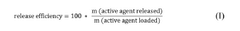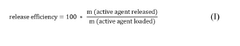Exploring Magnesium Carbonate in Bioactive Compound Delivery
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Carbonate Biodelivery Background
Magnesium carbonate has emerged as a promising material in the field of bioactive compound delivery, attracting significant attention from researchers and pharmaceutical companies alike. This inorganic compound, with its unique properties and biocompatibility, has opened up new avenues for drug delivery systems and therapeutic applications.
The exploration of magnesium carbonate in bioactive compound delivery stems from the growing need for more efficient and targeted drug delivery methods. Traditional drug delivery systems often face challenges such as poor bioavailability, rapid clearance from the body, and potential side effects. Magnesium carbonate offers potential solutions to these issues, owing to its versatile nature and ability to interact with various bioactive compounds.
Historically, magnesium carbonate has been widely used in industries such as food, cosmetics, and pharmaceuticals as an additive or excipient. However, its potential as a drug delivery vehicle has only recently been recognized and investigated. The shift towards exploring magnesium carbonate for bioactive compound delivery is driven by its favorable characteristics, including high surface area, porosity, and pH-dependent solubility.
The biocompatibility of magnesium carbonate is a key factor in its potential for drug delivery applications. As a naturally occurring mineral, it is generally well-tolerated by the human body and can be safely metabolized. This makes it an attractive option for developing drug carriers that can minimize adverse reactions and improve patient compliance.
One of the primary goals in exploring magnesium carbonate for bioactive compound delivery is to enhance the solubility and bioavailability of poorly water-soluble drugs. Many pharmaceutical compounds suffer from low solubility, which limits their effectiveness. Magnesium carbonate's ability to form stable complexes with various drugs and its pH-responsive behavior offer potential solutions to this challenge.
Another important aspect of magnesium carbonate in bioactive compound delivery is its potential for controlled release formulations. The porous structure of magnesium carbonate allows for the incorporation of drugs within its matrix, enabling sustained release over extended periods. This characteristic is particularly valuable for maintaining therapeutic drug levels and reducing dosing frequency.
The exploration of magnesium carbonate in this field also aligns with the growing trend towards developing environmentally friendly and sustainable drug delivery systems. As a naturally abundant material, magnesium carbonate offers a more eco-friendly alternative to some synthetic polymers commonly used in drug delivery.
The exploration of magnesium carbonate in bioactive compound delivery stems from the growing need for more efficient and targeted drug delivery methods. Traditional drug delivery systems often face challenges such as poor bioavailability, rapid clearance from the body, and potential side effects. Magnesium carbonate offers potential solutions to these issues, owing to its versatile nature and ability to interact with various bioactive compounds.
Historically, magnesium carbonate has been widely used in industries such as food, cosmetics, and pharmaceuticals as an additive or excipient. However, its potential as a drug delivery vehicle has only recently been recognized and investigated. The shift towards exploring magnesium carbonate for bioactive compound delivery is driven by its favorable characteristics, including high surface area, porosity, and pH-dependent solubility.
The biocompatibility of magnesium carbonate is a key factor in its potential for drug delivery applications. As a naturally occurring mineral, it is generally well-tolerated by the human body and can be safely metabolized. This makes it an attractive option for developing drug carriers that can minimize adverse reactions and improve patient compliance.
One of the primary goals in exploring magnesium carbonate for bioactive compound delivery is to enhance the solubility and bioavailability of poorly water-soluble drugs. Many pharmaceutical compounds suffer from low solubility, which limits their effectiveness. Magnesium carbonate's ability to form stable complexes with various drugs and its pH-responsive behavior offer potential solutions to this challenge.
Another important aspect of magnesium carbonate in bioactive compound delivery is its potential for controlled release formulations. The porous structure of magnesium carbonate allows for the incorporation of drugs within its matrix, enabling sustained release over extended periods. This characteristic is particularly valuable for maintaining therapeutic drug levels and reducing dosing frequency.
The exploration of magnesium carbonate in this field also aligns with the growing trend towards developing environmentally friendly and sustainable drug delivery systems. As a naturally abundant material, magnesium carbonate offers a more eco-friendly alternative to some synthetic polymers commonly used in drug delivery.
Market Analysis for Bioactive Delivery Systems
The bioactive compound delivery systems market is experiencing significant growth, driven by increasing demand for targeted drug delivery and enhanced therapeutic efficacy. This market segment encompasses a wide range of delivery technologies, including nanoparticles, liposomes, polymeric systems, and inorganic carriers such as magnesium carbonate. The global market for bioactive delivery systems is projected to expand at a compound annual growth rate (CAGR) of over 7% in the coming years, with a particularly strong growth trajectory in the pharmaceutical and nutraceutical sectors.
Magnesium carbonate, as a potential carrier for bioactive compounds, is gaining attention due to its biocompatibility, low toxicity, and versatile physicochemical properties. The increasing focus on sustainable and eco-friendly materials in drug delivery systems further enhances the market potential for magnesium carbonate-based carriers. This aligns with the growing consumer preference for natural and mineral-based products in both pharmaceutical and cosmeceutical applications.
The pharmaceutical industry remains the largest end-user segment for bioactive delivery systems, accounting for a substantial market share. The demand is primarily driven by the need for improved drug bioavailability, controlled release profiles, and reduced side effects. Magnesium carbonate's potential in this area, particularly for oral drug delivery, presents a significant market opportunity.
Emerging applications in the nutraceutical and functional food industries are also contributing to market growth. The ability of magnesium carbonate to encapsulate and protect sensitive bioactive compounds, such as vitamins, minerals, and probiotics, makes it an attractive option for fortified food products and dietary supplements. This segment is expected to show rapid growth, fueled by increasing consumer awareness of preventive healthcare and wellness.
Geographically, North America and Europe currently dominate the bioactive delivery systems market, owing to advanced healthcare infrastructure and high R&D investments. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by improving healthcare access, rising disposable incomes, and a large patient population. This regional diversity presents both opportunities and challenges for magnesium carbonate-based delivery systems, necessitating tailored market strategies.
Key market players in the bioactive delivery systems space include pharmaceutical giants, specialized drug delivery companies, and materials science firms. The competitive landscape is characterized by ongoing research and development activities, strategic collaborations, and patent filings. As the potential of magnesium carbonate in bioactive compound delivery becomes more recognized, it is likely to attract increased investment and research focus from both established players and innovative startups.
Magnesium carbonate, as a potential carrier for bioactive compounds, is gaining attention due to its biocompatibility, low toxicity, and versatile physicochemical properties. The increasing focus on sustainable and eco-friendly materials in drug delivery systems further enhances the market potential for magnesium carbonate-based carriers. This aligns with the growing consumer preference for natural and mineral-based products in both pharmaceutical and cosmeceutical applications.
The pharmaceutical industry remains the largest end-user segment for bioactive delivery systems, accounting for a substantial market share. The demand is primarily driven by the need for improved drug bioavailability, controlled release profiles, and reduced side effects. Magnesium carbonate's potential in this area, particularly for oral drug delivery, presents a significant market opportunity.
Emerging applications in the nutraceutical and functional food industries are also contributing to market growth. The ability of magnesium carbonate to encapsulate and protect sensitive bioactive compounds, such as vitamins, minerals, and probiotics, makes it an attractive option for fortified food products and dietary supplements. This segment is expected to show rapid growth, fueled by increasing consumer awareness of preventive healthcare and wellness.
Geographically, North America and Europe currently dominate the bioactive delivery systems market, owing to advanced healthcare infrastructure and high R&D investments. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by improving healthcare access, rising disposable incomes, and a large patient population. This regional diversity presents both opportunities and challenges for magnesium carbonate-based delivery systems, necessitating tailored market strategies.
Key market players in the bioactive delivery systems space include pharmaceutical giants, specialized drug delivery companies, and materials science firms. The competitive landscape is characterized by ongoing research and development activities, strategic collaborations, and patent filings. As the potential of magnesium carbonate in bioactive compound delivery becomes more recognized, it is likely to attract increased investment and research focus from both established players and innovative startups.
Current Challenges in Magnesium Carbonate Delivery
Despite the promising potential of magnesium carbonate in bioactive compound delivery, several significant challenges currently hinder its widespread application and efficacy. One of the primary obstacles is the control of release kinetics. Magnesium carbonate's inherent properties can lead to rapid dissolution and premature release of bioactive compounds, potentially reducing therapeutic efficacy and increasing the risk of side effects.
Another critical challenge lies in the stability of magnesium carbonate-based delivery systems. Environmental factors such as pH, temperature, and ionic strength can significantly impact the structural integrity of these systems, potentially compromising their ability to protect and deliver bioactive compounds effectively. This instability can result in reduced shelf life and inconsistent performance in various physiological conditions.
The biocompatibility and biodegradability of magnesium carbonate-based delivery systems also present ongoing concerns. While magnesium is generally considered safe, the long-term effects of sustained exposure to magnesium carbonate nanoparticles or microparticles in the body are not fully understood. Potential accumulation in tissues and organs, as well as the body's ability to metabolize and excrete these particles, requires further investigation.
Scalability and reproducibility in manufacturing processes pose additional challenges. Producing magnesium carbonate-based delivery systems with consistent size, morphology, and loading capacity on an industrial scale remains difficult. This variability can lead to batch-to-batch inconsistencies, affecting the overall quality and performance of the delivery systems.
The limited drug loading capacity of magnesium carbonate is another area of concern. Compared to some other inorganic carriers, magnesium carbonate may have lower surface area and pore volume, potentially restricting the amount of bioactive compounds that can be effectively loaded and delivered. This limitation may necessitate higher doses or more frequent administration, which could impact patient compliance and treatment efficacy.
Targeting and site-specific delivery remain significant challenges for magnesium carbonate-based systems. While surface modifications can improve targeting capabilities, achieving precise and efficient delivery to specific tissues or organs is still a complex task. This is particularly crucial for applications in cancer therapy or other localized treatments where off-target effects can be detrimental.
Lastly, regulatory hurdles and safety concerns present ongoing challenges in the development and commercialization of magnesium carbonate-based delivery systems. Stringent regulatory requirements for novel drug delivery systems, coupled with the need for extensive toxicological studies and clinical trials, can significantly delay the translation of these technologies from bench to bedside.
Another critical challenge lies in the stability of magnesium carbonate-based delivery systems. Environmental factors such as pH, temperature, and ionic strength can significantly impact the structural integrity of these systems, potentially compromising their ability to protect and deliver bioactive compounds effectively. This instability can result in reduced shelf life and inconsistent performance in various physiological conditions.
The biocompatibility and biodegradability of magnesium carbonate-based delivery systems also present ongoing concerns. While magnesium is generally considered safe, the long-term effects of sustained exposure to magnesium carbonate nanoparticles or microparticles in the body are not fully understood. Potential accumulation in tissues and organs, as well as the body's ability to metabolize and excrete these particles, requires further investigation.
Scalability and reproducibility in manufacturing processes pose additional challenges. Producing magnesium carbonate-based delivery systems with consistent size, morphology, and loading capacity on an industrial scale remains difficult. This variability can lead to batch-to-batch inconsistencies, affecting the overall quality and performance of the delivery systems.
The limited drug loading capacity of magnesium carbonate is another area of concern. Compared to some other inorganic carriers, magnesium carbonate may have lower surface area and pore volume, potentially restricting the amount of bioactive compounds that can be effectively loaded and delivered. This limitation may necessitate higher doses or more frequent administration, which could impact patient compliance and treatment efficacy.
Targeting and site-specific delivery remain significant challenges for magnesium carbonate-based systems. While surface modifications can improve targeting capabilities, achieving precise and efficient delivery to specific tissues or organs is still a complex task. This is particularly crucial for applications in cancer therapy or other localized treatments where off-target effects can be detrimental.
Lastly, regulatory hurdles and safety concerns present ongoing challenges in the development and commercialization of magnesium carbonate-based delivery systems. Stringent regulatory requirements for novel drug delivery systems, coupled with the need for extensive toxicological studies and clinical trials, can significantly delay the translation of these technologies from bench to bedside.
Existing Magnesium Carbonate Delivery Solutions
01 Oral delivery of magnesium carbonate
Magnesium carbonate can be formulated into oral dosage forms such as tablets, capsules, or powders for dietary supplementation. These formulations may include additional ingredients to enhance absorption or improve taste. The delivery system aims to provide a convenient and effective way to supplement magnesium intake.- Oral delivery of magnesium carbonate: Magnesium carbonate can be formulated into oral dosage forms such as tablets, capsules, or powders for dietary supplementation. These formulations may include additional ingredients to enhance absorption or improve taste. The delivery system aims to provide a convenient and effective way to supplement magnesium intake.
- Topical application of magnesium carbonate: Magnesium carbonate can be incorporated into topical formulations such as creams, lotions, or gels for transdermal delivery. These products may be used for various purposes, including skin care, muscle relaxation, or as a component in deodorants. The topical route allows for localized delivery of magnesium.
- Inhalation delivery of magnesium carbonate: Magnesium carbonate can be formulated for inhalation delivery, potentially for respiratory treatments or as a carrier for other active ingredients. This method may involve dry powder inhalers or nebulized solutions, allowing for direct delivery to the lungs and respiratory tract.
- Controlled release formulations of magnesium carbonate: Controlled release systems can be developed for magnesium carbonate to provide sustained delivery over an extended period. These formulations may involve matrix systems, coated particles, or other technologies to modulate the release profile of magnesium carbonate, potentially improving its efficacy and reducing dosing frequency.
- Magnesium carbonate in combination with other active ingredients: Magnesium carbonate can be combined with other active ingredients in various delivery systems to create multi-functional products. These combinations may enhance the overall efficacy of the formulation or provide complementary benefits. The delivery system is designed to ensure compatibility and optimal performance of all components.
02 Topical delivery of magnesium carbonate
Magnesium carbonate can be incorporated into topical formulations such as creams, lotions, or gels for transdermal delivery. These formulations may be designed to enhance skin absorption and provide localized effects. The topical delivery system can be used for various purposes, including skincare and pain relief.Expand Specific Solutions03 Inhalation delivery of magnesium carbonate
Magnesium carbonate can be formulated for inhalation delivery, such as in dry powder inhalers or nebulizers. This method of delivery may be used for respiratory conditions or to provide rapid systemic absorption of magnesium. The formulation may include additional excipients to improve flow properties and lung deposition.Expand Specific Solutions04 Controlled release delivery of magnesium carbonate
Controlled release formulations of magnesium carbonate can be developed to provide sustained or targeted delivery. These formulations may use various technologies such as matrix systems, coated pellets, or osmotic systems to modulate the release profile of magnesium carbonate over time or in specific areas of the gastrointestinal tract.Expand Specific Solutions05 Nanoparticle-based delivery of magnesium carbonate
Magnesium carbonate can be formulated into nanoparticles to enhance its delivery and bioavailability. Nanoparticle formulations may improve solubility, increase absorption, and allow for targeted delivery. Various methods can be used to prepare magnesium carbonate nanoparticles, including precipitation, milling, or encapsulation techniques.Expand Specific Solutions
Key Players in Bioactive Delivery Industry
The exploration of magnesium carbonate in bioactive compound delivery is in an emerging phase, with growing interest from both academic institutions and industry players. The market size is expanding as researchers and companies recognize the potential applications in drug delivery systems and biomedical engineering. Technologically, the field is still developing, with varying levels of maturity across different applications. Key players like Shandong Shunfeng Biotechnology, Omya International AG, and Nanyang Technological University are contributing to advancements in this area. Universities such as UNC Chapel Hill and Indian Institutes of Technology are also conducting significant research, indicating a collaborative ecosystem between academia and industry. As the technology progresses, we can expect increased commercialization efforts and potential breakthroughs in bioactive compound delivery methods.
Omya International AG
Technical Solution: Omya International AG has developed a range of functionalized calcium carbonate (FCC) and magnesium carbonate materials for controlled release applications in the pharmaceutical and nutraceutical industries. Their approach involves engineering the particle size, porosity, and surface properties of magnesium carbonate to create tailored carrier systems for various bioactive compounds. Omya's technology focuses on improving the solubility and bioavailability of poorly water-soluble drugs through co-processing with magnesium carbonate[13]. The company has demonstrated success in enhancing the dissolution rate of several BCS Class II and IV drugs using their proprietary magnesium carbonate-based formulations[15]. Additionally, Omya has explored the use of magnesium carbonate as a pH modifier in enteric coating formulations, providing targeted release in the intestinal tract[17].
Strengths: Extensive experience in mineral processing, customizable formulations, and broad applicability across different drug classes. Weaknesses: Potential for drug-excipient interactions and challenges in achieving ultra-high drug loading.
Nanyang Technological University
Technical Solution: Nanyang Technological University has developed a novel magnesium carbonate-based nanocarrier system for bioactive compound delivery. Their approach involves synthesizing porous magnesium carbonate nanoparticles with high surface area and controlled pore size distribution. These nanoparticles are then functionalized with specific ligands to enhance targeting and cellular uptake. The university's research has shown that these nanocarriers can effectively encapsulate a wide range of bioactive compounds, including small molecule drugs, proteins, and nucleic acids[1]. In vitro studies have demonstrated sustained release profiles and improved bioavailability of the encapsulated compounds[3]. The team has also explored the use of layer-by-layer coating techniques to further control the release kinetics and protect sensitive biomolecules from degradation[5].
Strengths: High loading capacity, controlled release, biocompatibility, and potential for targeted delivery. Weaknesses: Potential for premature degradation in acidic environments and limited long-term stability data.
Innovative Magnesium Carbonate Delivery Techniques
Carrier material for the release of one or more active agent(s) in a home care formulation
PatentWO2019145467A1
Innovation
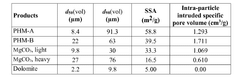
- A carrier material comprising magnesium carbonate with a specific surface area greater than 25 m2/g, measured using the BET method, and an intra-particle intruded specific pore volume in the range of 0.9 to 2.3 cm3/g, allowing for high loading and efficient release of active agents, suitable for both liquid and dry formulations.
Surface-reacted magnesium carbonate as carrier material for the release of one or more active agent(s) in a home care formulation
PatentWO2020225118A1
Innovation
- Surface-reacted magnesium carbonate is created by treating magnesium carbonate with acids like sulphuric acid, phosphoric acid, or carboxylic acids, enhancing its specific surface area and pore volume, allowing for higher loading and efficient release of active agents in home care formulations.
Regulatory Framework for Bioactive Delivery Systems
The regulatory framework for bioactive delivery systems involving magnesium carbonate is complex and multifaceted, encompassing various aspects of safety, efficacy, and quality control. At the forefront of this framework is the classification of magnesium carbonate-based delivery systems, which often fall under the category of novel excipients or drug delivery technologies. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established specific guidelines for the evaluation and approval of such systems.
One of the primary considerations in the regulatory process is the safety assessment of magnesium carbonate when used as a carrier for bioactive compounds. This includes evaluating potential toxicity, biocompatibility, and the impact on the pharmacokinetics of the delivered bioactive agents. Manufacturers must provide comprehensive data on the physical and chemical properties of the magnesium carbonate formulation, as well as its interaction with the bioactive compounds it is intended to deliver.
The regulatory framework also emphasizes the importance of quality control and manufacturing processes. Good Manufacturing Practices (GMP) must be strictly adhered to, ensuring consistency and reliability in the production of magnesium carbonate-based delivery systems. This includes establishing robust analytical methods for characterizing the final product and monitoring its stability throughout its shelf life.
Efficacy evaluation is another crucial aspect of the regulatory process. Manufacturers must demonstrate that the magnesium carbonate-based delivery system effectively enhances the bioavailability, targeted delivery, or controlled release of the bioactive compounds. This often requires extensive in vitro and in vivo studies, including pharmacokinetic and pharmacodynamic assessments.
The regulatory framework also addresses the environmental impact of magnesium carbonate-based delivery systems. As sustainability becomes an increasingly important consideration in pharmaceutical development, regulatory bodies are placing greater emphasis on the biodegradability and environmental fate of these materials.
Internationally, there is a push towards harmonization of regulatory requirements for novel delivery systems. Initiatives such as the International Conference on Harmonisation (ICH) aim to streamline the regulatory process across different regions, potentially facilitating faster global market access for innovative magnesium carbonate-based delivery technologies.
As the field of bioactive compound delivery continues to evolve, regulatory frameworks are adapting to keep pace with technological advancements. This includes the development of specific guidance documents for nanomaterial-based delivery systems, which may be relevant to certain magnesium carbonate formulations. Additionally, regulatory bodies are increasingly considering patient-centric approaches, emphasizing the importance of user-friendly and compliant delivery systems in the approval process.
One of the primary considerations in the regulatory process is the safety assessment of magnesium carbonate when used as a carrier for bioactive compounds. This includes evaluating potential toxicity, biocompatibility, and the impact on the pharmacokinetics of the delivered bioactive agents. Manufacturers must provide comprehensive data on the physical and chemical properties of the magnesium carbonate formulation, as well as its interaction with the bioactive compounds it is intended to deliver.
The regulatory framework also emphasizes the importance of quality control and manufacturing processes. Good Manufacturing Practices (GMP) must be strictly adhered to, ensuring consistency and reliability in the production of magnesium carbonate-based delivery systems. This includes establishing robust analytical methods for characterizing the final product and monitoring its stability throughout its shelf life.
Efficacy evaluation is another crucial aspect of the regulatory process. Manufacturers must demonstrate that the magnesium carbonate-based delivery system effectively enhances the bioavailability, targeted delivery, or controlled release of the bioactive compounds. This often requires extensive in vitro and in vivo studies, including pharmacokinetic and pharmacodynamic assessments.
The regulatory framework also addresses the environmental impact of magnesium carbonate-based delivery systems. As sustainability becomes an increasingly important consideration in pharmaceutical development, regulatory bodies are placing greater emphasis on the biodegradability and environmental fate of these materials.
Internationally, there is a push towards harmonization of regulatory requirements for novel delivery systems. Initiatives such as the International Conference on Harmonisation (ICH) aim to streamline the regulatory process across different regions, potentially facilitating faster global market access for innovative magnesium carbonate-based delivery technologies.
As the field of bioactive compound delivery continues to evolve, regulatory frameworks are adapting to keep pace with technological advancements. This includes the development of specific guidance documents for nanomaterial-based delivery systems, which may be relevant to certain magnesium carbonate formulations. Additionally, regulatory bodies are increasingly considering patient-centric approaches, emphasizing the importance of user-friendly and compliant delivery systems in the approval process.
Environmental Impact of Magnesium Carbonate Use
The use of magnesium carbonate in bioactive compound delivery systems has potential environmental implications that warrant careful consideration. As a naturally occurring mineral, magnesium carbonate is generally considered environmentally friendly. However, its increased utilization in pharmaceutical and biomedical applications may lead to elevated levels in waste streams and ecosystems.
One of the primary environmental concerns is the potential for magnesium carbonate to alter local pH levels when released into aquatic environments. While magnesium carbonate is not highly soluble in water, it can slowly dissolve and increase alkalinity. This pH change, even if minor, could impact sensitive aquatic organisms and ecosystems, particularly in freshwater habitats.
The mining and processing of magnesium carbonate for industrial use also carry environmental considerations. Extraction activities can lead to habitat disruption, soil erosion, and potential groundwater contamination if not managed properly. Additionally, the energy-intensive nature of processing and refining magnesium carbonate contributes to carbon emissions, albeit on a relatively small scale compared to other industrial processes.
On the positive side, the use of magnesium carbonate in bioactive compound delivery systems may indirectly benefit the environment by improving the efficacy of pharmaceuticals and reducing the overall quantity of drugs needed for treatment. This could potentially decrease the amount of pharmaceutical residues entering the environment through wastewater systems.
The biodegradability of magnesium carbonate is another important factor to consider. As a naturally occurring mineral, it does not persist in the environment like synthetic polymers. However, the rate of degradation and the potential impacts of its breakdown products on various ecosystems require further study.
In terms of lifecycle assessment, the environmental footprint of magnesium carbonate use in bioactive compound delivery should be evaluated holistically. This includes considering the energy and resources required for extraction, processing, and transportation, as well as the end-of-life disposal or potential for recycling.
Research into the ecotoxicological effects of magnesium carbonate nanoparticles, which may be used in advanced drug delivery systems, is still in its early stages. The potential for these nanoparticles to accumulate in food chains or interact with microorganisms in soil and water ecosystems needs thorough investigation to ensure long-term environmental safety.
As the use of magnesium carbonate in bioactive compound delivery expands, it will be crucial to develop sustainable sourcing practices and implement efficient recycling or safe disposal methods. This proactive approach can help mitigate potential negative environmental impacts while harnessing the benefits of this versatile material in medical applications.
One of the primary environmental concerns is the potential for magnesium carbonate to alter local pH levels when released into aquatic environments. While magnesium carbonate is not highly soluble in water, it can slowly dissolve and increase alkalinity. This pH change, even if minor, could impact sensitive aquatic organisms and ecosystems, particularly in freshwater habitats.
The mining and processing of magnesium carbonate for industrial use also carry environmental considerations. Extraction activities can lead to habitat disruption, soil erosion, and potential groundwater contamination if not managed properly. Additionally, the energy-intensive nature of processing and refining magnesium carbonate contributes to carbon emissions, albeit on a relatively small scale compared to other industrial processes.
On the positive side, the use of magnesium carbonate in bioactive compound delivery systems may indirectly benefit the environment by improving the efficacy of pharmaceuticals and reducing the overall quantity of drugs needed for treatment. This could potentially decrease the amount of pharmaceutical residues entering the environment through wastewater systems.
The biodegradability of magnesium carbonate is another important factor to consider. As a naturally occurring mineral, it does not persist in the environment like synthetic polymers. However, the rate of degradation and the potential impacts of its breakdown products on various ecosystems require further study.
In terms of lifecycle assessment, the environmental footprint of magnesium carbonate use in bioactive compound delivery should be evaluated holistically. This includes considering the energy and resources required for extraction, processing, and transportation, as well as the end-of-life disposal or potential for recycling.
Research into the ecotoxicological effects of magnesium carbonate nanoparticles, which may be used in advanced drug delivery systems, is still in its early stages. The potential for these nanoparticles to accumulate in food chains or interact with microorganisms in soil and water ecosystems needs thorough investigation to ensure long-term environmental safety.
As the use of magnesium carbonate in bioactive compound delivery expands, it will be crucial to develop sustainable sourcing practices and implement efficient recycling or safe disposal methods. This proactive approach can help mitigate potential negative environmental impacts while harnessing the benefits of this versatile material in medical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!