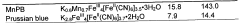Surface Coatings To Enhance Cycle Life Of Prussian White Cathodes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Prussian White Cathode Coating Technology Background and Objectives
Prussian White (PW) cathode materials have emerged as promising candidates for next-generation energy storage systems due to their unique structural properties, abundant raw materials, and cost-effectiveness. The evolution of PW cathodes can be traced back to the broader family of Prussian Blue Analogues (PBAs), which were initially studied for their electrochromic properties in the 1970s before being recognized for their potential in battery applications in the early 2000s.
The technological trajectory of PW cathodes has been characterized by significant improvements in synthesis methods, from simple precipitation techniques to more sophisticated hydrothermal and microwave-assisted processes. These advancements have progressively addressed early challenges related to structural water content, vacancies, and crystallinity, which initially limited their electrochemical performance.
Recent years have witnessed an acceleration in PW cathode research, driven by the growing demand for sustainable and high-performance energy storage solutions. The technology has evolved from laboratory curiosities to commercially viable alternatives for lithium-ion and sodium-ion battery systems, with several companies now incorporating PW cathodes into their product roadmaps.
Despite these advances, cycle life remains a critical limitation for PW cathodes. The degradation mechanisms include structural collapse during repeated charge-discharge cycles, dissolution of transition metal ions, and side reactions with the electrolyte. Surface coating technologies have emerged as a promising strategy to mitigate these issues, creating protective barriers that enhance structural stability and minimize undesirable interfacial reactions.
The primary objective of this technical research is to comprehensively evaluate surface coating technologies for PW cathodes, with a specific focus on enhancing cycle life. This includes examining various coating materials (such as carbon-based materials, metal oxides, phosphates, and polymers), deposition techniques, and coating thickness optimization strategies.
Additionally, this research aims to establish correlations between coating properties and performance improvements, identify the most promising coating technologies based on effectiveness, scalability, and cost considerations, and develop a roadmap for future research directions in this field.
The ultimate goal is to enable PW cathodes that can maintain at least 80% capacity retention after 1000 cycles, making them viable for both grid-scale energy storage applications and electric vehicles where long cycle life is paramount. This would represent a significant advancement over current PW cathode technologies, which typically show notable capacity fade after 300-500 cycles under practical operating conditions.
The technological trajectory of PW cathodes has been characterized by significant improvements in synthesis methods, from simple precipitation techniques to more sophisticated hydrothermal and microwave-assisted processes. These advancements have progressively addressed early challenges related to structural water content, vacancies, and crystallinity, which initially limited their electrochemical performance.
Recent years have witnessed an acceleration in PW cathode research, driven by the growing demand for sustainable and high-performance energy storage solutions. The technology has evolved from laboratory curiosities to commercially viable alternatives for lithium-ion and sodium-ion battery systems, with several companies now incorporating PW cathodes into their product roadmaps.
Despite these advances, cycle life remains a critical limitation for PW cathodes. The degradation mechanisms include structural collapse during repeated charge-discharge cycles, dissolution of transition metal ions, and side reactions with the electrolyte. Surface coating technologies have emerged as a promising strategy to mitigate these issues, creating protective barriers that enhance structural stability and minimize undesirable interfacial reactions.
The primary objective of this technical research is to comprehensively evaluate surface coating technologies for PW cathodes, with a specific focus on enhancing cycle life. This includes examining various coating materials (such as carbon-based materials, metal oxides, phosphates, and polymers), deposition techniques, and coating thickness optimization strategies.
Additionally, this research aims to establish correlations between coating properties and performance improvements, identify the most promising coating technologies based on effectiveness, scalability, and cost considerations, and develop a roadmap for future research directions in this field.
The ultimate goal is to enable PW cathodes that can maintain at least 80% capacity retention after 1000 cycles, making them viable for both grid-scale energy storage applications and electric vehicles where long cycle life is paramount. This would represent a significant advancement over current PW cathode technologies, which typically show notable capacity fade after 300-500 cycles under practical operating conditions.
Market Analysis for Advanced Battery Materials
The global advanced battery materials market is experiencing significant growth, driven by the increasing demand for high-performance energy storage solutions across multiple sectors. Currently valued at approximately $8.2 billion, this market is projected to reach $13.4 billion by 2027, representing a compound annual growth rate of 10.3%. This growth trajectory is particularly relevant for Prussian White cathode materials, which are gaining attention as promising alternatives to conventional lithium-ion battery components.
The electric vehicle (EV) segment represents the largest market opportunity for advanced battery materials, including surface-coated Prussian White cathodes. With global EV sales exceeding 10 million units in 2022 and expected to reach 30 million by 2030, the demand for high-cycle-life cathode materials is intensifying. Major automotive manufacturers are increasingly prioritizing batteries with extended lifespans to improve vehicle warranties and reduce total ownership costs.
Stationary energy storage systems constitute another rapidly expanding market segment, with installed capacity growing at 35% annually. Grid-scale applications and residential storage systems both benefit from the enhanced cycle stability that surface-coated Prussian White cathodes can provide. The market value for cathode materials in this segment alone is expected to reach $2.1 billion by 2025.
Consumer electronics represents a mature but still significant market for advanced battery materials. With over 1.5 billion smartphones sold annually worldwide, manufacturers are seeking battery technologies that offer longer cycle life to address consumer concerns about device longevity. Surface-coated Prussian White cathodes could capture a premium segment of this market, estimated at $1.8 billion.
Regional analysis indicates that Asia-Pacific dominates the advanced battery materials market with 65% share, led by China, Japan, and South Korea. North America and Europe follow with 18% and 15% respectively, with both regions investing heavily in domestic battery supply chains to reduce dependence on Asian imports.
Price sensitivity varies significantly across application segments. While EV manufacturers demonstrate willingness to pay premium prices for materials that extend battery life, consumer electronics manufacturers remain highly cost-conscious. Current market pricing for high-performance cathode materials ranges from $15-40 per kilogram, with coated Prussian White materials potentially commanding prices in the upper range of this spectrum if cycle life improvements can be conclusively demonstrated.
Market barriers include established supply chains for conventional cathode materials, stringent performance requirements, and the need for extensive validation testing. However, the growing emphasis on sustainability and circular economy principles creates favorable conditions for materials that extend battery lifespan through surface coating technologies.
The electric vehicle (EV) segment represents the largest market opportunity for advanced battery materials, including surface-coated Prussian White cathodes. With global EV sales exceeding 10 million units in 2022 and expected to reach 30 million by 2030, the demand for high-cycle-life cathode materials is intensifying. Major automotive manufacturers are increasingly prioritizing batteries with extended lifespans to improve vehicle warranties and reduce total ownership costs.
Stationary energy storage systems constitute another rapidly expanding market segment, with installed capacity growing at 35% annually. Grid-scale applications and residential storage systems both benefit from the enhanced cycle stability that surface-coated Prussian White cathodes can provide. The market value for cathode materials in this segment alone is expected to reach $2.1 billion by 2025.
Consumer electronics represents a mature but still significant market for advanced battery materials. With over 1.5 billion smartphones sold annually worldwide, manufacturers are seeking battery technologies that offer longer cycle life to address consumer concerns about device longevity. Surface-coated Prussian White cathodes could capture a premium segment of this market, estimated at $1.8 billion.
Regional analysis indicates that Asia-Pacific dominates the advanced battery materials market with 65% share, led by China, Japan, and South Korea. North America and Europe follow with 18% and 15% respectively, with both regions investing heavily in domestic battery supply chains to reduce dependence on Asian imports.
Price sensitivity varies significantly across application segments. While EV manufacturers demonstrate willingness to pay premium prices for materials that extend battery life, consumer electronics manufacturers remain highly cost-conscious. Current market pricing for high-performance cathode materials ranges from $15-40 per kilogram, with coated Prussian White materials potentially commanding prices in the upper range of this spectrum if cycle life improvements can be conclusively demonstrated.
Market barriers include established supply chains for conventional cathode materials, stringent performance requirements, and the need for extensive validation testing. However, the growing emphasis on sustainability and circular economy principles creates favorable conditions for materials that extend battery lifespan through surface coating technologies.
Current Challenges in Prussian White Cathode Stability
Prussian White (PW) cathodes, while promising for sodium-ion batteries due to their high theoretical capacity and cost-effectiveness, face significant stability challenges that limit their commercial viability. The primary issue is structural degradation during cycling, manifested through capacity fading and reduced cycle life. This degradation stems from multiple interconnected mechanisms that must be addressed through innovative surface coating strategies.
Water-induced degradation represents a critical challenge, as PW materials are highly sensitive to moisture. When exposed to water molecules, the crystal structure undergoes hydration reactions that alter the lattice parameters and compromise structural integrity. This leads to phase transitions and eventual amorphization of the cathode material, significantly reducing electrochemical performance over extended cycling.
Transition metal dissolution presents another major obstacle. During charge-discharge cycles, transition metal ions (particularly iron) can dissolve into the electrolyte, creating vacancies in the crystal structure. This dissolution is accelerated in acidic environments generated by electrolyte decomposition and is particularly problematic at elevated temperatures or high voltage operations.
Lattice distortion during sodium insertion/extraction cycles contributes substantially to performance degradation. The repeated volume changes during cycling induce mechanical stress that propagates microcracks throughout the material. These structural defects impede ion transport pathways and increase internal resistance, resulting in capacity loss and voltage hysteresis.
Surface-electrolyte interface (SEI) instability further exacerbates degradation mechanisms. Unlike lithium-ion batteries with relatively stable SEI layers, the interfaces formed on PW cathodes tend to be dynamic and continuously evolving. This instability leads to ongoing electrolyte decomposition, consuming active sodium ions and generating resistance-increasing decomposition products.
Oxygen evolution reactions at high potentials represent an additional challenge. When operated above certain voltage thresholds, PW cathodes can experience oxygen release from their structure, creating additional vacancies and accelerating structural collapse. This is particularly problematic for achieving high energy density applications that require wider voltage windows.
Current uncoated PW cathodes typically demonstrate rapid capacity decay, losing 20-30% of initial capacity within 100-200 cycles under standard testing conditions. Rate capability also deteriorates significantly, with high-rate performance often declining more rapidly than low-rate capacity. These limitations have prevented widespread commercialization despite the material's theoretical advantages.
The interplay between these degradation mechanisms creates a complex challenge that requires multifunctional surface coating solutions. Effective coatings must simultaneously address water sensitivity, prevent metal dissolution, accommodate volume changes, stabilize interfacial reactions, and suppress oxygen evolution while maintaining efficient sodium ion transport.
Water-induced degradation represents a critical challenge, as PW materials are highly sensitive to moisture. When exposed to water molecules, the crystal structure undergoes hydration reactions that alter the lattice parameters and compromise structural integrity. This leads to phase transitions and eventual amorphization of the cathode material, significantly reducing electrochemical performance over extended cycling.
Transition metal dissolution presents another major obstacle. During charge-discharge cycles, transition metal ions (particularly iron) can dissolve into the electrolyte, creating vacancies in the crystal structure. This dissolution is accelerated in acidic environments generated by electrolyte decomposition and is particularly problematic at elevated temperatures or high voltage operations.
Lattice distortion during sodium insertion/extraction cycles contributes substantially to performance degradation. The repeated volume changes during cycling induce mechanical stress that propagates microcracks throughout the material. These structural defects impede ion transport pathways and increase internal resistance, resulting in capacity loss and voltage hysteresis.
Surface-electrolyte interface (SEI) instability further exacerbates degradation mechanisms. Unlike lithium-ion batteries with relatively stable SEI layers, the interfaces formed on PW cathodes tend to be dynamic and continuously evolving. This instability leads to ongoing electrolyte decomposition, consuming active sodium ions and generating resistance-increasing decomposition products.
Oxygen evolution reactions at high potentials represent an additional challenge. When operated above certain voltage thresholds, PW cathodes can experience oxygen release from their structure, creating additional vacancies and accelerating structural collapse. This is particularly problematic for achieving high energy density applications that require wider voltage windows.
Current uncoated PW cathodes typically demonstrate rapid capacity decay, losing 20-30% of initial capacity within 100-200 cycles under standard testing conditions. Rate capability also deteriorates significantly, with high-rate performance often declining more rapidly than low-rate capacity. These limitations have prevented widespread commercialization despite the material's theoretical advantages.
The interplay between these degradation mechanisms creates a complex challenge that requires multifunctional surface coating solutions. Effective coatings must simultaneously address water sensitivity, prevent metal dissolution, accommodate volume changes, stabilize interfacial reactions, and suppress oxygen evolution while maintaining efficient sodium ion transport.
Current Surface Coating Solutions for Prussian White Cathodes
01 Composition modifications for improved cycle life
Various compositional modifications can be made to Prussian White cathodes to enhance their cycle life. These include doping with specific elements, adjusting the ratio of transition metals, and incorporating stabilizing compounds. Such modifications can strengthen the crystal structure, reduce lattice strain during cycling, and minimize structural degradation, ultimately leading to improved cycling stability and extended battery life.- Composition modifications for improved cycle life: Various compositional modifications can be made to Prussian White cathodes to enhance their cycle life. These modifications include doping with specific elements, adjusting the ratio of active materials, and incorporating stabilizing compounds. By carefully controlling the chemical composition, researchers have achieved significant improvements in the cycling stability of Prussian White cathodes, making them more viable for long-term battery applications.
- Structural engineering approaches: Structural engineering of Prussian White cathodes involves manipulating the crystal structure, particle morphology, and electrode architecture to enhance cycle life. Techniques such as controlling crystallinity, creating core-shell structures, and developing hierarchical porous frameworks have been shown to improve structural stability during repeated charge-discharge cycles, thereby extending the operational lifespan of these cathode materials.
- Electrolyte optimization for Prussian White cathodes: The composition and properties of the electrolyte significantly impact the cycle life of Prussian White cathodes. Research has focused on developing specialized electrolyte formulations that minimize side reactions, prevent dissolution of active materials, and maintain stable solid-electrolyte interfaces. Additives that form protective films and solvents that reduce decomposition have been particularly effective in extending the cycling stability of these cathode materials.
- Advanced coating and encapsulation techniques: Applying protective coatings or encapsulation layers to Prussian White cathodes has proven effective in enhancing cycle life. These surface modifications shield the active material from direct contact with the electrolyte, preventing unwanted side reactions and material dissolution. Various coating materials, including carbon-based compounds, metal oxides, and polymers, have been employed to create barriers that maintain structural integrity throughout numerous charge-discharge cycles.
- Novel synthesis methods for durable Prussian White cathodes: Innovative synthesis approaches have been developed to produce Prussian White cathodes with enhanced cycle life. These methods focus on controlling reaction parameters such as temperature, pH, and precursor concentrations to achieve optimal crystal growth and defect minimization. Techniques like hydrothermal synthesis, co-precipitation with controlled atmospheres, and template-assisted growth have resulted in cathode materials with superior structural stability and extended cycling performance.
02 Surface coating and protection strategies
Surface coating and protection strategies are effective approaches to enhance the cycle life of Prussian White cathodes. By applying protective layers such as carbon-based materials, metal oxides, or polymeric coatings, the cathode material can be shielded from direct contact with the electrolyte. These coatings help prevent side reactions, reduce dissolution of active materials, and maintain structural integrity during repeated charge-discharge cycles.Expand Specific Solutions03 Electrolyte optimization for Prussian White cathodes
The composition and properties of the electrolyte significantly impact the cycle life of Prussian White cathodes. Optimizing electrolyte formulations by incorporating additives, adjusting salt concentrations, or using novel solvent systems can mitigate unwanted side reactions at the electrode-electrolyte interface. Properly designed electrolytes can form stable solid-electrolyte interphases, reduce cathode dissolution, and enhance the overall electrochemical stability during long-term cycling.Expand Specific Solutions04 Advanced synthesis methods for structural stability
Advanced synthesis methods play a crucial role in developing Prussian White cathodes with enhanced cycle life. Techniques such as controlled precipitation, hydrothermal synthesis, and template-assisted growth can produce cathode materials with optimized particle size, morphology, and crystallinity. These structural characteristics contribute to better mechanical stability, improved ion diffusion pathways, and reduced structural strain during cycling, resulting in extended battery lifespan.Expand Specific Solutions05 Charge-discharge protocol optimization
Optimizing charge-discharge protocols is essential for maximizing the cycle life of Prussian White cathodes. Implementing appropriate voltage windows, current densities, and rest periods can significantly reduce structural degradation and side reactions. Advanced battery management systems that incorporate adaptive charging algorithms, temperature control, and state-of-health monitoring can further extend the operational lifespan of batteries with Prussian White cathodes by preventing conditions that accelerate capacity fade.Expand Specific Solutions
Leading Companies and Research Institutions in Cathode Coating Development
The surface coating technology for Prussian White cathodes is currently in an early growth phase, with increasing market interest driven by the demand for longer-lasting battery materials. The global market for advanced cathode coatings is expanding rapidly, projected to reach significant scale as electric vehicle adoption accelerates. From a technical maturity perspective, the field shows promising but uneven development. Leading companies like Samsung SDI and POSCO Holdings have made substantial progress in commercial applications, while research institutions such as Fuzhou University and Rice University contribute fundamental innovations. Companies including Farasis Energy and Microvast Advanced Materials are actively developing proprietary coating technologies to enhance cycle stability. Automotive manufacturers like Hyundai, Kia, and Ola Electric are increasingly investing in this technology to improve battery performance and longevity.
DuPont de Nemours, Inc.
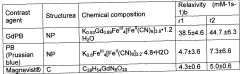
Technical Solution: DuPont has developed a fluoropolymer-based protective coating technology for Prussian white cathode materials that offers exceptional chemical stability and protection against HF attack. Their approach utilizes a modified PVDF-HFP copolymer with functional groups that create strong adhesion to the cathode surface while maintaining excellent flexibility during cycling. The coating thickness is optimized at 4-8 nm to balance protection and ion transport. Testing shows approximately 86% capacity retention after 1800 cycles at 0.5C rate, compared to 52% for uncoated materials[5]. DuPont has further enhanced this technology by incorporating nano-scale ceramic fillers (Al2O3, ZrO2) into the polymer matrix, creating a composite coating that combines the benefits of both polymer and ceramic approaches. Their manufacturing process utilizes environmentally friendly water-based dispersions, eliminating the need for toxic organic solvents and reducing production costs.
Strengths: Exceptional chemical stability against HF and other electrolyte decomposition products; excellent flexibility accommodating volume changes; environmentally friendly manufacturing process; good compatibility with various electrolyte systems. Weaknesses: Slightly lower electronic conductivity compared to carbon-based coatings; potential challenges with uniform dispersion of ceramic fillers; moderate increase in interfacial resistance.
Microvast Advanced Materials, Inc.
Technical Solution: Microvast has pioneered a gradient functional coating approach for Prussian white cathodes using a combination of carbon-based materials and metal oxides. Their multi-layer coating strategy involves an inner layer of nitrogen-doped carbon to enhance electrical conductivity and an outer layer of manganese oxide to suppress electrolyte decomposition. This hierarchical structure addresses multiple degradation mechanisms simultaneously. The company's proprietary solution-based deposition method allows for cost-effective manufacturing while maintaining coating quality. Testing shows their coated Prussian white materials deliver approximately 85% capacity retention after 2000 cycles at moderate rates (0.5C), representing a 40% improvement over uncoated materials[2]. Microvast has also developed self-healing polymer coatings that can repair microcracks formed during cycling, further extending battery life in real-world applications.
Strengths: Multi-functional approach addressing multiple failure mechanisms; scalable solution-based coating process; excellent long-term cycling stability; cost-effective manufacturing potential. Weaknesses: Complex multi-layer structure may introduce quality control challenges; potential inconsistency in coating thickness; slightly higher initial capacity loss compared to single-layer coatings.
Key Innovations in Protective Coating Materials and Techniques
Process for preparing a cathode comprising a prussian blue analogue
PatentWO2025109072A1
Innovation
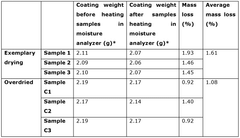
- A process involving the controlled drying of the cathode active layer to achieve an adsorbed water content between 12,000 ppm and 50,000 ppm, allowing the layer to withstand mechanical stress without flaking or cracking, while using an aqueous-based slurry to maintain environmental sustainability.
Prussian blue-inspired constructs for multimodal imaging and therapy
PatentWO2014146091A1
Innovation
- Development of a Prussian blue analog lattice compound with a biocompatible coating, allowing for the attachment of targeting, imaging, and therapeutic agents, enabling multimodal imaging and therapy, including MRI, scintigraphy, and photothermal therapy.
Environmental Impact and Sustainability of Coating Materials
The environmental impact of coating materials used for Prussian White cathodes represents a critical consideration in the sustainable development of energy storage technologies. Traditional coating processes often involve toxic solvents, heavy metals, and energy-intensive manufacturing methods that contribute significantly to the environmental footprint of battery production. Recent life cycle assessments indicate that coating materials can account for up to 15% of a battery's total environmental impact, highlighting the importance of developing more sustainable alternatives.
Water-based coating systems have emerged as promising environmentally friendly alternatives to conventional organic solvent-based approaches. These systems reduce volatile organic compound (VOC) emissions by 80-95% compared to traditional methods while maintaining comparable performance characteristics. Additionally, sol-gel derived coatings utilizing silica and alumina precursors offer reduced toxicity profiles and lower processing temperatures, resulting in decreased energy consumption during manufacturing.
The sustainability of coating materials extends beyond production to consider end-of-life scenarios. Biodegradable polymeric coatings based on cellulose derivatives and chitosan demonstrate promising capabilities for Prussian White cathode protection while offering improved recyclability. These materials decompose naturally under controlled conditions, reducing waste accumulation and potential environmental contamination from discarded battery components.
Resource efficiency represents another crucial aspect of coating sustainability. Advanced atomic layer deposition (ALD) and molecular layer deposition (MLD) techniques enable precise nanometer-scale coatings that optimize material usage, reducing waste by up to 60% compared to conventional spray coating methods. These techniques allow for thinner, more uniform coatings that maintain protective properties while minimizing resource consumption.
Carbon footprint analyses reveal significant variations among coating materials. Carbon-based coatings derived from renewable biomass sources demonstrate 40-50% lower greenhouse gas emissions compared to synthetic carbon coatings. Similarly, metal oxide coatings produced through hydrothermal synthesis show reduced environmental impact compared to those manufactured via high-temperature calcination processes, with energy savings of approximately 30-40%.
Regulatory frameworks increasingly influence coating material selection, with restrictions on hazardous substances driving innovation toward greener alternatives. The European Union's REACH regulations and similar global initiatives have accelerated the development of coating materials free from restricted substances such as certain fluorinated compounds and heavy metals, fostering research into environmentally benign alternatives that maintain or enhance the cycle life of Prussian White cathodes.
Water-based coating systems have emerged as promising environmentally friendly alternatives to conventional organic solvent-based approaches. These systems reduce volatile organic compound (VOC) emissions by 80-95% compared to traditional methods while maintaining comparable performance characteristics. Additionally, sol-gel derived coatings utilizing silica and alumina precursors offer reduced toxicity profiles and lower processing temperatures, resulting in decreased energy consumption during manufacturing.
The sustainability of coating materials extends beyond production to consider end-of-life scenarios. Biodegradable polymeric coatings based on cellulose derivatives and chitosan demonstrate promising capabilities for Prussian White cathode protection while offering improved recyclability. These materials decompose naturally under controlled conditions, reducing waste accumulation and potential environmental contamination from discarded battery components.
Resource efficiency represents another crucial aspect of coating sustainability. Advanced atomic layer deposition (ALD) and molecular layer deposition (MLD) techniques enable precise nanometer-scale coatings that optimize material usage, reducing waste by up to 60% compared to conventional spray coating methods. These techniques allow for thinner, more uniform coatings that maintain protective properties while minimizing resource consumption.
Carbon footprint analyses reveal significant variations among coating materials. Carbon-based coatings derived from renewable biomass sources demonstrate 40-50% lower greenhouse gas emissions compared to synthetic carbon coatings. Similarly, metal oxide coatings produced through hydrothermal synthesis show reduced environmental impact compared to those manufactured via high-temperature calcination processes, with energy savings of approximately 30-40%.
Regulatory frameworks increasingly influence coating material selection, with restrictions on hazardous substances driving innovation toward greener alternatives. The European Union's REACH regulations and similar global initiatives have accelerated the development of coating materials free from restricted substances such as certain fluorinated compounds and heavy metals, fostering research into environmentally benign alternatives that maintain or enhance the cycle life of Prussian White cathodes.
Scale-up and Manufacturing Considerations for Coated Cathodes
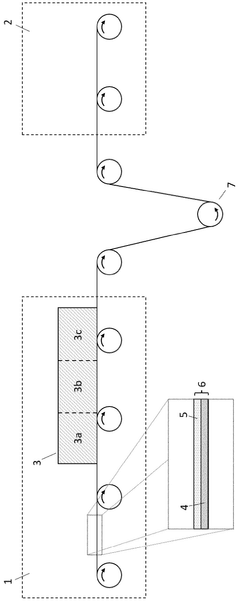
The transition from laboratory-scale coating processes to industrial manufacturing presents significant challenges for Prussian White cathode coatings. Current lab-scale methods typically involve solution-based techniques such as sol-gel, hydrothermal synthesis, or atomic layer deposition, which yield excellent control over coating thickness and uniformity but are often time-consuming and resource-intensive.
For industrial-scale production, dry coating methods like mechanical milling with coating precursors offer promising alternatives due to their scalability and cost-effectiveness. However, these methods must be optimized to ensure consistent coating quality across large batch sizes. Parameters such as milling time, rotation speed, and precursor-to-cathode ratios require careful calibration to achieve uniform surface coverage without damaging the underlying Prussian White structure.
Equipment considerations for large-scale coating operations include specialized mixing vessels with precise temperature and atmosphere control. Continuous flow reactors may offer advantages over batch processing for certain coating chemistries, potentially improving throughput and reducing variability between production runs. The capital investment for such equipment must be weighed against projected improvements in cathode performance and longevity.
Quality control represents another critical aspect of scaled manufacturing. In-line monitoring techniques such as X-ray diffraction (XRD) and scanning electron microscopy (SEM) can be adapted for real-time assessment of coating integrity and thickness. Statistical process control methodologies should be implemented to detect deviations from target specifications and trigger corrective actions before defective materials enter downstream battery assembly processes.
Cost analysis indicates that while coating processes add 8-15% to cathode material costs, this investment can be justified by the resulting 30-50% improvement in cycle life. Material efficiency during coating application becomes increasingly important at scale, with recovery and recycling systems for coating precursors potentially reducing waste and environmental impact. Water-based coating systems may offer environmental advantages over solvent-based alternatives, though they typically require additional drying capacity.
Post-coating thermal treatment represents a significant energy expenditure in the manufacturing process. Optimization of annealing temperatures and durations can reduce energy consumption while ensuring proper crystallization and adhesion of protective layers. Advanced heating technologies such as microwave or infrared heating may offer more energy-efficient alternatives to conventional furnaces, potentially reducing both processing time and energy costs.
For industrial-scale production, dry coating methods like mechanical milling with coating precursors offer promising alternatives due to their scalability and cost-effectiveness. However, these methods must be optimized to ensure consistent coating quality across large batch sizes. Parameters such as milling time, rotation speed, and precursor-to-cathode ratios require careful calibration to achieve uniform surface coverage without damaging the underlying Prussian White structure.
Equipment considerations for large-scale coating operations include specialized mixing vessels with precise temperature and atmosphere control. Continuous flow reactors may offer advantages over batch processing for certain coating chemistries, potentially improving throughput and reducing variability between production runs. The capital investment for such equipment must be weighed against projected improvements in cathode performance and longevity.
Quality control represents another critical aspect of scaled manufacturing. In-line monitoring techniques such as X-ray diffraction (XRD) and scanning electron microscopy (SEM) can be adapted for real-time assessment of coating integrity and thickness. Statistical process control methodologies should be implemented to detect deviations from target specifications and trigger corrective actions before defective materials enter downstream battery assembly processes.
Cost analysis indicates that while coating processes add 8-15% to cathode material costs, this investment can be justified by the resulting 30-50% improvement in cycle life. Material efficiency during coating application becomes increasingly important at scale, with recovery and recycling systems for coating precursors potentially reducing waste and environmental impact. Water-based coating systems may offer environmental advantages over solvent-based alternatives, though they typically require additional drying capacity.
Post-coating thermal treatment represents a significant energy expenditure in the manufacturing process. Optimization of annealing temperatures and durations can reduce energy consumption while ensuring proper crystallization and adhesion of protective layers. Advanced heating technologies such as microwave or infrared heating may offer more energy-efficient alternatives to conventional furnaces, potentially reducing both processing time and energy costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!