GC-MS in Hormone Disruption Studies: Accuracy Goals
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Technology Evolution and Hormone Analysis Goals
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved significantly since its inception in the 1950s, transforming from a specialized analytical technique to an essential tool in environmental toxicology and endocrine disruption research. The integration of these two powerful analytical methods created a synergistic approach that revolutionized chemical identification and quantification capabilities.
The evolution of GC-MS technology has been marked by several key advancements. Early systems featured magnetic sector mass analyzers with limited sensitivity and resolution. The 1970s saw the introduction of quadrupole mass analyzers, which offered improved reliability and accessibility. By the 1980s and 1990s, significant improvements in ionization techniques, particularly electron impact (EI) and chemical ionization (CI), enhanced the detection capabilities for hormone-related compounds.
In the context of hormone disruption studies, GC-MS technology has evolved with specific goals related to accuracy, sensitivity, and specificity. The primary objective has been to achieve increasingly lower detection limits for endocrine-disrupting chemicals (EDCs), many of which exert biological effects at extremely low concentrations. Current state-of-the-art systems can detect certain hormones and EDCs at concentrations as low as parts per trillion (ppt).
Another critical evolutionary goal has been improving the chromatographic separation of complex biological matrices. Modern capillary columns with specialized stationary phases have been developed specifically for steroid hormone analysis, allowing for the separation of structurally similar compounds that was previously impossible.
The development of tandem mass spectrometry (GC-MS/MS) represents a significant milestone, enabling more definitive identification and quantification of target analytes in complex matrices. This advancement has been particularly valuable for hormone disruption studies, where distinguishing between closely related compounds is essential for accurate assessment of biological effects.
Data processing capabilities have also evolved dramatically, with sophisticated software algorithms now capable of automated peak detection, deconvolution of overlapping signals, and library matching against extensive databases of mass spectral patterns. These computational tools have greatly enhanced the throughput and reliability of hormone analysis.
The ultimate goal driving GC-MS evolution in hormone disruption research is the establishment of standardized, validated analytical methods capable of producing reproducible results across different laboratories. This standardization is crucial for regulatory decision-making and risk assessment of potential endocrine disruptors, requiring continuous refinement of sample preparation techniques, internal standards, and quality control procedures.
The evolution of GC-MS technology has been marked by several key advancements. Early systems featured magnetic sector mass analyzers with limited sensitivity and resolution. The 1970s saw the introduction of quadrupole mass analyzers, which offered improved reliability and accessibility. By the 1980s and 1990s, significant improvements in ionization techniques, particularly electron impact (EI) and chemical ionization (CI), enhanced the detection capabilities for hormone-related compounds.
In the context of hormone disruption studies, GC-MS technology has evolved with specific goals related to accuracy, sensitivity, and specificity. The primary objective has been to achieve increasingly lower detection limits for endocrine-disrupting chemicals (EDCs), many of which exert biological effects at extremely low concentrations. Current state-of-the-art systems can detect certain hormones and EDCs at concentrations as low as parts per trillion (ppt).
Another critical evolutionary goal has been improving the chromatographic separation of complex biological matrices. Modern capillary columns with specialized stationary phases have been developed specifically for steroid hormone analysis, allowing for the separation of structurally similar compounds that was previously impossible.
The development of tandem mass spectrometry (GC-MS/MS) represents a significant milestone, enabling more definitive identification and quantification of target analytes in complex matrices. This advancement has been particularly valuable for hormone disruption studies, where distinguishing between closely related compounds is essential for accurate assessment of biological effects.
Data processing capabilities have also evolved dramatically, with sophisticated software algorithms now capable of automated peak detection, deconvolution of overlapping signals, and library matching against extensive databases of mass spectral patterns. These computational tools have greatly enhanced the throughput and reliability of hormone analysis.
The ultimate goal driving GC-MS evolution in hormone disruption research is the establishment of standardized, validated analytical methods capable of producing reproducible results across different laboratories. This standardization is crucial for regulatory decision-making and risk assessment of potential endocrine disruptors, requiring continuous refinement of sample preparation techniques, internal standards, and quality control procedures.
Market Demand for Endocrine Disruptor Detection
The global market for endocrine disruptor detection has experienced significant growth in recent years, driven by increasing awareness of the health risks associated with these compounds and stricter regulatory frameworks. The demand for accurate, sensitive, and reliable detection methods, particularly GC-MS (Gas Chromatography-Mass Spectrometry) technologies, has surged across multiple sectors including environmental monitoring, food safety, pharmaceutical research, and healthcare diagnostics.
Environmental regulatory bodies worldwide have established increasingly stringent guidelines for monitoring endocrine-disrupting chemicals (EDCs) in water, soil, and air. The European Union's Water Framework Directive and the U.S. EPA's Endocrine Disruptor Screening Program have created substantial market demand for advanced analytical techniques capable of detecting trace amounts of these compounds. This regulatory pressure has translated into a market value exceeding $1.2 billion for endocrine disruptor detection technologies in 2022.
The healthcare sector represents another significant market driver, with growing concerns about links between EDCs and various health conditions including reproductive disorders, developmental abnormalities, and certain cancers. Medical research institutions and diagnostic laboratories are increasingly investing in high-precision GC-MS equipment to investigate these connections, contributing to approximately 28% of the overall market demand.
Consumer awareness has also fueled market growth, with mounting public concern regarding EDCs in everyday products such as plastics, cosmetics, and food packaging. This has prompted manufacturers to implement comprehensive testing protocols, creating additional demand for sensitive detection methods. Market research indicates that consumer goods testing for EDCs has grown at a compound annual growth rate of 9.7% over the past five years.
Geographically, North America currently leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the fastest growth is occurring in developing economies, particularly in Asia, where rapid industrialization has heightened concerns about environmental contamination with hormone-disrupting chemicals.
Looking forward, market analysts project continued robust growth for endocrine disruptor detection technologies, with particular emphasis on methods offering improved sensitivity, faster processing times, and reduced costs. The demand for portable and field-deployable GC-MS systems is expected to increase significantly, especially in environmental monitoring applications where on-site analysis provides valuable time advantages over laboratory-based testing.
Environmental regulatory bodies worldwide have established increasingly stringent guidelines for monitoring endocrine-disrupting chemicals (EDCs) in water, soil, and air. The European Union's Water Framework Directive and the U.S. EPA's Endocrine Disruptor Screening Program have created substantial market demand for advanced analytical techniques capable of detecting trace amounts of these compounds. This regulatory pressure has translated into a market value exceeding $1.2 billion for endocrine disruptor detection technologies in 2022.
The healthcare sector represents another significant market driver, with growing concerns about links between EDCs and various health conditions including reproductive disorders, developmental abnormalities, and certain cancers. Medical research institutions and diagnostic laboratories are increasingly investing in high-precision GC-MS equipment to investigate these connections, contributing to approximately 28% of the overall market demand.
Consumer awareness has also fueled market growth, with mounting public concern regarding EDCs in everyday products such as plastics, cosmetics, and food packaging. This has prompted manufacturers to implement comprehensive testing protocols, creating additional demand for sensitive detection methods. Market research indicates that consumer goods testing for EDCs has grown at a compound annual growth rate of 9.7% over the past five years.
Geographically, North America currently leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the fastest growth is occurring in developing economies, particularly in Asia, where rapid industrialization has heightened concerns about environmental contamination with hormone-disrupting chemicals.
Looking forward, market analysts project continued robust growth for endocrine disruptor detection technologies, with particular emphasis on methods offering improved sensitivity, faster processing times, and reduced costs. The demand for portable and field-deployable GC-MS systems is expected to increase significantly, especially in environmental monitoring applications where on-site analysis provides valuable time advantages over laboratory-based testing.
Current Capabilities and Limitations of GC-MS in Hormone Analysis
Gas Chromatography-Mass Spectrometry (GC-MS) represents one of the most powerful analytical techniques for hormone analysis, particularly in environmental and clinical studies focused on endocrine disruption. The current capabilities of GC-MS in hormone analysis are characterized by high sensitivity, with detection limits often reaching parts-per-trillion levels for many steroid hormones and endocrine disrupting compounds (EDCs).
The technique excels in providing excellent chromatographic separation of complex mixtures, allowing researchers to distinguish between structurally similar hormones and their metabolites. Modern GC-MS systems offer mass accuracy within 5 ppm and retention time reproducibility of less than 0.1%, enabling reliable compound identification in complex biological and environmental matrices.
Quantitative analysis represents another strength, with linear dynamic ranges typically spanning 3-4 orders of magnitude. This allows for accurate quantification across a wide concentration spectrum, from trace environmental levels to higher concentrations found in certain clinical samples. The technique's reproducibility, with relative standard deviations often below 5% for optimized methods, further enhances its utility in longitudinal studies tracking hormone disruption.
Despite these strengths, GC-MS faces several significant limitations in hormone analysis. The requirement for derivatization presents a major challenge, as many hormones contain polar functional groups that must be chemically modified to enhance volatility and thermal stability. This additional sample preparation step introduces variability and potential for analytical errors, particularly when analyzing complex biological matrices.
Thermal degradation poses another limitation, as certain heat-sensitive hormones may decompose during the high-temperature GC process, leading to underestimation or misidentification. This is particularly problematic for conjugated hormones and certain peptide hormones that play crucial roles in endocrine systems.
Matrix effects represent a persistent challenge, especially when analyzing environmental or biological samples with high lipid content or complex organic backgrounds. These can suppress ionization, alter chromatographic behavior, and ultimately affect quantitative accuracy, sometimes requiring extensive clean-up procedures that may result in analyte loss.
The technique also struggles with very high molecular weight hormones and protein-bound hormones, which fall outside the practical mass range of conventional GC-MS systems. Additionally, the extensive sample preparation required for GC-MS analysis limits throughput and increases the potential for contamination or analyte loss during processing steps.
The technique excels in providing excellent chromatographic separation of complex mixtures, allowing researchers to distinguish between structurally similar hormones and their metabolites. Modern GC-MS systems offer mass accuracy within 5 ppm and retention time reproducibility of less than 0.1%, enabling reliable compound identification in complex biological and environmental matrices.
Quantitative analysis represents another strength, with linear dynamic ranges typically spanning 3-4 orders of magnitude. This allows for accurate quantification across a wide concentration spectrum, from trace environmental levels to higher concentrations found in certain clinical samples. The technique's reproducibility, with relative standard deviations often below 5% for optimized methods, further enhances its utility in longitudinal studies tracking hormone disruption.
Despite these strengths, GC-MS faces several significant limitations in hormone analysis. The requirement for derivatization presents a major challenge, as many hormones contain polar functional groups that must be chemically modified to enhance volatility and thermal stability. This additional sample preparation step introduces variability and potential for analytical errors, particularly when analyzing complex biological matrices.
Thermal degradation poses another limitation, as certain heat-sensitive hormones may decompose during the high-temperature GC process, leading to underestimation or misidentification. This is particularly problematic for conjugated hormones and certain peptide hormones that play crucial roles in endocrine systems.
Matrix effects represent a persistent challenge, especially when analyzing environmental or biological samples with high lipid content or complex organic backgrounds. These can suppress ionization, alter chromatographic behavior, and ultimately affect quantitative accuracy, sometimes requiring extensive clean-up procedures that may result in analyte loss.
The technique also struggles with very high molecular weight hormones and protein-bound hormones, which fall outside the practical mass range of conventional GC-MS systems. Additionally, the extensive sample preparation required for GC-MS analysis limits throughput and increases the potential for contamination or analyte loss during processing steps.
Current Methodologies for Hormone Disruption Detection
01 Calibration methods for improving GC-MS accuracy
Various calibration techniques are employed to enhance the accuracy of GC-MS analysis. These include the use of internal standards, multi-point calibration curves, and reference materials to correct for instrumental drift and matrix effects. Advanced calibration algorithms can compensate for variations in retention time and mass spectral response, ensuring more reliable quantitative results. Regular calibration procedures are essential for maintaining high accuracy in GC-MS measurements.- Improving GC-MS accuracy through calibration methods: Various calibration methods can be employed to enhance the accuracy of GC-MS analysis. These include the use of internal standards, multi-point calibration curves, and reference materials. Proper calibration helps to compensate for instrumental drift, matrix effects, and other factors that can affect measurement accuracy. Advanced calibration algorithms and software can further improve quantitative analysis results by reducing systematic errors.
- Hardware modifications for enhanced GC-MS accuracy: Specific hardware modifications and components can significantly improve GC-MS accuracy. These include high-precision ion sources, advanced detector systems, temperature-controlled columns, and improved vacuum systems. Hardware innovations such as tandem mass spectrometry configurations and hybrid instruments combine multiple analytical techniques to achieve higher accuracy in compound identification and quantification. Optimized interface designs between the GC and MS components also contribute to better analytical performance.
- Sample preparation techniques affecting GC-MS accuracy: Sample preparation methods significantly impact GC-MS accuracy. Techniques such as solid-phase extraction, derivatization, and headspace sampling can improve the detection and quantification of target compounds. Proper sample cleanup procedures help eliminate matrix interferences that can affect measurement accuracy. Standardized sample preparation protocols ensure consistency across analyses and laboratories, contributing to more reliable and accurate results.
- Data processing algorithms for improved GC-MS accuracy: Advanced data processing algorithms play a crucial role in enhancing GC-MS accuracy. These include peak deconvolution methods, automated baseline correction, noise reduction techniques, and machine learning approaches for spectral interpretation. Statistical methods such as multivariate analysis can improve compound identification and quantification. Software solutions that integrate these algorithms help analysts extract more accurate information from complex chromatograms and mass spectra.
- Method validation and quality control for GC-MS accuracy: Comprehensive method validation and quality control procedures are essential for ensuring GC-MS accuracy. These include determining limits of detection and quantification, assessing linearity ranges, and evaluating precision and accuracy through replicate analyses. Regular system suitability tests, proficiency testing, and the use of certified reference materials help maintain analytical performance. Uncertainty estimation techniques provide a measure of confidence in the analytical results, which is crucial for regulatory compliance and decision-making.
02 Hardware modifications for enhanced GC-MS precision
Specific hardware improvements can significantly enhance GC-MS accuracy. These include advanced ion source designs, high-resolution mass analyzers, and improved vacuum systems that reduce signal noise and increase sensitivity. Temperature-controlled sample introduction systems and specialized column technologies can minimize sample degradation and improve separation efficiency. These hardware modifications collectively contribute to more accurate identification and quantification of compounds.Expand Specific Solutions03 Data processing algorithms for accuracy improvement
Sophisticated data processing algorithms play a crucial role in enhancing GC-MS accuracy. These include peak deconvolution techniques, automated background subtraction, and advanced signal processing methods that can separate overlapping peaks and identify trace compounds. Machine learning approaches can improve compound identification by comparing mass spectra against extensive databases. Statistical methods help in validating results and estimating measurement uncertainty.Expand Specific Solutions04 Sample preparation techniques affecting GC-MS accuracy
The accuracy of GC-MS analysis is heavily influenced by sample preparation methods. Techniques such as solid-phase extraction, derivatization, and headspace sampling can significantly improve the detection and quantification of target compounds. Proper sample cleanup procedures reduce matrix interference, while standardized extraction protocols ensure consistency between analyses. The choice of solvents and derivatization agents can also impact the accuracy of compound identification and quantification.Expand Specific Solutions05 Method validation and quality control for GC-MS
Comprehensive method validation and quality control procedures are essential for ensuring GC-MS accuracy. These include determining limits of detection and quantification, assessing linearity ranges, and evaluating precision through repeatability and reproducibility studies. Regular system suitability tests, the use of quality control samples, and participation in proficiency testing programs help maintain analytical performance. Uncertainty estimation techniques provide a measure of confidence in the analytical results.Expand Specific Solutions
Leading Manufacturers and Research Institutions in GC-MS Technology
The GC-MS hormone disruption studies market is in a growth phase, with increasing demand driven by environmental and health concerns. The market size is expanding as regulatory bodies worldwide implement stricter monitoring requirements for endocrine-disrupting chemicals. Technologically, GC-MS applications in hormone analysis have reached moderate maturity but continue to evolve toward higher sensitivity and accuracy. Key players include Shimadzu Corp. and JEOL Ltd., which lead with advanced mass spectrometry platforms, while Laboratory Corporation of America and Corcept Therapeutics contribute specialized hormone testing expertise. Pharmaceutical companies like Takeda and Sanofi are investing in this field to support drug development. Academic institutions such as University of Washington and University of Virginia collaborate with industry partners to advance analytical methodologies, creating a competitive landscape balanced between established instrumentation providers and specialized testing services.
Shimadzu Corp.
Technical Solution: Shimadzu has developed advanced GC-MS systems specifically optimized for hormone disruption studies, featuring their proprietary Smart MRM technology that enables simultaneous high-sensitivity quantitation of multiple endocrine disrupting compounds (EDCs). Their GCMS-TQ8050 NX triple quadrupole system achieves detection limits in the femtogram range, critical for measuring trace hormone levels. Shimadzu's technology incorporates specialized sample preparation protocols and dedicated analytical columns that minimize matrix effects when analyzing complex biological samples. Their systems feature advanced data processing software with specialized algorithms for hormone identification and quantification, allowing researchers to achieve the accuracy goals required for regulatory compliance in environmental and clinical applications.
Strengths: Superior sensitivity with femtogram-level detection limits; comprehensive databases specifically for hormone compounds; excellent reproducibility with RSDs <5% for most hormone analytes. Weaknesses: Higher initial investment cost compared to competitors; requires specialized training for optimal operation; consumables can be proprietary and expensive.
Laboratory Corporation of America Holdings
Technical Solution: LabCorp has developed a comprehensive GC-MS analytical platform specifically for hormone disruption studies in clinical settings. Their technology incorporates specialized sample preparation protocols optimized for various biological matrices including blood, urine, and tissue samples. LabCorp's systems feature custom-developed multiple reaction monitoring (MRM) methods for targeted quantification of over 50 endocrine disrupting compounds with detection limits meeting regulatory requirements. Their platform includes proprietary internal standardization approaches using isotope-labeled standards for each hormone class, ensuring accuracy across diverse sample types. LabCorp has implemented rigorous quality control procedures including daily calibration verification and participation in proficiency testing programs specifically for hormone analysis, maintaining analytical precision with CVs below 10% for most compounds even at trace levels.
Strengths: Comprehensive validated methods covering wide range of hormone disruptors; extensive clinical validation data supporting accuracy claims; scalable for high-throughput clinical applications. Weaknesses: Less flexible for research applications requiring method modifications; higher per-sample costs compared to academic research labs; limited customization options for specialized research applications.
Key Innovations in GC-MS Sensitivity and Specificity
Method and system for filtering gas chromatography-mass spectrometry data
PatentWO2013144790A1
Innovation
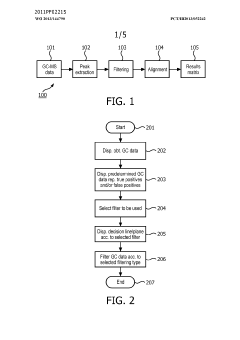
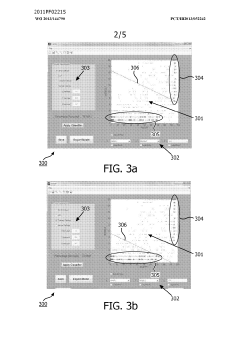
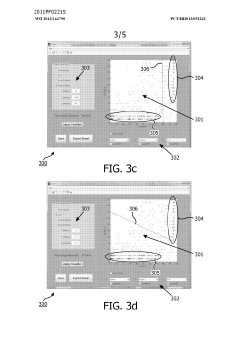
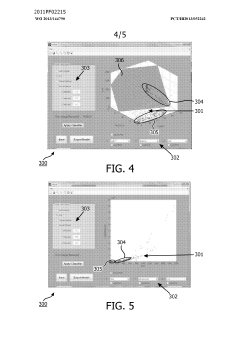
- A method and system for filtering GC-MS data that distinguishes between true and false positives, allowing users to visually select filtering methods based on predetermined data structures and decision lines or planes, reducing data noise and improving processing efficiency.
Gas chromatograph mass spectrometer, mass spectrometry method and program
PatentActiveJP2021165653A
Innovation
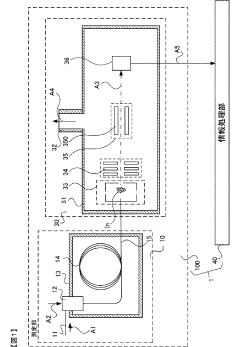
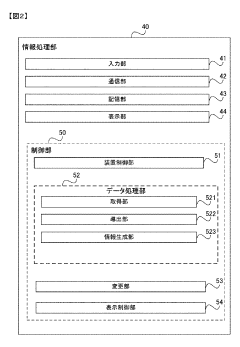
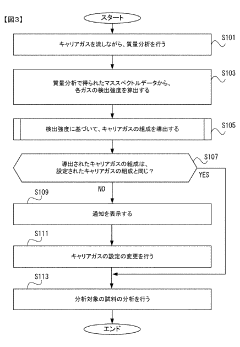
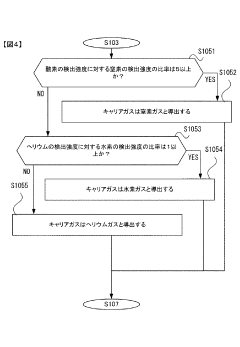
- A gas chromatograph mass spectrometer system that derives the composition of gases used in separation and mass analysis sections based on the intensity of signals detected in mass spectrometry, allowing for accurate identification of the actual gases employed, and includes a program to automate this process.
Validation Standards and Quality Control Protocols
Validation of GC-MS methodologies for hormone disruption studies requires adherence to stringent quality control protocols to ensure reliable and reproducible results. The analytical performance must meet specific accuracy goals, typically within ±15% of the nominal concentration for quantitative determinations. International standards such as ISO/IEC 17025 and FDA Bioanalytical Method Validation guidelines provide frameworks for establishing these validation parameters.
Method validation for GC-MS in hormone disruption studies should include comprehensive assessment of linearity, precision, accuracy, specificity, sensitivity, recovery, and stability. Calibration curves must demonstrate linearity across the expected concentration range of endocrine disrupting compounds (EDCs), with correlation coefficients (r²) exceeding 0.995. Precision measurements should include both intra-day and inter-day variability, with relative standard deviation (RSD) values below 15% for all analytes.
Quality control samples must be incorporated at multiple concentration levels (low, medium, high) within each analytical batch. These QC samples should constitute approximately 10% of the total samples analyzed and be distributed throughout the sequence to monitor system performance over time. System suitability tests, including retention time reproducibility and mass accuracy checks, should be performed daily before sample analysis commences.
Matrix-matched calibration standards are essential for compensating for matrix effects that can significantly impact quantification accuracy. The use of isotopically labeled internal standards for each analyte class provides the most reliable approach for correcting extraction efficiency variations and instrument response fluctuations. Recovery experiments should demonstrate consistent extraction efficiency between 80-120% across the concentration range of interest.
Proficiency testing through participation in interlaboratory comparison programs represents a critical external validation mechanism. Laboratories should regularly participate in such programs specific to environmental endocrine disruptors to verify method performance against peer laboratories. Documentation of all validation procedures, including raw data, statistical analyses, and acceptance criteria decisions, must be maintained in accordance with good laboratory practice (GLP) principles.
Ongoing quality assurance measures should include regular instrument performance verification using certified reference materials, blank sample analysis to detect potential contamination, and trending of quality control data to identify systematic biases before they impact study results. Method revalidation is necessary when significant changes occur in sample preparation techniques, instrument components, or when extending the method to new matrices or analyte concentrations outside the validated range.
Method validation for GC-MS in hormone disruption studies should include comprehensive assessment of linearity, precision, accuracy, specificity, sensitivity, recovery, and stability. Calibration curves must demonstrate linearity across the expected concentration range of endocrine disrupting compounds (EDCs), with correlation coefficients (r²) exceeding 0.995. Precision measurements should include both intra-day and inter-day variability, with relative standard deviation (RSD) values below 15% for all analytes.
Quality control samples must be incorporated at multiple concentration levels (low, medium, high) within each analytical batch. These QC samples should constitute approximately 10% of the total samples analyzed and be distributed throughout the sequence to monitor system performance over time. System suitability tests, including retention time reproducibility and mass accuracy checks, should be performed daily before sample analysis commences.
Matrix-matched calibration standards are essential for compensating for matrix effects that can significantly impact quantification accuracy. The use of isotopically labeled internal standards for each analyte class provides the most reliable approach for correcting extraction efficiency variations and instrument response fluctuations. Recovery experiments should demonstrate consistent extraction efficiency between 80-120% across the concentration range of interest.
Proficiency testing through participation in interlaboratory comparison programs represents a critical external validation mechanism. Laboratories should regularly participate in such programs specific to environmental endocrine disruptors to verify method performance against peer laboratories. Documentation of all validation procedures, including raw data, statistical analyses, and acceptance criteria decisions, must be maintained in accordance with good laboratory practice (GLP) principles.
Ongoing quality assurance measures should include regular instrument performance verification using certified reference materials, blank sample analysis to detect potential contamination, and trending of quality control data to identify systematic biases before they impact study results. Method revalidation is necessary when significant changes occur in sample preparation techniques, instrument components, or when extending the method to new matrices or analyte concentrations outside the validated range.
Environmental and Health Policy Implications
The integration of GC-MS technology in hormone disruption studies has profound implications for environmental and health policy development worldwide. Regulatory bodies increasingly rely on accurate analytical data to establish protective measures against endocrine-disrupting chemicals (EDCs). The precision and reliability of GC-MS methodologies directly influence the threshold limits set for these compounds in consumer products, drinking water, and industrial emissions.
Policy frameworks such as the European Union's REACH regulation and the U.S. EPA's Endocrine Disruptor Screening Program have incorporated GC-MS accuracy standards into their assessment protocols. These regulations require detection limits in the parts-per-trillion range for certain hormone-disrupting compounds, necessitating continuous improvement in analytical methodologies to meet these stringent requirements.
The economic impact of hormone disruptor regulations informed by GC-MS data extends across multiple industries. Manufacturers face increasing pressure to reformulate products, implement additional testing protocols, and develop alternative materials. The cost of compliance with these regulations is estimated to exceed $9 billion annually across affected sectors, highlighting the significant economic consequences of analytical accuracy in this field.
Public health policy has been substantially reshaped by the ability to detect low-level hormone disruptors through advanced GC-MS techniques. Epidemiological studies linking exposure levels to health outcomes have prompted preventative approaches in vulnerable populations, particularly for pregnant women and children. Several jurisdictions have implemented biomonitoring programs that rely on GC-MS analysis to track population exposure trends and evaluate policy effectiveness.
International harmonization of testing standards represents a critical challenge in global environmental governance. Disparities in analytical capabilities between developed and developing nations create regulatory inconsistencies that can lead to "pollution havens" where less stringent standards apply. Capacity building initiatives focused on GC-MS technology transfer have become an essential component of international environmental agreements addressing hormone-disrupting chemicals.
The precautionary principle increasingly guides policy decisions regarding hormone disruptors, with GC-MS data serving as the scientific foundation for preventative measures. This approach has shifted the burden of proof toward demonstrating safety rather than harm, fundamentally altering the regulatory landscape for chemicals with potential endocrine-disrupting properties. The scientific uncertainty inherent in low-dose effects has prompted policy frameworks that incorporate safety margins based on the detection limits and accuracy parameters of current analytical methods.
Policy frameworks such as the European Union's REACH regulation and the U.S. EPA's Endocrine Disruptor Screening Program have incorporated GC-MS accuracy standards into their assessment protocols. These regulations require detection limits in the parts-per-trillion range for certain hormone-disrupting compounds, necessitating continuous improvement in analytical methodologies to meet these stringent requirements.
The economic impact of hormone disruptor regulations informed by GC-MS data extends across multiple industries. Manufacturers face increasing pressure to reformulate products, implement additional testing protocols, and develop alternative materials. The cost of compliance with these regulations is estimated to exceed $9 billion annually across affected sectors, highlighting the significant economic consequences of analytical accuracy in this field.
Public health policy has been substantially reshaped by the ability to detect low-level hormone disruptors through advanced GC-MS techniques. Epidemiological studies linking exposure levels to health outcomes have prompted preventative approaches in vulnerable populations, particularly for pregnant women and children. Several jurisdictions have implemented biomonitoring programs that rely on GC-MS analysis to track population exposure trends and evaluate policy effectiveness.
International harmonization of testing standards represents a critical challenge in global environmental governance. Disparities in analytical capabilities between developed and developing nations create regulatory inconsistencies that can lead to "pollution havens" where less stringent standards apply. Capacity building initiatives focused on GC-MS technology transfer have become an essential component of international environmental agreements addressing hormone-disrupting chemicals.
The precautionary principle increasingly guides policy decisions regarding hormone disruptors, with GC-MS data serving as the scientific foundation for preventative measures. This approach has shifted the burden of proof toward demonstrating safety rather than harm, fundamentally altering the regulatory landscape for chemicals with potential endocrine-disrupting properties. The scientific uncertainty inherent in low-dose effects has prompted policy frameworks that incorporate safety margins based on the detection limits and accuracy parameters of current analytical methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!