Electrospun Filtration For Medical Facilities: Sterilization Compatibility
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrospun Filtration Technology Background and Objectives
Electrospun filtration technology has evolved significantly over the past three decades, transitioning from laboratory curiosity to commercial application. The process, which utilizes electrostatic forces to create ultra-fine fibers from polymer solutions, was first patented in 1934 by Anton Formhals but remained largely unexplored until the 1990s when nanotechnology research accelerated. The resulting non-woven mats of fibers with diameters ranging from nanometers to micrometers exhibit exceptional filtration properties due to their high surface area-to-volume ratio, controllable porosity, and ability to incorporate functional additives.
In medical facilities, air and fluid filtration systems represent critical infrastructure for preventing healthcare-associated infections (HAIs) and maintaining sterile environments. Traditional filtration technologies such as melt-blown polypropylene filters, while effective, often present trade-offs between filtration efficiency, pressure drop, and durability. Electrospun filters have emerged as promising alternatives due to their superior filtration efficiency at equivalent or lower pressure drops compared to conventional technologies.
The current technological trajectory focuses on developing electrospun filters that can withstand rigorous sterilization processes required in medical settings. These include autoclave sterilization (121-134°C under pressure), ethylene oxide exposure, gamma irradiation, and hydrogen peroxide plasma treatments. Each sterilization method presents unique challenges to filter integrity, potentially causing polymer degradation, fiber morphology changes, or reduction in filtration performance.
Recent advancements have focused on polymer selection and modification strategies to enhance sterilization compatibility. Polymers such as polyimide, PEEK (polyether ether ketone), and certain fluoropolymers demonstrate superior thermal and chemical resistance. Additionally, cross-linking techniques and the incorporation of stabilizing additives have shown promise in preserving filter integrity during sterilization cycles.
The primary objective of current research is to develop electrospun filtration media that maintain structural integrity and filtration performance after multiple sterilization cycles while meeting the stringent requirements of medical environments. Secondary objectives include reducing manufacturing costs, improving scalability of production processes, and developing sustainable alternatives to petroleum-based polymers without compromising sterilization compatibility.
Achieving these objectives would represent a significant advancement in healthcare infrastructure, potentially reducing HAIs, decreasing healthcare costs, and improving patient outcomes. The technology also has broader implications for cleanroom environments, pharmaceutical manufacturing, and biological research facilities where sterile filtration is essential.
In medical facilities, air and fluid filtration systems represent critical infrastructure for preventing healthcare-associated infections (HAIs) and maintaining sterile environments. Traditional filtration technologies such as melt-blown polypropylene filters, while effective, often present trade-offs between filtration efficiency, pressure drop, and durability. Electrospun filters have emerged as promising alternatives due to their superior filtration efficiency at equivalent or lower pressure drops compared to conventional technologies.
The current technological trajectory focuses on developing electrospun filters that can withstand rigorous sterilization processes required in medical settings. These include autoclave sterilization (121-134°C under pressure), ethylene oxide exposure, gamma irradiation, and hydrogen peroxide plasma treatments. Each sterilization method presents unique challenges to filter integrity, potentially causing polymer degradation, fiber morphology changes, or reduction in filtration performance.
Recent advancements have focused on polymer selection and modification strategies to enhance sterilization compatibility. Polymers such as polyimide, PEEK (polyether ether ketone), and certain fluoropolymers demonstrate superior thermal and chemical resistance. Additionally, cross-linking techniques and the incorporation of stabilizing additives have shown promise in preserving filter integrity during sterilization cycles.
The primary objective of current research is to develop electrospun filtration media that maintain structural integrity and filtration performance after multiple sterilization cycles while meeting the stringent requirements of medical environments. Secondary objectives include reducing manufacturing costs, improving scalability of production processes, and developing sustainable alternatives to petroleum-based polymers without compromising sterilization compatibility.
Achieving these objectives would represent a significant advancement in healthcare infrastructure, potentially reducing HAIs, decreasing healthcare costs, and improving patient outcomes. The technology also has broader implications for cleanroom environments, pharmaceutical manufacturing, and biological research facilities where sterile filtration is essential.
Healthcare Facility Filtration Market Analysis
The global healthcare facility filtration market is experiencing robust growth, driven by increasing awareness of airborne infections and stringent regulations for healthcare environments. Currently valued at approximately 3.2 billion USD, the market is projected to reach 4.7 billion USD by 2027, representing a compound annual growth rate of 7.9%. This growth trajectory is particularly pronounced in regions with expanding healthcare infrastructure, such as Asia-Pacific and the Middle East.
Electrospun filtration technologies are emerging as a significant segment within this market, with an estimated current share of 12% and growing rapidly. The technology's ability to create nanofiber membranes with high filtration efficiency while maintaining breathability positions it favorably against traditional filtration methods. Market research indicates that hospitals and surgical centers constitute the largest end-user segment, accounting for 58% of the total market demand.
The COVID-19 pandemic has substantially accelerated market growth, with a 34% increase in demand for advanced filtration systems in 2020 alone. This surge has created both opportunities and challenges, as healthcare facilities seek solutions that can be rapidly deployed and easily sterilized without compromising filtration integrity. Post-pandemic, the market is expected to stabilize but maintain higher growth rates than pre-pandemic projections.
Key market drivers include the rising incidence of hospital-acquired infections (HAIs), which affect approximately 1 in 31 hospital patients in the United States daily. The economic burden of HAIs, estimated at 28-45 billion USD annually, provides strong incentive for investment in superior filtration technologies. Additionally, regulatory bodies worldwide are implementing stricter air quality standards for healthcare facilities, further stimulating market demand.
Market segmentation reveals distinct preferences across different healthcare settings. While large hospitals prioritize comprehensive HVAC filtration systems with integrated electrospun components, ambulatory surgical centers and specialized clinics show greater interest in portable, modular filtration units that can be easily sterilized between uses. This segmentation presents opportunities for targeted product development and marketing strategies.
Consumer behavior analysis indicates that purchasing decisions are increasingly influenced by total cost of ownership rather than initial investment. Healthcare facilities are willing to pay premium prices for filtration systems that demonstrate superior sterilization compatibility, longer service life, and reduced maintenance requirements. This trend favors electrospun filtration technologies, which typically offer longer replacement cycles and better resistance to sterilization procedures compared to conventional filters.
Electrospun filtration technologies are emerging as a significant segment within this market, with an estimated current share of 12% and growing rapidly. The technology's ability to create nanofiber membranes with high filtration efficiency while maintaining breathability positions it favorably against traditional filtration methods. Market research indicates that hospitals and surgical centers constitute the largest end-user segment, accounting for 58% of the total market demand.
The COVID-19 pandemic has substantially accelerated market growth, with a 34% increase in demand for advanced filtration systems in 2020 alone. This surge has created both opportunities and challenges, as healthcare facilities seek solutions that can be rapidly deployed and easily sterilized without compromising filtration integrity. Post-pandemic, the market is expected to stabilize but maintain higher growth rates than pre-pandemic projections.
Key market drivers include the rising incidence of hospital-acquired infections (HAIs), which affect approximately 1 in 31 hospital patients in the United States daily. The economic burden of HAIs, estimated at 28-45 billion USD annually, provides strong incentive for investment in superior filtration technologies. Additionally, regulatory bodies worldwide are implementing stricter air quality standards for healthcare facilities, further stimulating market demand.
Market segmentation reveals distinct preferences across different healthcare settings. While large hospitals prioritize comprehensive HVAC filtration systems with integrated electrospun components, ambulatory surgical centers and specialized clinics show greater interest in portable, modular filtration units that can be easily sterilized between uses. This segmentation presents opportunities for targeted product development and marketing strategies.
Consumer behavior analysis indicates that purchasing decisions are increasingly influenced by total cost of ownership rather than initial investment. Healthcare facilities are willing to pay premium prices for filtration systems that demonstrate superior sterilization compatibility, longer service life, and reduced maintenance requirements. This trend favors electrospun filtration technologies, which typically offer longer replacement cycles and better resistance to sterilization procedures compared to conventional filters.
Current Challenges in Medical-Grade Electrospun Filtration
Despite significant advancements in electrospun filtration technology for medical facilities, several critical challenges persist in achieving optimal sterilization compatibility. The primary obstacle lies in maintaining the structural integrity and filtration efficiency of electrospun nanofibers during sterilization processes. Conventional sterilization methods such as autoclaving (121-134°C steam under pressure) often cause melting, shrinkage, or degradation of polymer nanofibers, particularly those composed of temperature-sensitive materials like polycaprolactone (PCL) or polyvinyl alcohol (PVA).
Ethylene oxide (EtO) sterilization, while operating at lower temperatures, introduces chemical interactions that may alter surface properties of electrospun filters, potentially reducing filtration efficiency or introducing toxic residues. Studies have shown that EtO exposure can reduce the hydrophilicity of certain polymer nanofibers, affecting their performance in humid medical environments.
Gamma irradiation presents another challenge, as the high-energy radiation can induce chain scission or cross-linking in polymer structures, leading to mechanical weakening or embrittlement of nanofibers. Research indicates that doses above 25 kGy—the standard for medical sterilization—can significantly compromise the tensile strength of electrospun membranes composed of polyurethane and similar elastomeric polymers.
Material selection constraints further complicate development efforts. While polymers like PTFE (polytetrafluoroethylene) demonstrate excellent sterilization resistance, they present significant challenges in the electrospinning process due to their limited solubility in conventional solvents and high electrostatic properties.
The incorporation of antimicrobial agents into electrospun filters introduces additional compatibility issues. Silver nanoparticles and quaternary ammonium compounds, commonly used for antimicrobial properties, may leach during sterilization or exhibit reduced efficacy after repeated sterilization cycles, compromising long-term performance.
Scale-up manufacturing presents persistent challenges in maintaining consistent fiber diameter, porosity, and mechanical properties across large-area filters required for hospital HVAC systems and clean rooms. Current production methods struggle to deliver the uniformity necessary for medical-grade applications when scaling beyond laboratory dimensions.
Regulatory hurdles compound these technical challenges. The FDA and equivalent international bodies require extensive validation of both initial filtration efficiency and post-sterilization performance, with stringent requirements for particulate shedding, biocompatibility, and maintenance of filtration parameters through multiple sterilization cycles.
Cost-effectiveness remains a significant barrier to widespread adoption. Current medical-grade electrospun filters with sterilization compatibility typically cost 3-5 times more than conventional melt-blown alternatives, limiting their implementation despite superior filtration performance at the sub-micron level.
Ethylene oxide (EtO) sterilization, while operating at lower temperatures, introduces chemical interactions that may alter surface properties of electrospun filters, potentially reducing filtration efficiency or introducing toxic residues. Studies have shown that EtO exposure can reduce the hydrophilicity of certain polymer nanofibers, affecting their performance in humid medical environments.
Gamma irradiation presents another challenge, as the high-energy radiation can induce chain scission or cross-linking in polymer structures, leading to mechanical weakening or embrittlement of nanofibers. Research indicates that doses above 25 kGy—the standard for medical sterilization—can significantly compromise the tensile strength of electrospun membranes composed of polyurethane and similar elastomeric polymers.
Material selection constraints further complicate development efforts. While polymers like PTFE (polytetrafluoroethylene) demonstrate excellent sterilization resistance, they present significant challenges in the electrospinning process due to their limited solubility in conventional solvents and high electrostatic properties.
The incorporation of antimicrobial agents into electrospun filters introduces additional compatibility issues. Silver nanoparticles and quaternary ammonium compounds, commonly used for antimicrobial properties, may leach during sterilization or exhibit reduced efficacy after repeated sterilization cycles, compromising long-term performance.
Scale-up manufacturing presents persistent challenges in maintaining consistent fiber diameter, porosity, and mechanical properties across large-area filters required for hospital HVAC systems and clean rooms. Current production methods struggle to deliver the uniformity necessary for medical-grade applications when scaling beyond laboratory dimensions.
Regulatory hurdles compound these technical challenges. The FDA and equivalent international bodies require extensive validation of both initial filtration efficiency and post-sterilization performance, with stringent requirements for particulate shedding, biocompatibility, and maintenance of filtration parameters through multiple sterilization cycles.
Cost-effectiveness remains a significant barrier to widespread adoption. Current medical-grade electrospun filters with sterilization compatibility typically cost 3-5 times more than conventional melt-blown alternatives, limiting their implementation despite superior filtration performance at the sub-micron level.
Current Sterilization-Compatible Electrospun Solutions
01 Electrospun nanofiber filtration materials for sterilization
Electrospun nanofiber materials can be used to create highly efficient filtration media for sterilization purposes. These materials offer advantages such as high surface area, controllable pore size, and excellent filtration efficiency. The nanofiber structure allows for effective capture of microorganisms and particles while maintaining good air permeability. These materials can be engineered with specific properties to enhance their sterilization capabilities while remaining compatible with various applications.- Electrospun nanofiber filtration materials for sterilization: Electrospun nanofiber materials can be engineered for filtration applications with sterilization capabilities. These materials offer high surface area-to-volume ratios and controllable pore sizes that enable efficient capture of microorganisms and particulates. The nanoscale fibers can be functionalized with antimicrobial agents to provide active sterilization properties while maintaining high filtration efficiency. These materials are particularly valuable in medical applications, water purification, and air filtration systems where both filtration and sterilization are required simultaneously.
- Sterilization compatibility of electrospun filter materials: Electrospun filter materials can be designed to withstand various sterilization methods including heat, radiation, and chemical treatments without compromising their filtration performance. By selecting appropriate polymer compositions and incorporating stabilizing additives, these materials maintain structural integrity and filtration efficiency after sterilization processes. This compatibility enables reusable filtration systems in medical and laboratory settings where sterility is critical. The development of sterilization-resistant electrospun materials extends filter lifespan and reduces waste in applications requiring repeated sterilization cycles.
- Antimicrobial functionalization of electrospun filters: Electrospun filtration materials can be functionalized with antimicrobial agents to enhance their sterilization capabilities. These agents include metal nanoparticles (silver, copper), quaternary ammonium compounds, and natural antimicrobial substances that can be incorporated directly into the polymer solution before electrospinning or applied as post-treatments. The antimicrobial components provide active sterilization by inhibiting or killing microorganisms that come into contact with the filter surface. This approach creates multifunctional filters that not only physically trap contaminants but also actively neutralize biological threats.
- Composite electrospun structures for enhanced filtration and sterilization: Composite electrospun structures combining multiple materials or layers can achieve superior filtration and sterilization performance. These structures may incorporate hydrophobic and hydrophilic layers, gradient density configurations, or hybrid materials combining electrospun fibers with other filter media. The layered or composite approach allows optimization of different properties such as mechanical strength, filtration efficiency, and antimicrobial activity in a single filter system. These advanced structures can be tailored for specific applications requiring both high filtration performance and sterilization capabilities.
- Environmental and operational stability of sterilizable electrospun filters: Electrospun filters for sterilization applications can be engineered for stability under various environmental and operational conditions. By incorporating stabilizers, crosslinking agents, or selecting inherently stable polymers, these filters maintain performance under challenging conditions such as high humidity, temperature fluctuations, or exposure to harsh chemicals. The stability enables consistent filtration and sterilization performance throughout the filter's operational life. Advanced manufacturing techniques can also improve the mechanical properties of electrospun filters to withstand pressure differentials and flow rates encountered in practical applications.
02 Sterilization compatibility of electrospun filters
Electrospun filtration materials can be designed to withstand various sterilization methods without compromising their structural integrity or filtration performance. These materials can be made compatible with heat sterilization, gamma radiation, ethylene oxide, or other sterilization techniques commonly used in medical and industrial applications. The compatibility with sterilization processes is crucial for applications requiring repeated use or those in sterile environments, ensuring that the filter maintains its effectiveness after sterilization.Expand Specific Solutions03 Composite electrospun filters with antimicrobial properties
Electrospun filters can be enhanced with antimicrobial agents or materials to create composite structures with inherent sterilization capabilities. These composite filters combine the physical filtration properties of electrospun fibers with chemical or biological agents that can inactivate or kill microorganisms. The incorporation of antimicrobial agents such as silver nanoparticles, copper compounds, or specific polymers can provide additional protection against microbial contamination while maintaining compatibility with the filtration system.Expand Specific Solutions04 Electrospun filter systems for liquid sterilization
Specialized electrospun filtration systems can be designed specifically for liquid sterilization applications. These systems utilize the unique properties of electrospun nanofibers to effectively remove microorganisms and contaminants from liquid media. The high surface area and controllable pore structure of electrospun filters make them particularly effective for liquid filtration while maintaining flow rates. These systems can be optimized for compatibility with various liquid compositions and sterilization requirements in pharmaceutical, food, and water treatment industries.Expand Specific Solutions05 Sterilizable electrospun filters for medical applications
Electrospun filtration materials can be specifically engineered for medical applications where both sterilization effectiveness and biocompatibility are essential. These filters can be designed to remove pathogens while being compatible with biological systems and medical devices. The materials used in these filters must withstand medical-grade sterilization processes while maintaining their filtration efficiency and structural integrity. Applications include respiratory protection, surgical environments, medical device components, and biopharmaceutical processing where sterility is critical.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The electrospun filtration market for medical facilities is currently in a growth phase, with increasing demand driven by heightened infection control requirements in healthcare settings. The market size is expanding at a significant rate as medical facilities seek advanced filtration solutions compatible with various sterilization methods. Technologically, the field is moderately mature but continues to evolve, with companies like NovaSterilis, Pall Corp., and W.L. Gore & Associates leading innovation in sterilization-compatible filtration materials. Established medical device manufacturers such as Medtronic, B. Braun, and Ethicon are integrating these technologies into their product lines, while research institutions like Donghua University and University of South Carolina are advancing fundamental knowledge in electrospun materials that can withstand sterilization processes without degradation.
NovaSterilis, Inc.
Technical Solution: NovaSterilis has developed innovative electrospun filtration technology specifically designed for medical facilities with superior sterilization compatibility. Their proprietary approach utilizes a blend of high-temperature resistant polymers including modified PEEK (polyether ether ketone) and PPS (polyphenylene sulfide) to create nanofibers with diameters ranging from 100-400 nm. These materials maintain structural integrity through multiple sterilization cycles, including their patented supercritical CO₂ sterilization process which operates at lower temperatures (35-40°C) than traditional methods, preserving filter integrity. NovaSterilis' electrospun membranes feature a unique three-dimensional architecture that maximizes surface area (>20 m²/g) while maintaining high porosity (>75%) and controlled pore size distribution. Their filters incorporate antimicrobial agents directly into the fiber structure, providing persistent microbial resistance even after multiple sterilization cycles. The technology is compatible with multiple sterilization methods including steam autoclaving (up to 50 cycles at 121°C), gamma irradiation (25-45 kGy), and various chemical sterilants without significant performance degradation.
Strengths: Exceptional resistance to multiple sterilization methods; integrated antimicrobial properties that persist through sterilization cycles; compatibility with their proprietary supercritical CO₂ sterilization technology. Weaknesses: Higher cost compared to conventional filtration systems; limited availability in some global markets; requires specialized handling during installation to prevent damage to the nanofiber structure.
Medtronic, Inc.
Technical Solution: Medtronic has developed specialized electrospun filtration technology for medical environments that maintains integrity through multiple sterilization cycles. Their approach utilizes a composite nanofiber structure combining polysulfone (PSU) and polyethersulfone (PES) fibers with diameters ranging from 150-600 nm, creating a filtration matrix with precisely controlled pore size distribution (typically 0.2-1.0 μm). The company's proprietary electrospinning process incorporates stabilizing additives that prevent fiber degradation during thermal and chemical sterilization processes. Medtronic's filters can withstand steam sterilization at temperatures up to 134°C for 30+ cycles, gamma irradiation at doses of 25-40 kGy, and chemical sterilants including hydrogen peroxide vapor and peracetic acid. Their technology incorporates a gradient density structure that optimizes both filtration efficiency and air permeability, critical for maintaining appropriate air exchange rates in medical facilities. Recent innovations include integration of their electrospun filters with smart monitoring systems that track filter performance and predict replacement needs based on sterilization cycle history.
Strengths: Excellent mechanical stability through multiple sterilization cycles; consistent filtration performance across varying humidity conditions; integration capabilities with facility monitoring systems. Weaknesses: Higher pressure drop compared to some conventional filters; requires specific handling protocols during sterilization to maintain optimal performance; limited customization options for specialized applications.
Key Patents and Innovations in Medical Electrospun Filtration
Method of sterilizing medical appliance
PatentWO2020012837A1
Innovation
- A method involving electron beam transparent films and controlled electron beam irradiation is used to sterilize small, biocompatible medical devices, ensuring high sterility without damaging the devices, by covering them with an electron beam transparent film and irradiating with an electron beam from an electron beam irradiator, followed by storage in a sterile bag.
Method for sterilizing medical instrument kit
PatentPendingEP4529930A1
Innovation
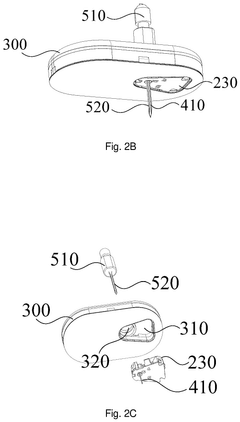
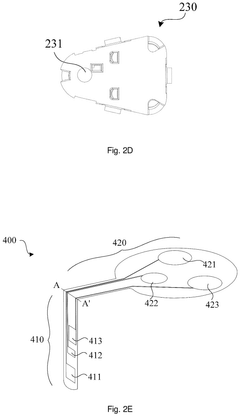
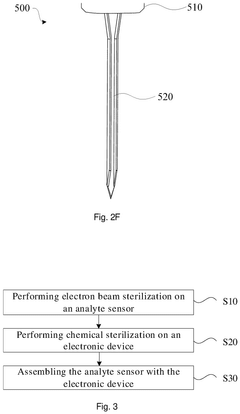
- A sterilization method that seals the sensing portion of the analyte sensor in an accommodating chamber and irradiates it with an electron beam, while keeping the electronic device separate to prevent damage, followed by chemical sterilization of the device, ensuring a simple and convenient assembly process.
Regulatory Framework for Medical Filtration Systems
The regulatory landscape governing medical filtration systems is complex and multifaceted, requiring manufacturers of electrospun filtration technologies to navigate numerous standards and compliance requirements. At the international level, ISO 13485 serves as the cornerstone for quality management systems in medical device manufacturing, including filtration systems used in healthcare facilities. This standard ensures consistent design, development, production, and service processes that meet both customer and regulatory requirements.
In the United States, the FDA classifies medical filtration systems under various device categories depending on their intended use, with most falling under Class II, requiring 510(k) clearance. The FDA's guidance document on "Reprocessing Medical Devices in Health Care Settings" specifically addresses sterilization compatibility concerns that are critical for electrospun filtration materials. Additionally, the CDC's "Guidelines for Environmental Infection Control in Health-Care Facilities" provides recommendations for air filtration systems that manufacturers must consider.
European regulations are governed by the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive and imposes stricter requirements for clinical evaluation and post-market surveillance. Electrospun filtration products must demonstrate compliance with Essential Requirements, including biocompatibility and sterilization validation according to EN ISO 14971 for risk management.
Sterilization compatibility represents a critical regulatory focus area, with standards such as ISO 11135 (ethylene oxide sterilization), ISO 11137 (radiation sterilization), and ISO 17665 (steam sterilization) defining validation requirements. Manufacturers must demonstrate that their electrospun filtration materials maintain structural integrity and filtration efficiency after undergoing these sterilization processes, with documented evidence of material stability.
Environmental regulations also impact medical filtration systems, particularly regarding disposal of used filters. The EU's Waste Electrical and Electronic Equipment (WEEE) Directive and Restriction of Hazardous Substances (RoHS) Directive limit certain hazardous substances in electrical and electronic equipment, including medical devices with electronic components that may incorporate filtration systems.
Emerging regulatory trends indicate increasing scrutiny of nanomaterials used in medical applications, which directly impacts electrospun filtration technologies. The FDA's guidance on "Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology" suggests additional testing requirements for nanoscale materials, potentially affecting regulatory pathways for advanced electrospun filtration systems.
Compliance with these regulatory frameworks requires comprehensive documentation, including technical files, risk management reports, clinical evaluations, and post-market surveillance plans. Manufacturers must establish robust quality management systems that address the entire product lifecycle, from design and development through production, installation, and servicing.
In the United States, the FDA classifies medical filtration systems under various device categories depending on their intended use, with most falling under Class II, requiring 510(k) clearance. The FDA's guidance document on "Reprocessing Medical Devices in Health Care Settings" specifically addresses sterilization compatibility concerns that are critical for electrospun filtration materials. Additionally, the CDC's "Guidelines for Environmental Infection Control in Health-Care Facilities" provides recommendations for air filtration systems that manufacturers must consider.
European regulations are governed by the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive and imposes stricter requirements for clinical evaluation and post-market surveillance. Electrospun filtration products must demonstrate compliance with Essential Requirements, including biocompatibility and sterilization validation according to EN ISO 14971 for risk management.
Sterilization compatibility represents a critical regulatory focus area, with standards such as ISO 11135 (ethylene oxide sterilization), ISO 11137 (radiation sterilization), and ISO 17665 (steam sterilization) defining validation requirements. Manufacturers must demonstrate that their electrospun filtration materials maintain structural integrity and filtration efficiency after undergoing these sterilization processes, with documented evidence of material stability.
Environmental regulations also impact medical filtration systems, particularly regarding disposal of used filters. The EU's Waste Electrical and Electronic Equipment (WEEE) Directive and Restriction of Hazardous Substances (RoHS) Directive limit certain hazardous substances in electrical and electronic equipment, including medical devices with electronic components that may incorporate filtration systems.
Emerging regulatory trends indicate increasing scrutiny of nanomaterials used in medical applications, which directly impacts electrospun filtration technologies. The FDA's guidance on "Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology" suggests additional testing requirements for nanoscale materials, potentially affecting regulatory pathways for advanced electrospun filtration systems.
Compliance with these regulatory frameworks requires comprehensive documentation, including technical files, risk management reports, clinical evaluations, and post-market surveillance plans. Manufacturers must establish robust quality management systems that address the entire product lifecycle, from design and development through production, installation, and servicing.
Cost-Benefit Analysis of Electrospun vs. Conventional Filtration
When evaluating the economic viability of electrospun filtration systems for medical facilities, a comprehensive cost-benefit analysis reveals significant differences compared to conventional filtration technologies. Initial capital expenditure for electrospun filtration systems typically exceeds that of traditional HEPA or melt-blown filter systems by 30-45%, primarily due to specialized manufacturing equipment and higher-grade polymeric materials required for nanofiber production.
However, the operational cost trajectory demonstrates a different pattern. Electrospun filters exhibit extended service life—approximately 2.5 times longer than conventional filters when subjected to similar airflow and particulate loads. This longevity translates to reduced replacement frequency and associated labor costs, with maintenance intervals extending from typical 3-6 month cycles to 9-15 months in medical environments.
Energy efficiency represents another critical economic factor. The high porosity and low air resistance characteristics of electrospun filters reduce HVAC system pressure drop by an average of 18-22% compared to conventional filters of equivalent filtration efficiency. For large medical facilities, this translates to potential energy savings of $5,000-12,000 annually per building, depending on facility size and local energy costs.
Sterilization compatibility further influences the cost-benefit equation. Conventional filters often require replacement after sterilization procedures, while properly engineered electrospun filters can withstand multiple sterilization cycles using ethylene oxide, gamma irradiation, or hydrogen peroxide vapor without significant degradation in filtration performance. This compatibility reduces waste generation by approximately 60% and decreases disposal costs proportionally.
The enhanced filtration efficiency of electrospun media—capturing 99.97% of particles at 0.3 microns versus 95-97% for many conventional filters—contributes to reduced healthcare-associated infection rates. Published studies indicate potential reductions of 15-30% in airborne pathogen transmission within controlled environments. While difficult to quantify precisely, the economic impact of prevented infections represents substantial cost avoidance, estimated at $14,000-38,000 per prevented case depending on pathogen type.
Return on investment calculations indicate that despite higher initial costs, electrospun filtration systems typically achieve financial break-even within 18-24 months in high-acuity medical settings. This timeline shortens in facilities with continuous operation or those located in regions with high energy costs. The total cost of ownership over a five-year period demonstrates a 22-28% advantage for electrospun systems when accounting for all direct and indirect operational factors.
However, the operational cost trajectory demonstrates a different pattern. Electrospun filters exhibit extended service life—approximately 2.5 times longer than conventional filters when subjected to similar airflow and particulate loads. This longevity translates to reduced replacement frequency and associated labor costs, with maintenance intervals extending from typical 3-6 month cycles to 9-15 months in medical environments.
Energy efficiency represents another critical economic factor. The high porosity and low air resistance characteristics of electrospun filters reduce HVAC system pressure drop by an average of 18-22% compared to conventional filters of equivalent filtration efficiency. For large medical facilities, this translates to potential energy savings of $5,000-12,000 annually per building, depending on facility size and local energy costs.
Sterilization compatibility further influences the cost-benefit equation. Conventional filters often require replacement after sterilization procedures, while properly engineered electrospun filters can withstand multiple sterilization cycles using ethylene oxide, gamma irradiation, or hydrogen peroxide vapor without significant degradation in filtration performance. This compatibility reduces waste generation by approximately 60% and decreases disposal costs proportionally.
The enhanced filtration efficiency of electrospun media—capturing 99.97% of particles at 0.3 microns versus 95-97% for many conventional filters—contributes to reduced healthcare-associated infection rates. Published studies indicate potential reductions of 15-30% in airborne pathogen transmission within controlled environments. While difficult to quantify precisely, the economic impact of prevented infections represents substantial cost avoidance, estimated at $14,000-38,000 per prevented case depending on pathogen type.
Return on investment calculations indicate that despite higher initial costs, electrospun filtration systems typically achieve financial break-even within 18-24 months in high-acuity medical settings. This timeline shortens in facilities with continuous operation or those located in regions with high energy costs. The total cost of ownership over a five-year period demonstrates a 22-28% advantage for electrospun systems when accounting for all direct and indirect operational factors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!