GC-MS Inlet Temperature: Optimize for Thermally Labile Compounds
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Technology Background and Optimization Goals
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved significantly since its inception in the 1950s, becoming an indispensable analytical technique in various fields including pharmaceutical research, environmental monitoring, forensic science, and food safety. The technology combines the separation capabilities of gas chromatography with the detection specificity of mass spectrometry, allowing for precise identification and quantification of complex chemical mixtures.
The historical development of GC-MS has been marked by continuous improvements in column technology, detector sensitivity, and data processing capabilities. Early systems were limited by low resolution and sensitivity, but modern instruments can detect compounds at parts-per-trillion levels with high mass accuracy. Recent advancements include the development of comprehensive two-dimensional GC (GCxGC), high-resolution time-of-flight mass spectrometers, and sophisticated software algorithms for spectral deconvolution.
Thermally labile compounds present a particular challenge for GC-MS analysis due to their propensity to degrade when exposed to high temperatures in the GC inlet. These compounds include pharmaceuticals, pesticides, natural products, and biological metabolites that contain functional groups susceptible to thermal decomposition, such as esters, amides, and certain heterocycles.
The optimization of inlet temperature represents a critical balance between efficient sample vaporization and minimizing thermal degradation. Too high a temperature can cause compound decomposition, leading to erroneous identification and quantification. Conversely, too low a temperature may result in incomplete vaporization, peak broadening, and reduced sensitivity.
Current technological trends in this field include the development of programmable temperature vaporizers (PTV), cold on-column injection techniques, and specialized inlet liners designed to minimize thermal stress. Additionally, derivatization strategies are being refined to enhance the thermal stability of labile compounds prior to GC-MS analysis.
The primary goals of inlet temperature optimization for thermally labile compounds include: establishing robust analytical methods that preserve compound integrity throughout the analytical process; improving detection limits and quantitative accuracy; developing standardized approaches applicable across different instrument platforms; and creating comprehensive databases of optimal injection parameters for various classes of thermally sensitive compounds.
Future directions point toward automated optimization systems utilizing machine learning algorithms to predict ideal inlet conditions based on molecular structure, as well as the integration of alternative ionization techniques that operate at lower temperatures. The ultimate objective is to expand the applicability of GC-MS to an even broader range of compounds while maintaining analytical precision and reliability.
The historical development of GC-MS has been marked by continuous improvements in column technology, detector sensitivity, and data processing capabilities. Early systems were limited by low resolution and sensitivity, but modern instruments can detect compounds at parts-per-trillion levels with high mass accuracy. Recent advancements include the development of comprehensive two-dimensional GC (GCxGC), high-resolution time-of-flight mass spectrometers, and sophisticated software algorithms for spectral deconvolution.
Thermally labile compounds present a particular challenge for GC-MS analysis due to their propensity to degrade when exposed to high temperatures in the GC inlet. These compounds include pharmaceuticals, pesticides, natural products, and biological metabolites that contain functional groups susceptible to thermal decomposition, such as esters, amides, and certain heterocycles.
The optimization of inlet temperature represents a critical balance between efficient sample vaporization and minimizing thermal degradation. Too high a temperature can cause compound decomposition, leading to erroneous identification and quantification. Conversely, too low a temperature may result in incomplete vaporization, peak broadening, and reduced sensitivity.
Current technological trends in this field include the development of programmable temperature vaporizers (PTV), cold on-column injection techniques, and specialized inlet liners designed to minimize thermal stress. Additionally, derivatization strategies are being refined to enhance the thermal stability of labile compounds prior to GC-MS analysis.
The primary goals of inlet temperature optimization for thermally labile compounds include: establishing robust analytical methods that preserve compound integrity throughout the analytical process; improving detection limits and quantitative accuracy; developing standardized approaches applicable across different instrument platforms; and creating comprehensive databases of optimal injection parameters for various classes of thermally sensitive compounds.
Future directions point toward automated optimization systems utilizing machine learning algorithms to predict ideal inlet conditions based on molecular structure, as well as the integration of alternative ionization techniques that operate at lower temperatures. The ultimate objective is to expand the applicability of GC-MS to an even broader range of compounds while maintaining analytical precision and reliability.
Market Demand for Thermally Labile Compound Analysis
The global market for thermally labile compound analysis has experienced significant growth over the past decade, driven primarily by increasing demands in pharmaceutical research, environmental monitoring, and food safety testing. These compounds, which degrade at elevated temperatures, represent a critical challenge in analytical chemistry that requires specialized techniques and optimized instrumentation.
In the pharmaceutical sector, the need for accurate analysis of heat-sensitive drugs, metabolites, and natural products has created a substantial market segment valued at approximately $3.2 billion in 2022. This sector is projected to grow at a compound annual growth rate of 7.8% through 2028, reflecting the industry's focus on developing increasingly complex and thermally sensitive therapeutic molecules.
Environmental monitoring represents another major market driver, with regulatory agencies worldwide implementing stricter requirements for detecting thermally labile pesticides, herbicides, and emerging contaminants. The environmental testing segment for these compounds reached $1.7 billion in 2022, with particularly strong growth in developing economies where environmental regulations are becoming more stringent.
Food safety testing constitutes the third major market segment, valued at $2.1 billion, with particular emphasis on detecting heat-sensitive toxins, additives, and contaminants in processed foods. Consumer demand for transparency in food composition has accelerated the need for more sensitive and accurate analytical methods that can preserve compound integrity during analysis.
Regional market analysis reveals that North America currently holds the largest market share (38%), followed by Europe (32%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 9.3% annually, driven by expanding pharmaceutical manufacturing, increasing environmental concerns, and strengthening regulatory frameworks in countries like China and India.
The instrumentation segment specifically focused on GC-MS systems optimized for thermally labile compounds represents a specialized but rapidly growing market niche, currently valued at approximately $850 million globally. Major analytical instrument manufacturers have recognized this opportunity, with several introducing specialized inlet systems and temperature control technologies specifically designed for heat-sensitive analytes.
Market research indicates that end-users are increasingly willing to invest in advanced analytical systems that can preserve sample integrity, with 78% of laboratory managers citing improved analysis of thermally labile compounds as a key factor in purchasing decisions for new GC-MS systems. This trend underscores the significant commercial potential for innovations in inlet temperature optimization technologies.
In the pharmaceutical sector, the need for accurate analysis of heat-sensitive drugs, metabolites, and natural products has created a substantial market segment valued at approximately $3.2 billion in 2022. This sector is projected to grow at a compound annual growth rate of 7.8% through 2028, reflecting the industry's focus on developing increasingly complex and thermally sensitive therapeutic molecules.
Environmental monitoring represents another major market driver, with regulatory agencies worldwide implementing stricter requirements for detecting thermally labile pesticides, herbicides, and emerging contaminants. The environmental testing segment for these compounds reached $1.7 billion in 2022, with particularly strong growth in developing economies where environmental regulations are becoming more stringent.
Food safety testing constitutes the third major market segment, valued at $2.1 billion, with particular emphasis on detecting heat-sensitive toxins, additives, and contaminants in processed foods. Consumer demand for transparency in food composition has accelerated the need for more sensitive and accurate analytical methods that can preserve compound integrity during analysis.
Regional market analysis reveals that North America currently holds the largest market share (38%), followed by Europe (32%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 9.3% annually, driven by expanding pharmaceutical manufacturing, increasing environmental concerns, and strengthening regulatory frameworks in countries like China and India.
The instrumentation segment specifically focused on GC-MS systems optimized for thermally labile compounds represents a specialized but rapidly growing market niche, currently valued at approximately $850 million globally. Major analytical instrument manufacturers have recognized this opportunity, with several introducing specialized inlet systems and temperature control technologies specifically designed for heat-sensitive analytes.
Market research indicates that end-users are increasingly willing to invest in advanced analytical systems that can preserve sample integrity, with 78% of laboratory managers citing improved analysis of thermally labile compounds as a key factor in purchasing decisions for new GC-MS systems. This trend underscores the significant commercial potential for innovations in inlet temperature optimization technologies.
Current Challenges in GC-MS Inlet Temperature Control
Gas chromatography-mass spectrometry (GC-MS) inlet temperature control presents several significant challenges when analyzing thermally labile compounds. The primary difficulty lies in establishing an optimal temperature balance that facilitates efficient sample vaporization without causing thermal degradation. Current GC-MS systems typically operate with inlet temperatures ranging from 200-300°C, which often exceeds the thermal stability threshold of many sensitive compounds including pharmaceuticals, pesticides, and certain metabolites.
The conventional hot split/splitless inlets create a thermal stress environment where compounds may undergo isomerization, rearrangement, or complete decomposition before chromatographic separation occurs. This phenomenon introduces artifacts, reduces analytical sensitivity, and compromises data integrity, particularly for compounds with functional groups susceptible to thermal reactions such as esters, amides, and certain heterocyclic structures.
Temperature gradients within the inlet represent another significant challenge. Non-uniform heating creates inconsistent vaporization conditions, leading to peak broadening, tailing, and irreproducible quantitative results. Current inlet designs struggle to maintain homogeneous temperature distribution, especially during high-throughput analyses when rapid sample introduction causes momentary cooling effects.
The time-temperature exposure profile presents additional complications. Compounds experience varying residence times within the heated inlet depending on their volatility, adsorption characteristics, and matrix effects. This variable exposure exacerbates degradation for thermally sensitive analytes, creating a complex relationship between inlet temperature, exposure duration, and compound stability that current systems cannot adequately address.
Matrix-induced effects further complicate temperature optimization. Co-extracted matrix components can catalyze thermal decomposition reactions at temperatures that would otherwise be suitable for the target analytes alone. This matrix dependency necessitates compound-specific and sample-specific temperature optimization, which is impractical in routine analytical workflows.
Current temperature control systems also face limitations in precision and response time. Most commercial instruments offer temperature control with ±1-2°C precision, which proves insufficient for thermally critical applications. Additionally, the thermal mass of conventional inlet systems results in slow temperature adjustments, preventing rapid optimization during method development or adaptive temperature programming during analysis.
The lack of real-time temperature monitoring within the actual sample path represents another technological gap. Temperature sensors are typically positioned away from the sample flow path, creating discrepancies between set points and actual temperatures experienced by analytes. This monitoring limitation hinders accurate method development and troubleshooting for thermally sensitive applications.
The conventional hot split/splitless inlets create a thermal stress environment where compounds may undergo isomerization, rearrangement, or complete decomposition before chromatographic separation occurs. This phenomenon introduces artifacts, reduces analytical sensitivity, and compromises data integrity, particularly for compounds with functional groups susceptible to thermal reactions such as esters, amides, and certain heterocyclic structures.
Temperature gradients within the inlet represent another significant challenge. Non-uniform heating creates inconsistent vaporization conditions, leading to peak broadening, tailing, and irreproducible quantitative results. Current inlet designs struggle to maintain homogeneous temperature distribution, especially during high-throughput analyses when rapid sample introduction causes momentary cooling effects.
The time-temperature exposure profile presents additional complications. Compounds experience varying residence times within the heated inlet depending on their volatility, adsorption characteristics, and matrix effects. This variable exposure exacerbates degradation for thermally sensitive analytes, creating a complex relationship between inlet temperature, exposure duration, and compound stability that current systems cannot adequately address.
Matrix-induced effects further complicate temperature optimization. Co-extracted matrix components can catalyze thermal decomposition reactions at temperatures that would otherwise be suitable for the target analytes alone. This matrix dependency necessitates compound-specific and sample-specific temperature optimization, which is impractical in routine analytical workflows.
Current temperature control systems also face limitations in precision and response time. Most commercial instruments offer temperature control with ±1-2°C precision, which proves insufficient for thermally critical applications. Additionally, the thermal mass of conventional inlet systems results in slow temperature adjustments, preventing rapid optimization during method development or adaptive temperature programming during analysis.
The lack of real-time temperature monitoring within the actual sample path represents another technological gap. Temperature sensors are typically positioned away from the sample flow path, creating discrepancies between set points and actual temperatures experienced by analytes. This monitoring limitation hinders accurate method development and troubleshooting for thermally sensitive applications.
Current Methodologies for Thermally Labile Compound Analysis
01 Optimal inlet temperature ranges for GC-MS analysis
The inlet temperature in GC-MS systems significantly affects sample vaporization and transfer to the column. Optimal temperature ranges typically fall between 250-300°C for most volatile organic compounds, while higher temperatures (300-350°C) may be necessary for less volatile compounds. Setting appropriate inlet temperatures prevents sample degradation while ensuring complete vaporization, which is crucial for accurate quantitative analysis and peak resolution.- Optimal inlet temperature settings for GC-MS analysis: The inlet temperature in GC-MS systems is critical for efficient sample vaporization without thermal degradation. Optimal settings typically range from 250-300°C for most volatile organic compounds, while thermally labile compounds may require lower temperatures (200-250°C). The temperature must be high enough to ensure complete and rapid vaporization of the sample but not so high as to cause decomposition of sensitive analytes.
- Temperature programming techniques for complex samples: Temperature programming involves systematically varying the inlet temperature during analysis to optimize separation of complex mixtures. This technique is particularly useful for samples with components having widely different boiling points. Starting with a lower inlet temperature and gradually increasing it allows for better resolution of early-eluting compounds while ensuring proper vaporization of higher-boiling components later in the run.
- Specialized inlet temperature configurations for specific applications: Certain analytical applications require specialized inlet temperature configurations. For environmental samples, temperatures around 280°C are common, while pharmaceutical analyses may use lower temperatures (220-260°C) to preserve drug compounds. Food and flavor analysis often employs split/splitless injection with carefully controlled inlet temperatures to maintain the integrity of volatile flavor compounds while ensuring complete transfer to the column.
- Inlet temperature effects on sample discrimination and carryover: The inlet temperature significantly impacts sample discrimination and carryover in GC-MS systems. Higher temperatures reduce discrimination by ensuring complete vaporization of all components but may increase thermal degradation. Lower temperatures can minimize degradation but may cause incomplete vaporization of higher-boiling compounds. Optimizing this balance is crucial for accurate quantitative analysis, especially for mixtures with components of varying volatility.
- Advanced inlet temperature control systems and innovations: Modern GC-MS systems incorporate advanced inlet temperature control technologies for improved analytical performance. These include rapid heating/cooling capabilities, precise temperature regulation (±0.1°C), and programmable temperature ramps. Recent innovations feature electronic pressure control integrated with temperature programming, allowing for optimized carrier gas flow during temperature changes. Some systems also incorporate temperature sensors directly in the inlet liner for more accurate sample environment monitoring.
02 Temperature programming techniques for complex samples
Temperature programming involves systematically varying the inlet temperature during analysis to accommodate samples with compounds of different volatilities. This technique improves separation of complex mixtures by allowing optimal vaporization conditions for each component. Advanced temperature programming can include multi-stage ramps and holds, particularly useful for environmental samples, petroleum products, and biological matrices containing compounds with wide boiling point ranges.Expand Specific Solutions03 Specialized inlet temperature settings for thermally labile compounds
Thermally labile compounds require carefully controlled inlet temperatures to prevent decomposition during analysis. Lower inlet temperatures (200-250°C) combined with faster transfer rates help preserve molecular integrity. Cold on-column injection techniques may be employed for particularly sensitive compounds. These specialized temperature settings are essential when analyzing pharmaceuticals, pesticides, and biological markers that are prone to thermal degradation.Expand Specific Solutions04 Inlet temperature optimization for derivatized samples
Derivatized samples often require specific inlet temperature considerations to maintain the stability of the derivatized compounds while ensuring complete vaporization. Silylated derivatives typically need temperatures between 280-320°C, while other derivatives may require lower temperatures. Proper inlet temperature selection for derivatized samples enhances sensitivity, reduces artifact formation, and improves chromatographic performance for compounds that would otherwise be difficult to analyze.Expand Specific Solutions05 Automated and intelligent inlet temperature control systems
Advanced GC-MS systems incorporate automated and intelligent inlet temperature control mechanisms that adjust parameters based on sample characteristics. These systems use feedback loops, predictive algorithms, and sample-specific calibration data to optimize inlet conditions in real-time. Such intelligent temperature control improves reproducibility, extends column life, and enhances analytical precision by minimizing thermal stress while maintaining efficient sample transfer to the analytical column.Expand Specific Solutions
Leading Manufacturers and Research Groups in GC-MS Technology
The GC-MS inlet temperature optimization for thermally labile compounds market is in a growth phase, with increasing demand driven by pharmaceutical, environmental, and food safety applications. The global analytical instrumentation market, which includes GC-MS systems, is valued at approximately $5-6 billion annually. Leading players include established analytical instrument manufacturers like Agilent Technologies, PerkinElmer, and Revvity Health Sciences, who offer advanced GC-MS systems with optimized inlet technologies. Research institutions such as Beijing Technology & Business University and University of Connecticut contribute significant innovations in methodology development. Technical maturity varies across applications, with continuous improvements focusing on reducing thermal degradation, enhancing sensitivity, and developing specialized inlet systems for increasingly complex thermally labile compounds.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced GC-MS inlet temperature optimization solutions specifically designed for thermally labile compounds. Their Multi-Mode Inlet (MMI) technology allows for programmable temperature vaporization (PTV), enabling analysts to start at low temperatures (as low as 40°C) and gradually increase to the optimal temperature for specific compounds. This approach minimizes thermal degradation while maximizing analytical sensitivity. Agilent's systems incorporate cold splitless injection techniques that maintain sample integrity by rapidly transferring analytes to the column while minimizing their exposure to high temperatures. Their JetCool technology provides active cooling capabilities, allowing the inlet to rapidly cool between analyses, which is particularly beneficial for sequential analyses of heat-sensitive compounds. Additionally, Agilent has implemented intelligent software algorithms that can suggest optimal inlet parameters based on compound properties and historical performance data.
Strengths: Superior temperature control precision (±1°C) across a wide temperature range; integrated electronic pressure control systems that maintain consistent carrier gas flow during temperature programming; comprehensive software support for method development. Weaknesses: Higher initial investment cost compared to basic GC-MS systems; requires more technical expertise to fully utilize advanced features; cooling systems add complexity to maintenance routines.
Shanghai Micro-Spectial Chem Analysis & Test Tech Co. Ltd.
Technical Solution: Shanghai Micro-Spectial has developed a specialized GC-MS inlet system tailored for thermally sensitive compounds, featuring their MicroCool technology. Their approach incorporates a dual-zone temperature control system that creates a thermal gradient within the inlet, allowing for gentle vaporization of thermally labile compounds. The company's proprietary inlet design includes ultra-inert flow paths with specialized deactivation treatments that minimize catalytic degradation of sensitive analytes. Their system features rapid cooling capabilities that can reduce inlet temperatures from 350°C to 50°C in under 3 minutes, enabling efficient analysis of multiple samples with different thermal requirements. Shanghai Micro-Spectial has also developed specialized low-mass inlet components that provide faster thermal response and more precise temperature control compared to conventional systems. Their technology includes automated temperature optimization protocols that can systematically evaluate multiple inlet temperatures to determine ideal conditions for specific compound classes while monitoring degradation products to verify sample integrity.
Strengths: Cost-effective solution compared to Western manufacturers while maintaining good performance specifications; specialized focus on thermally labile natural products common in traditional Chinese medicine applications; excellent technical support within Asian markets. Weaknesses: More limited global service network compared to larger international competitors; fewer integration options with third-party software platforms; less extensive validation documentation for regulated industries.
Key Innovations in Inlet Temperature Control Systems
Phospholipid containing garlic, curry leaves and turmeric extracts for treatment of adipogenesis
PatentPendingIN202141048482A
Innovation
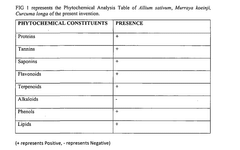
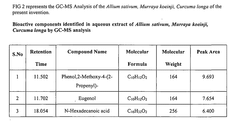
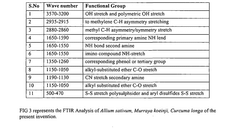
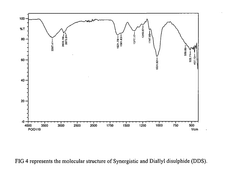
- A synergistic extract derived from Allium sativum, Murraya koenji, and Curcuma longa, combined with phospholipid as a Phytosome complex, is developed for enhanced bioavailability and therapeutic potential, involving a method of extraction, purification, and characterization using GC-MS, FTIR, and SEM, demonstrating the presence of bioactive compounds and antioxidant activity.
Full-scanning analysis method for organic matters in leaching liquor of medical apparatus and instruments
PatentActiveCN115684379A
Innovation
- An acidic leach solution is used to simulate the contact environment between medical devices and acidic drugs, combined with a specific chromatographic column and temperature program, GC-MS is used for analysis, and the detection sensitivity is improved through a combination of dichloromethane, ethyl acetate, and ethanol extraction agents. , precision and accuracy.
Sample Preparation Techniques for Thermally Sensitive Analytes
Sample preparation is a critical step in the analytical workflow for thermally sensitive compounds, as these analytes can degrade during the GC-MS inlet heating process. Conventional sample preparation methods often fail to adequately protect these compounds, resulting in poor recovery rates and unreliable analytical results. The development of specialized techniques has become essential to preserve the structural integrity of thermally labile molecules prior to their introduction into the GC-MS system.
Derivatization stands as one of the most effective approaches for thermally sensitive analytes. This chemical modification process converts unstable compounds into more thermally stable derivatives by protecting reactive functional groups. Silylation agents such as BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide) and MSTFA (N-Methyl-N-(trimethylsilyl)trifluoroacetamide) are particularly valuable for compounds containing hydroxyl, carboxyl, and amine groups, significantly improving their thermal stability during GC analysis.
Cold on-column injection techniques represent another significant advancement, allowing samples to be introduced directly onto the column at lower temperatures, thus minimizing thermal stress. This approach is particularly beneficial for compounds with decomposition points below standard inlet temperatures, as it bypasses the hot inlet environment altogether. The technique requires specialized instrumentation but offers superior results for highly thermally labile compounds.
Solid-phase microextraction (SPME) has emerged as a valuable solvent-free preparation technique that combines sampling, extraction, and concentration into a single step. When coupled with cold injection techniques, SPME significantly reduces thermal degradation risks. The fiber coating can be selected based on the specific properties of the target analytes, providing an additional layer of method optimization for thermally sensitive compounds.
Headspace sampling techniques offer another viable approach, particularly for volatile thermally labile compounds. By extracting only the volatile components from the sample matrix without heating the entire sample, these methods minimize thermal exposure. Static headspace, dynamic headspace, and headspace SPME variants can be tailored to specific analytical requirements, balancing sensitivity needs against thermal stability concerns.
Cryogenic focusing techniques, including liquid nitrogen trapping systems, enable the concentration of analytes at sub-zero temperatures before GC separation. This approach is particularly valuable for highly volatile and thermally sensitive compounds that might otherwise be lost during conventional sample preparation steps. When combined with programmed temperature vaporization (PTV) injection, these techniques offer exceptional protection for thermally labile analytes.
Derivatization stands as one of the most effective approaches for thermally sensitive analytes. This chemical modification process converts unstable compounds into more thermally stable derivatives by protecting reactive functional groups. Silylation agents such as BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide) and MSTFA (N-Methyl-N-(trimethylsilyl)trifluoroacetamide) are particularly valuable for compounds containing hydroxyl, carboxyl, and amine groups, significantly improving their thermal stability during GC analysis.
Cold on-column injection techniques represent another significant advancement, allowing samples to be introduced directly onto the column at lower temperatures, thus minimizing thermal stress. This approach is particularly beneficial for compounds with decomposition points below standard inlet temperatures, as it bypasses the hot inlet environment altogether. The technique requires specialized instrumentation but offers superior results for highly thermally labile compounds.
Solid-phase microextraction (SPME) has emerged as a valuable solvent-free preparation technique that combines sampling, extraction, and concentration into a single step. When coupled with cold injection techniques, SPME significantly reduces thermal degradation risks. The fiber coating can be selected based on the specific properties of the target analytes, providing an additional layer of method optimization for thermally sensitive compounds.
Headspace sampling techniques offer another viable approach, particularly for volatile thermally labile compounds. By extracting only the volatile components from the sample matrix without heating the entire sample, these methods minimize thermal exposure. Static headspace, dynamic headspace, and headspace SPME variants can be tailored to specific analytical requirements, balancing sensitivity needs against thermal stability concerns.
Cryogenic focusing techniques, including liquid nitrogen trapping systems, enable the concentration of analytes at sub-zero temperatures before GC separation. This approach is particularly valuable for highly volatile and thermally sensitive compounds that might otherwise be lost during conventional sample preparation steps. When combined with programmed temperature vaporization (PTV) injection, these techniques offer exceptional protection for thermally labile analytes.
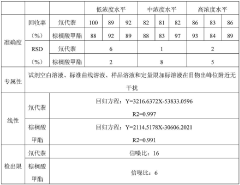
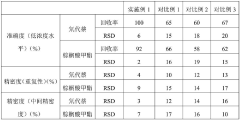
Validation Protocols for Optimized GC-MS Methods
Validation protocols for optimized GC-MS methods targeting thermally labile compounds require systematic approaches that ensure both analytical reliability and reproducibility. These protocols must specifically address the unique challenges posed by compounds susceptible to thermal degradation during analysis.
The validation process begins with establishing system suitability parameters tailored to thermally sensitive analytes. This includes verification of chromatographic resolution, peak symmetry, and detector response at the optimized inlet temperature. For thermally labile compounds, these parameters must demonstrate stability across multiple injections at the selected lower inlet temperature without compromising analytical performance.
Method specificity validation requires demonstrating that the optimized inlet temperature effectively preserves the molecular integrity of target compounds. This involves comparative analysis of known degradation products at both standard and optimized temperatures, with acceptance criteria typically requiring less than 5% formation of thermal degradation products at the optimized conditions.
Linearity assessment protocols must account for potential temperature-dependent response variations. Calibration curves should be established using at least five concentration levels, with each level analyzed in triplicate at the optimized inlet temperature. The validation should confirm that correlation coefficients exceed 0.995 across the working range despite the modified inlet conditions.
Precision validation requires particular attention when working with thermally labile compounds. Repeatability studies should include at least six replicate injections at three concentration levels (low, medium, high), while intermediate precision should evaluate day-to-day and analyst-to-analyst variations. For thermally sensitive analytes, acceptance criteria typically specify RSD values below 5% for high concentrations and below 10% for concentrations near the quantitation limit.
Accuracy validation protocols must incorporate recovery studies using matrix-matched samples spiked with known quantities of thermally labile analytes. Recovery rates between 85-115% are generally considered acceptable, with particular emphasis on demonstrating that the optimized inlet temperature improves recovery compared to standard conditions.
Robustness testing specifically examines the stability of the method when small variations in the optimized inlet temperature occur (typically ±5°C). This critical validation component ensures that minor fluctuations in inlet temperature do not significantly impact analytical results, with acceptance criteria requiring that such variations produce less than 10% change in quantitative results.
The validation process begins with establishing system suitability parameters tailored to thermally sensitive analytes. This includes verification of chromatographic resolution, peak symmetry, and detector response at the optimized inlet temperature. For thermally labile compounds, these parameters must demonstrate stability across multiple injections at the selected lower inlet temperature without compromising analytical performance.
Method specificity validation requires demonstrating that the optimized inlet temperature effectively preserves the molecular integrity of target compounds. This involves comparative analysis of known degradation products at both standard and optimized temperatures, with acceptance criteria typically requiring less than 5% formation of thermal degradation products at the optimized conditions.
Linearity assessment protocols must account for potential temperature-dependent response variations. Calibration curves should be established using at least five concentration levels, with each level analyzed in triplicate at the optimized inlet temperature. The validation should confirm that correlation coefficients exceed 0.995 across the working range despite the modified inlet conditions.
Precision validation requires particular attention when working with thermally labile compounds. Repeatability studies should include at least six replicate injections at three concentration levels (low, medium, high), while intermediate precision should evaluate day-to-day and analyst-to-analyst variations. For thermally sensitive analytes, acceptance criteria typically specify RSD values below 5% for high concentrations and below 10% for concentrations near the quantitation limit.
Accuracy validation protocols must incorporate recovery studies using matrix-matched samples spiked with known quantities of thermally labile analytes. Recovery rates between 85-115% are generally considered acceptable, with particular emphasis on demonstrating that the optimized inlet temperature improves recovery compared to standard conditions.
Robustness testing specifically examines the stability of the method when small variations in the optimized inlet temperature occur (typically ±5°C). This critical validation component ensures that minor fluctuations in inlet temperature do not significantly impact analytical results, with acceptance criteria requiring that such variations produce less than 10% change in quantitative results.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!