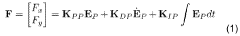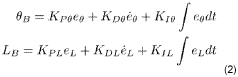Exploring Ferrofluid's Role in Precision Medical Devices
Ferrofluid in Medicine: Background and Objectives
Ferrofluids, a unique class of magnetic nanomaterials, have emerged as a promising technology in the field of precision medical devices. These colloidal suspensions of magnetic nanoparticles in a carrier fluid exhibit remarkable properties that bridge the gap between liquid and magnetic states, opening up new possibilities in medical applications.
The development of ferrofluids can be traced back to the 1960s when NASA scientists sought to create a magnetic liquid for controlling fluids in space. Since then, ferrofluids have found applications in various industries, including electronics, mechanical engineering, and more recently, medicine. The medical field has shown particular interest in ferrofluids due to their potential for targeted drug delivery, magnetic hyperthermia, and contrast enhancement in magnetic resonance imaging (MRI).
In the context of precision medical devices, ferrofluids offer unique advantages. Their ability to be manipulated by external magnetic fields allows for precise control and positioning within the body. This property is particularly valuable in developing minimally invasive surgical tools, drug delivery systems, and diagnostic devices that require high accuracy and specificity.
The primary objective of exploring ferrofluids in precision medical devices is to enhance the efficacy, safety, and precision of medical interventions. Researchers aim to leverage the magnetic properties of ferrofluids to create smart medical devices that can navigate through complex biological environments, target specific tissues or organs, and perform therapeutic or diagnostic functions with unprecedented accuracy.
One of the key technological trends in this field is the development of biocompatible ferrofluids that can be safely used within the human body. This involves engineering nanoparticles with appropriate surface coatings and carrier fluids that minimize toxicity and immune responses. Another trend is the integration of ferrofluids with other emerging technologies, such as microfluidics and nanotechnology, to create multifunctional medical devices.
The potential applications of ferrofluids in precision medicine are vast and diverse. They range from targeted cancer therapies and localized drug delivery to advanced imaging techniques and biosensors. As research progresses, it is anticipated that ferrofluids will play a crucial role in developing next-generation medical devices that offer personalized, precise, and minimally invasive treatment options.
However, the journey from laboratory research to clinical application faces several challenges. These include optimizing the stability and biocompatibility of ferrofluids, developing reliable methods for their controlled manipulation within the body, and addressing regulatory and safety concerns associated with nanomaterials in medical applications.
Market Analysis for Ferrofluid-Based Medical Devices
The market for ferrofluid-based medical devices is experiencing significant growth, driven by the increasing demand for precision and minimally invasive medical procedures. Ferrofluids, with their unique magnetic properties and ability to be controlled by external magnetic fields, offer innovative solutions in various medical applications.
In the diagnostic imaging sector, ferrofluid-enhanced contrast agents are gaining traction for magnetic resonance imaging (MRI). These agents provide superior image quality and improved diagnostic accuracy, particularly in detecting small tumors and vascular abnormalities. The global MRI contrast agents market, which includes ferrofluid-based products, is projected to grow steadily over the next five years.
Drug delivery systems represent another promising area for ferrofluid applications. Magnetic nanoparticles in ferrofluids can be used to target specific tissues or organs, allowing for more precise and efficient drug delivery. This technology is particularly valuable in cancer treatment, where targeted therapy can minimize side effects and improve patient outcomes. The targeted drug delivery market is expected to expand rapidly, with ferrofluid-based systems playing a crucial role.
Microfluidic devices incorporating ferrofluids are emerging as powerful tools for point-of-care diagnostics. These devices enable rapid, sensitive, and cost-effective detection of various biomarkers, pathogens, and diseases. The growing emphasis on personalized medicine and the need for quick, accurate diagnostics are driving the adoption of ferrofluid-based microfluidic platforms.
In the field of regenerative medicine, ferrofluids are being explored for tissue engineering applications. Magnetic scaffolds incorporating ferrofluids can provide dynamic stimulation to cells, promoting tissue growth and regeneration. This technology shows promise in orthopedics, wound healing, and organ reconstruction.
The surgical instruments market is also benefiting from ferrofluid innovations. Magnetically controlled surgical tools using ferrofluids offer enhanced precision and maneuverability during minimally invasive procedures. These instruments are particularly valuable in neurosurgery and ophthalmology, where micron-level accuracy is critical.
While the market potential for ferrofluid-based medical devices is substantial, challenges remain. Regulatory hurdles, long development cycles, and the need for extensive clinical validation can slow market entry for new devices. Additionally, concerns about the long-term safety and biocompatibility of ferrofluids in the human body need to be addressed through rigorous testing and research.
Despite these challenges, the overall market outlook for ferrofluid-based medical devices is positive. As research advances and more clinical evidence accumulates, the adoption of these innovative technologies is expected to accelerate across various medical specialties, contributing to improved patient care and outcomes.
Current Challenges in Ferrofluid Medical Applications
Despite the promising potential of ferrofluids in precision medical devices, several significant challenges currently hinder their widespread adoption and application. One of the primary obstacles is the long-term stability of ferrofluids in biological environments. The complex and dynamic nature of the human body can lead to degradation or aggregation of magnetic nanoparticles, potentially compromising the performance and safety of ferrofluid-based medical devices.
Another critical challenge lies in the biocompatibility and toxicity of ferrofluids. While efforts have been made to develop biocompatible coatings for magnetic nanoparticles, concerns persist regarding their long-term effects on human tissues and organs. Ensuring that ferrofluids remain non-toxic and do not trigger adverse immune responses over extended periods is crucial for their successful integration into medical applications.
The precise control and manipulation of ferrofluids within the body present additional technical hurdles. Achieving accurate spatial and temporal control of ferrofluid behavior in complex biological systems requires advanced engineering solutions. This includes developing sophisticated magnetic field generation and control systems that can operate effectively within the constraints of medical environments.
Furthermore, the scalability and reproducibility of ferrofluid-based medical devices pose significant challenges. Ensuring consistent performance across different batches of ferrofluids and maintaining their properties during the manufacturing and sterilization processes of medical devices are critical for regulatory approval and clinical adoption.
The integration of ferrofluids with existing medical imaging technologies also presents challenges. While ferrofluids offer potential enhancements in imaging contrast, optimizing their compatibility with various imaging modalities such as MRI, CT, and ultrasound requires further research and development.
Regulatory hurdles represent another significant challenge in the application of ferrofluids in medical devices. The novel nature of these materials necessitates rigorous safety and efficacy testing, potentially leading to lengthy and costly approval processes. Establishing standardized protocols for the evaluation and validation of ferrofluid-based medical devices is essential for their successful commercialization.
Lastly, the cost-effectiveness of ferrofluid technologies in medical applications remains a concern. The production of high-quality, medical-grade ferrofluids and the development of specialized equipment for their manipulation can be expensive. Demonstrating clear clinical benefits and cost advantages over existing technologies is crucial for driving adoption in the healthcare industry.
Existing Ferrofluid Solutions in Precision Medicine
01 Composition and preparation of ferrofluids
Ferrofluids are colloidal suspensions of magnetic nanoparticles in a carrier fluid. They are typically composed of magnetite or other ferromagnetic materials coated with surfactants to prevent agglomeration. The preparation process involves careful control of particle size and distribution to maintain stability and magnetic properties.- Composition and preparation of ferrofluids: Ferrofluids are colloidal suspensions of magnetic nanoparticles in a carrier fluid. They are typically composed of magnetite or other ferromagnetic materials coated with surfactants to prevent agglomeration. The preparation process involves careful control of particle size and distribution to maintain stability and magnetic properties.
- Applications in sealing and lubrication: Ferrofluids are widely used in sealing and lubrication systems, particularly in rotating shaft seals. They provide effective sealing against pressure differentials and contaminants while reducing friction. These applications leverage the fluid's ability to be held in place by magnetic fields and its low-friction properties.
- Thermal management and heat transfer: Ferrofluids exhibit enhanced heat transfer properties due to their magnetic nature. They are used in cooling systems for electronic devices and in thermal management applications. The fluid's movement can be controlled by magnetic fields, allowing for targeted cooling and improved heat dissipation in various systems.
- Damping and vibration control: The unique properties of ferrofluids make them effective in damping and vibration control applications. They can be used in shock absorbers, vibration isolators, and inertial dampers. The fluid's response to magnetic fields allows for adaptive damping systems that can adjust to different conditions.
- Sensing and measurement applications: Ferrofluids are employed in various sensing and measurement devices. They can be used in accelerometers, inclinometers, and position sensors. The fluid's response to magnetic fields and gravity allows for precise measurements of orientation and movement, making them valuable in scientific instruments and industrial applications.
02 Applications in sealing and lubrication
Ferrofluids are widely used in sealing and lubrication systems, particularly in rotating shaft seals. They provide effective sealing against pressure differentials and contaminants while reducing friction. These applications leverage the fluid's ability to be held in place by magnetic fields and its low-friction properties.Expand Specific Solutions03 Thermal management and heat transfer
Ferrofluids exhibit enhanced heat transfer properties due to their magnetic nature. They are used in cooling systems for electronic devices and in thermal management applications. The fluid's movement can be controlled by external magnetic fields, allowing for directed heat transfer and improved cooling efficiency.Expand Specific Solutions04 Damping and vibration control
The unique properties of ferrofluids make them suitable for damping applications and vibration control. They can be used in shock absorbers, inertial dampers, and other mechanical systems to reduce unwanted vibrations and improve system stability. The fluid's response to magnetic fields allows for adaptive damping characteristics.Expand Specific Solutions05 Sensing and measurement applications
Ferrofluids are employed in various sensing and measurement devices. They can be used in accelerometers, inclinometers, and other instruments that rely on fluid movement or position detection. The fluid's response to magnetic fields and gravity enables precise measurements and sensing capabilities.Expand Specific Solutions
Key Players in Ferrofluid Medical Device Industry
The ferrofluid market in precision medical devices is in an early growth stage, with increasing applications driving market expansion. While the technology shows promise, it is still maturing, with key players at various stages of development. Companies like Yale University, Arizona State University, and Koninklijke Philips NV are conducting research to advance ferrofluid applications in medical diagnostics and treatments. Established firms such as Unilever and Western Digital are exploring potential uses in their respective fields. Specialized companies like Nano4Imaging and MagnaMedics are focusing on niche applications, indicating a diversifying market. As the technology evolves, collaboration between academic institutions and industry players will likely accelerate innovation and market growth.
Koninklijke Philips NV
Nano4Imaging GmbH
Core Innovations in Ferrofluid Medical Technology
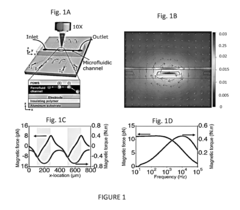
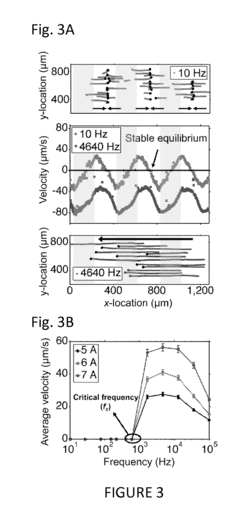
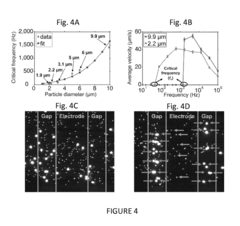
- A microfluidic platform using biocompatible ferrofluids with a microfluidic channel and electrodes that generate a magnetic field pattern, allowing for the controlled manipulation and separation of microparticles and live cells based on size, shape, and elasticity, with high efficiency and rapid separation capabilities.
- A system comprising a ferrofluid droplet and an electromagnetic field generation system with a controller that determines and applies necessary magnetic field parameters to control the position and shape of the ferrofluid droplet, using PID controllers for precise manipulation, allowing for shape and position control, and simultaneous position and shape control through the generation and manipulation of controlled magnetic fields.
Regulatory Framework for Ferrofluid Medical Devices
The regulatory framework for ferrofluid medical devices is a complex and evolving landscape that requires careful navigation by manufacturers, healthcare providers, and regulatory bodies. As ferrofluids gain traction in precision medical applications, the need for comprehensive and specific regulations becomes increasingly apparent.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development, approval, and marketing of medical devices incorporating ferrofluids. These devices typically fall under Class II or Class III classifications, depending on their intended use and potential risks. Class II devices may require a 510(k) premarket notification, while Class III devices often necessitate a more rigorous Premarket Approval (PMA) process.
The European Union's regulatory approach is guided by the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). These regulations emphasize the importance of clinical evidence, post-market surveillance, and risk management throughout the device lifecycle. Ferrofluid-based medical devices must comply with these stringent requirements to obtain CE marking for market access in the EU.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established a regulatory framework that focuses on safety and efficacy. The approval process for ferrofluid medical devices in Japan often involves extensive clinical trials and quality management system assessments.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory standards across different regions. This effort aims to streamline the approval process for innovative technologies like ferrofluid-based devices, potentially accelerating their market entry while maintaining high safety standards.
Key regulatory considerations for ferrofluid medical devices include biocompatibility, stability, and long-term safety. Manufacturers must demonstrate that these devices do not pose unacceptable risks of toxicity, immune response, or unintended interactions with biological systems. Additionally, the unique properties of ferrofluids, such as their response to magnetic fields, require specific safety protocols and testing methodologies.
As the field advances, regulatory bodies are likely to develop more tailored guidelines for ferrofluid-based medical devices. This may include specialized testing requirements, risk assessment frameworks, and post-market surveillance protocols. Manufacturers and researchers should actively engage with regulatory agencies to help shape these emerging standards and ensure that they adequately address the unique characteristics of ferrofluid technologies.
Biocompatibility and Safety Considerations
The integration of ferrofluids into precision medical devices necessitates a thorough examination of biocompatibility and safety considerations. Ferrofluids, composed of nanoscale magnetic particles suspended in a carrier fluid, present unique challenges when introduced to biological systems.
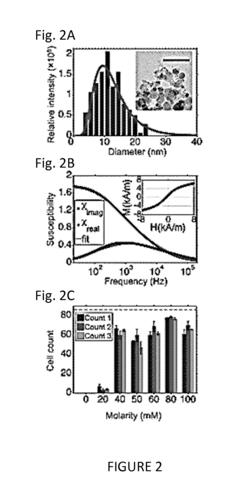
Biocompatibility is a primary concern, as these nanoparticles may interact with living tissues and cells in complex ways. The size, shape, and surface properties of the magnetic particles play crucial roles in determining their biological interactions. Particles smaller than 10 nm may be able to cross biological barriers, including the blood-brain barrier, raising potential safety concerns. Conversely, larger particles may be more easily recognized and cleared by the immune system.
The chemical composition of the magnetic nanoparticles, typically iron oxides such as magnetite (Fe3O4) or maghemite (γ-Fe2O3), must be carefully evaluated for potential toxicity. While iron oxides are generally considered to have low toxicity, their long-term effects and potential for accumulation in tissues require extensive study. The carrier fluid, often a synthetic or natural oil, must also be assessed for biocompatibility and potential allergenic properties.
Surface modifications of the nanoparticles, such as coatings or functionalization, can significantly impact their biocompatibility. These modifications may be designed to improve stability, reduce aggregation, or enhance specific targeting capabilities. However, they may also introduce additional chemical components that require safety evaluation.
The potential for ferrofluids to generate heat under alternating magnetic fields, known as magnetic hyperthermia, must be carefully controlled in medical applications to prevent unintended tissue damage. Additionally, the long-term fate of these nanoparticles in the body, including their biodegradation and excretion pathways, needs to be thoroughly investigated to ensure safety over extended periods.
Regulatory considerations for ferrofluid-based medical devices are complex, requiring extensive in vitro and in vivo testing to demonstrate safety and efficacy. This includes cytotoxicity assays, genotoxicity studies, and long-term animal studies to assess potential systemic effects. The FDA's guidance on nanotechnology in medical products provides a framework for evaluating the safety of these novel materials.
Standardization of ferrofluid characterization and testing protocols is essential to ensure consistent safety evaluations across different research groups and manufacturers. This includes developing robust methods for assessing particle size distribution, magnetic properties, and surface chemistry in biological environments.
As ferrofluids find increasing applications in precision medical devices, ongoing research into their biocompatibility and safety profiles will be crucial. This includes investigating potential interactions with specific tissues and organs, as well as evaluating their impact on cellular functions and genetic material. The development of advanced imaging techniques to track nanoparticle distribution and fate in vivo will be instrumental in addressing long-term safety concerns.