach maximizes performance while minimizing resource consumption.
The carbon footprint associated with sodium-ion battery production is estimated to be 60-80% lower than conventional lithium-ion batteries. This reduction stems from both the accessibility of raw materials and the lower processing temperatures required for sodium-based cathode materials like Prussian White. Manufacturing processes for these materials typically operate at temperatures below 600°C, compared to the 800-900°C needed for many lithium cathode materials.
Water consumption metrics also favor sodium-ion technology, with production requiring approximately 30-40% less water than comparable lithium-ion batteries. This advantage becomes particularly crucial in regions facing water scarcity challenges, where battery manufacturing facilities might otherwise place additional strain on limited resources.
End-of-life considerations further highlight the sustainability benefits of sodium-ion technology. The absence of cobalt and nickel in Prussian White cathodes eliminates toxic heavy metal concerns during recycling or disposal. Additionally, the recycling process for sodium-ion batteries is potentially more straightforward, as the materials can be recovered at lower temperatures with reduced energy requirements.
From a circular economy perspective, sodium vacancy engineered batteries offer promising opportunities. The materials used are more readily recyclable, and the technology's compatibility with existing manufacturing infrastructure means that transitioning to sodium-ion production could utilize much of the same equipment currently employed for lithium-ion batteries, reducing waste from obsolete manufacturing assets.
Sodium vacancy engineering in Prussian White compounds further enhances this sustainability profile by optimizing material efficiency. By strategically creating and controlling sodium vacancies, researchers can improve the rate capability of these batteries without requiring additional rare materials or energy-intensive manufacturing processes. This approach maximizes performance while minimizing resource consumption.
The carbon footprint associated with sodium-ion battery production is estimated to be 60-80% lower than conventional lithium-ion batteries. This reduction stems from both the accessibility of raw materials and the lower processing temperatures required for sodium-based cathode materials like Prussian White. Manufacturing processes for these materials typically operate at temperatures below 600°C, compared to the 800-900°C needed for many lithium cathode materials.
Water consumption metrics also favor sodium-ion technology, with production requiring approximately 30-40% less water than comparable lithium-ion batteries. This advantage becomes particularly crucial in regions facing water scarcity challenges, where battery manufacturing facilities might otherwise place additional strain on limited resources.
End-of-life considerations further highlight the sustainability benefits of sodium-ion technology. The absence of cobalt and nickel in Prussian White cathodes eliminates toxic heavy metal concerns during recycling or disposal. Additionally, the recycling process for sodium-ion batteries is potentially more straightforward, as the materials can be recovered at lower temperatures with reduced energy requirements.
From a circular economy perspective, sodium vacancy engineered batteries offer promising opportunities. The materials used are more readily recyclable, and the technology's compatibility with existing manufacturing infrastructure means that transitioning to sodium-ion production could utilize much of the same equipment currently employed for lithium-ion batteries, reducing waste from obsolete manufacturing assets.
Scalability and Manufacturing Considerations
The scalability of sodium vacancy engineering in Prussian White materials presents both significant opportunities and challenges for industrial applications. Current laboratory-scale synthesis methods have demonstrated promising results for enhancing rate capability through controlled vacancy formation, but transitioning these processes to mass production requires careful consideration of several manufacturing factors.
Production scaling of Prussian White materials with engineered sodium vacancies demands precise control over reaction parameters including temperature, concentration, and mixing conditions. Conventional batch processing methods may introduce inconsistencies in vacancy distribution when scaled up, potentially compromising the electrochemical performance advantages observed in laboratory settings. Continuous flow synthesis approaches offer a promising alternative, allowing for more uniform reaction conditions and better control over sodium vacancy formation across large production volumes.
Raw material sourcing represents another critical consideration for commercial viability. The purity of precursors significantly impacts the formation of well-defined sodium vacancies in the crystal structure. Trace impurities can disrupt the intended vacancy engineering process, leading to inconsistent electrochemical performance. Establishing reliable supply chains for high-purity precursors at industrial scales will be essential for maintaining quality control in mass production scenarios.
Energy consumption during synthesis presents a notable challenge for sustainable manufacturing. Current methods for creating controlled sodium vacancies often require precise thermal treatments or electrochemical processing steps that may be energy-intensive when scaled. Developing more energy-efficient approaches to vacancy engineering will be crucial for reducing production costs and environmental impact, particularly as demand for high-rate capability sodium-ion batteries increases.
Quality control and characterization methods must also evolve to accommodate industrial-scale production. Laboratory techniques such as synchrotron-based X-ray diffraction or advanced microscopy, while valuable for fundamental research, are impractical for routine quality assessment in manufacturing environments. Developing rapid, reliable, and cost-effective characterization methods to verify sodium vacancy concentration and distribution will be essential for ensuring consistent product quality.
Cost considerations ultimately determine commercial viability. While sodium-based systems offer inherent cost advantages over lithium-ion technologies due to abundant raw materials, the additional processing steps required for vacancy engineering may introduce new expenses. Economic analysis suggests that the performance benefits of enhanced rate capability must be balanced against any increased manufacturing complexity and cost. Optimizing synthesis protocols to minimize additional processing steps while maintaining the desired vacancy engineering effects represents a key challenge for commercialization.
Production scaling of Prussian White materials with engineered sodium vacancies demands precise control over reaction parameters including temperature, concentration, and mixing conditions. Conventional batch processing methods may introduce inconsistencies in vacancy distribution when scaled up, potentially compromising the electrochemical performance advantages observed in laboratory settings. Continuous flow synthesis approaches offer a promising alternative, allowing for more uniform reaction conditions and better control over sodium vacancy formation across large production volumes.
Raw material sourcing represents another critical consideration for commercial viability. The purity of precursors significantly impacts the formation of well-defined sodium vacancies in the crystal structure. Trace impurities can disrupt the intended vacancy engineering process, leading to inconsistent electrochemical performance. Establishing reliable supply chains for high-purity precursors at industrial scales will be essential for maintaining quality control in mass production scenarios.
Energy consumption during synthesis presents a notable challenge for sustainable manufacturing. Current methods for creating controlled sodium vacancies often require precise thermal treatments or electrochemical processing steps that may be energy-intensive when scaled. Developing more energy-efficient approaches to vacancy engineering will be crucial for reducing production costs and environmental impact, particularly as demand for high-rate capability sodium-ion batteries increases.
Quality control and characterization methods must also evolve to accommodate industrial-scale production. Laboratory techniques such as synchrotron-based X-ray diffraction or advanced microscopy, while valuable for fundamental research, are impractical for routine quality assessment in manufacturing environments. Developing rapid, reliable, and cost-effective characterization methods to verify sodium vacancy concentration and distribution will be essential for ensuring consistent product quality.
Cost considerations ultimately determine commercial viability. While sodium-based systems offer inherent cost advantages over lithium-ion technologies due to abundant raw materials, the additional processing steps required for vacancy engineering may introduce new expenses. Economic analysis suggests that the performance benefits of enhanced rate capability must be balanced against any increased manufacturing complexity and cost. Optimizing synthesis protocols to minimize additional processing steps while maintaining the desired vacancy engineering effects represents a key challenge for commercialization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!