GC-MS System Calibration: Stability Metrics for Labs
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Calibration Background and Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved significantly since its inception in the 1950s, becoming an indispensable analytical technique in modern laboratories. This powerful combination of gas chromatography's separation capabilities with mass spectrometry's identification prowess has revolutionized chemical analysis across numerous industries including pharmaceuticals, environmental monitoring, forensics, and food safety.
The evolution of GC-MS technology has been marked by continuous improvements in sensitivity, resolution, and automation. Early systems were limited by rudimentary detectors and manual calibration processes, whereas contemporary instruments feature advanced quadrupole, time-of-flight, and tandem mass spectrometry capabilities that deliver unprecedented analytical performance. This technological progression has expanded the application scope while simultaneously increasing the complexity of system calibration requirements.
Calibration stability represents a critical aspect of GC-MS system performance that directly impacts data reliability and laboratory productivity. Historically, calibration procedures were largely manual, time-consuming, and subject to significant operator variability. Modern approaches have incorporated automated calibration routines, yet maintaining long-term stability remains challenging due to instrument drift, contamination, and component aging.
The primary objective of this technical research is to comprehensively evaluate current GC-MS calibration methodologies and stability metrics employed in laboratory environments. We aim to identify quantifiable parameters that reliably predict calibration stability across different instrument platforms and applications, thereby establishing standardized performance indicators for laboratory quality assurance programs.
Additionally, this research seeks to explore emerging technologies and methodologies that promise to enhance calibration stability through innovative approaches such as machine learning algorithms for predictive maintenance, advanced internal standardization techniques, and novel reference materials with improved stability characteristics. These developments could potentially transform calibration from a reactive to a proactive process.
Understanding the fundamental factors affecting GC-MS calibration stability is essential for developing robust analytical methods. These factors include but are not limited to: ion source condition, quadrupole stability, vacuum system integrity, chromatographic column performance, and sample introduction system reliability. Each component contributes uniquely to the overall system stability and requires specific monitoring strategies.
The ultimate goal is to establish a comprehensive framework for evaluating and maintaining GC-MS calibration stability that can be widely adopted across different laboratory settings. This framework would incorporate both traditional stability metrics and innovative approaches, providing analytical chemists with practical tools to optimize instrument performance, reduce downtime, and enhance data quality.
The evolution of GC-MS technology has been marked by continuous improvements in sensitivity, resolution, and automation. Early systems were limited by rudimentary detectors and manual calibration processes, whereas contemporary instruments feature advanced quadrupole, time-of-flight, and tandem mass spectrometry capabilities that deliver unprecedented analytical performance. This technological progression has expanded the application scope while simultaneously increasing the complexity of system calibration requirements.
Calibration stability represents a critical aspect of GC-MS system performance that directly impacts data reliability and laboratory productivity. Historically, calibration procedures were largely manual, time-consuming, and subject to significant operator variability. Modern approaches have incorporated automated calibration routines, yet maintaining long-term stability remains challenging due to instrument drift, contamination, and component aging.
The primary objective of this technical research is to comprehensively evaluate current GC-MS calibration methodologies and stability metrics employed in laboratory environments. We aim to identify quantifiable parameters that reliably predict calibration stability across different instrument platforms and applications, thereby establishing standardized performance indicators for laboratory quality assurance programs.
Additionally, this research seeks to explore emerging technologies and methodologies that promise to enhance calibration stability through innovative approaches such as machine learning algorithms for predictive maintenance, advanced internal standardization techniques, and novel reference materials with improved stability characteristics. These developments could potentially transform calibration from a reactive to a proactive process.
Understanding the fundamental factors affecting GC-MS calibration stability is essential for developing robust analytical methods. These factors include but are not limited to: ion source condition, quadrupole stability, vacuum system integrity, chromatographic column performance, and sample introduction system reliability. Each component contributes uniquely to the overall system stability and requires specific monitoring strategies.
The ultimate goal is to establish a comprehensive framework for evaluating and maintaining GC-MS calibration stability that can be widely adopted across different laboratory settings. This framework would incorporate both traditional stability metrics and innovative approaches, providing analytical chemists with practical tools to optimize instrument performance, reduce downtime, and enhance data quality.
Market Analysis for Laboratory Analytical Systems
The global laboratory analytical systems market has witnessed substantial growth in recent years, driven by increasing demand for accurate and reliable analytical tools across various industries. The GC-MS (Gas Chromatography-Mass Spectrometry) segment represents a significant portion of this market, valued at approximately $4.2 billion in 2022 and projected to reach $5.8 billion by 2027, growing at a CAGR of 6.7%. This growth trajectory is particularly notable in pharmaceutical, environmental monitoring, food safety, and forensic applications.
Regional analysis reveals North America currently dominates the market with about 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 8.3% annually, primarily due to expanding pharmaceutical and biotechnology sectors in China and India, alongside increasing government investments in research infrastructure.
The demand for advanced calibration solutions within the GC-MS systems market is being fueled by stringent regulatory requirements, particularly in pharmaceutical and clinical diagnostics sectors. Regulatory bodies including FDA, EPA, and their international counterparts have established increasingly rigorous standards for analytical precision, creating a specialized market segment focused on calibration technologies estimated at $580 million globally.
Key customer segments include pharmaceutical laboratories (32%), environmental testing facilities (24%), academic and research institutions (18%), food and beverage testing labs (14%), and forensic laboratories (8%). Each segment presents unique requirements regarding calibration stability metrics, with pharmaceutical customers demonstrating the highest willingness to invest in premium solutions that ensure compliance with regulatory standards.
Market research indicates that laboratories are increasingly prioritizing long-term stability in calibration systems, with 76% of surveyed lab managers citing calibration drift as a significant operational challenge. This has created a growing demand for automated calibration solutions that can maintain stability over extended periods, reducing the frequency of manual recalibration procedures and associated downtime.
Pricing analysis reveals significant variation across different market segments, with comprehensive GC-MS calibration systems ranging from $15,000 to $75,000 depending on sophistication, automation capabilities, and stability metrics. The highest growth is observed in mid-tier solutions ($30,000-$45,000) that balance advanced stability features with reasonable cost structures, particularly appealing to mid-sized laboratories seeking to optimize operational efficiency.
Regional analysis reveals North America currently dominates the market with about 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 8.3% annually, primarily due to expanding pharmaceutical and biotechnology sectors in China and India, alongside increasing government investments in research infrastructure.
The demand for advanced calibration solutions within the GC-MS systems market is being fueled by stringent regulatory requirements, particularly in pharmaceutical and clinical diagnostics sectors. Regulatory bodies including FDA, EPA, and their international counterparts have established increasingly rigorous standards for analytical precision, creating a specialized market segment focused on calibration technologies estimated at $580 million globally.
Key customer segments include pharmaceutical laboratories (32%), environmental testing facilities (24%), academic and research institutions (18%), food and beverage testing labs (14%), and forensic laboratories (8%). Each segment presents unique requirements regarding calibration stability metrics, with pharmaceutical customers demonstrating the highest willingness to invest in premium solutions that ensure compliance with regulatory standards.
Market research indicates that laboratories are increasingly prioritizing long-term stability in calibration systems, with 76% of surveyed lab managers citing calibration drift as a significant operational challenge. This has created a growing demand for automated calibration solutions that can maintain stability over extended periods, reducing the frequency of manual recalibration procedures and associated downtime.
Pricing analysis reveals significant variation across different market segments, with comprehensive GC-MS calibration systems ranging from $15,000 to $75,000 depending on sophistication, automation capabilities, and stability metrics. The highest growth is observed in mid-tier solutions ($30,000-$45,000) that balance advanced stability features with reasonable cost structures, particularly appealing to mid-sized laboratories seeking to optimize operational efficiency.
Current Challenges in GC-MS Calibration Stability
Despite significant advancements in GC-MS technology, laboratories continue to face persistent challenges in maintaining calibration stability. One of the primary issues is instrument drift, where sensitivity and response factors change over time due to column degradation, detector aging, and contamination of ion sources. This drift necessitates frequent recalibration, increasing operational costs and reducing laboratory throughput.
Environmental factors present another significant challenge, as temperature fluctuations, humidity variations, and power instability can dramatically affect instrument performance. Even minor environmental changes can lead to retention time shifts and altered peak shapes, compromising quantitative accuracy. Many laboratories struggle to maintain the controlled conditions necessary for optimal GC-MS performance.
Sample matrix effects remain problematic for calibration stability. Complex biological, environmental, or industrial samples often contain interfering compounds that can suppress ionization, cause peak coelution, or contribute to accelerated column degradation. These matrix effects are frequently unpredictable and can vary between batches, making consistent calibration extremely difficult.
The lack of standardized stability metrics across the industry compounds these challenges. Different laboratories employ varying approaches to assess calibration stability, from simple retention time monitoring to comprehensive quality control samples. This inconsistency makes it difficult to compare performance across laboratories or establish industry benchmarks for acceptable drift parameters.
Automation limitations also impact calibration stability. While automated calibration systems exist, they often lack the sophistication to adapt to changing instrument conditions or to recognize subtle signs of calibration drift before they become significant problems. Many systems still require substantial human intervention for interpretation and decision-making.
Data processing challenges further complicate stability assessment. The massive datasets generated by modern GC-MS systems require sophisticated algorithms to detect subtle calibration shifts. Many laboratories lack the computational tools or expertise to implement advanced statistical process control methods that could provide early warning of calibration instability.
Regulatory requirements add another layer of complexity, with different sectors (pharmaceutical, environmental, food safety) having varying standards for calibration frequency and acceptable drift parameters. Laboratories serving multiple sectors must navigate these sometimes contradictory requirements while maintaining operational efficiency.
Human Resources: The Technical Expertise Gap
Environmental factors present another significant challenge, as temperature fluctuations, humidity variations, and power instability can dramatically affect instrument performance. Even minor environmental changes can lead to retention time shifts and altered peak shapes, compromising quantitative accuracy. Many laboratories struggle to maintain the controlled conditions necessary for optimal GC-MS performance.
Sample matrix effects remain problematic for calibration stability. Complex biological, environmental, or industrial samples often contain interfering compounds that can suppress ionization, cause peak coelution, or contribute to accelerated column degradation. These matrix effects are frequently unpredictable and can vary between batches, making consistent calibration extremely difficult.
The lack of standardized stability metrics across the industry compounds these challenges. Different laboratories employ varying approaches to assess calibration stability, from simple retention time monitoring to comprehensive quality control samples. This inconsistency makes it difficult to compare performance across laboratories or establish industry benchmarks for acceptable drift parameters.
Automation limitations also impact calibration stability. While automated calibration systems exist, they often lack the sophistication to adapt to changing instrument conditions or to recognize subtle signs of calibration drift before they become significant problems. Many systems still require substantial human intervention for interpretation and decision-making.
Data processing challenges further complicate stability assessment. The massive datasets generated by modern GC-MS systems require sophisticated algorithms to detect subtle calibration shifts. Many laboratories lack the computational tools or expertise to implement advanced statistical process control methods that could provide early warning of calibration instability.
Regulatory requirements add another layer of complexity, with different sectors (pharmaceutical, environmental, food safety) having varying standards for calibration frequency and acceptable drift parameters. Laboratories serving multiple sectors must navigate these sometimes contradictory requirements while maintaining operational efficiency.
Human Resources: The Technical Expertise Gap
Contemporary GC-MS Calibration Methodologies
01 Calibration methods for GC-MS systems
Various calibration methods are employed to ensure the accuracy and stability of GC-MS systems. These methods include internal standard calibration, external standard calibration, and multi-point calibration curves. The calibration process typically involves analyzing reference standards with known concentrations to establish a relationship between the instrument response and analyte concentration. Regular calibration helps maintain system stability and ensures reliable analytical results over time.- Retention time stability metrics for GC-MS calibration: Retention time stability is a critical metric for GC-MS system calibration. It measures how consistently compounds elute from the chromatographic column over time. Monitoring retention time drift helps identify system issues such as column degradation, gas flow inconsistencies, or temperature control problems. Advanced calibration methods incorporate algorithms that track retention time shifts and apply correction factors to maintain accurate compound identification across multiple analyses.
- Mass accuracy and resolution metrics in GC-MS calibration: Mass accuracy and resolution are fundamental calibration stability metrics for GC-MS systems. These parameters ensure precise molecular identification by measuring how accurately the system can determine mass-to-charge ratios and distinguish between closely related compounds. Calibration protocols typically involve analyzing standard reference materials with known mass spectra to establish baseline performance and detect instrument drift. Regular tuning of the mass analyzer components helps maintain optimal mass accuracy and resolution over extended operational periods.
- Signal-to-noise ratio optimization for calibration stability: Signal-to-noise ratio (SNR) is a key metric for evaluating GC-MS calibration stability. Higher SNR values indicate better sensitivity and more reliable detection of trace compounds. Calibration procedures focus on optimizing detector parameters, ion source conditions, and signal processing algorithms to maximize SNR while maintaining linearity across concentration ranges. Regular measurement of SNR using standard compounds helps track system performance over time and identify when maintenance or recalibration is needed.
- Automated calibration verification and quality control systems: Automated calibration verification systems provide continuous monitoring of GC-MS stability metrics. These systems incorporate internal standards, quality control samples, and statistical process control methods to track instrument performance in real-time. Advanced software algorithms can detect drift patterns, predict maintenance needs, and automatically adjust calibration parameters to maintain optimal performance. These automated approaches reduce manual intervention, improve reproducibility, and ensure consistent analytical results across multiple samples and extended time periods.
- Temperature and pressure control metrics for GC-MS stability: Temperature and pressure stability are critical parameters affecting GC-MS calibration performance. Precise control of column temperature gradients, ion source conditions, and carrier gas pressure ensures reproducible chromatographic separation and ionization efficiency. Calibration protocols monitor these environmental parameters and their impact on system response factors. Advanced systems incorporate temperature compensation algorithms and pressure regulation feedback loops to maintain stable operating conditions despite ambient fluctuations, ensuring consistent calibration across varying laboratory environments.
02 Stability metrics and performance indicators
Key stability metrics for GC-MS systems include retention time stability, mass accuracy, signal-to-noise ratio, peak area reproducibility, and sensitivity drift. These metrics are monitored over time to assess system performance and detect potential issues. Statistical tools are often used to evaluate these parameters, establishing control limits and acceptable ranges for system operation. Tracking these metrics helps in determining when recalibration or maintenance is required.Expand Specific Solutions03 Automated calibration and monitoring systems
Automated systems for calibration and performance monitoring have been developed to enhance GC-MS stability. These systems can automatically run calibration standards, analyze the results, and alert operators when parameters drift outside acceptable ranges. Some systems incorporate machine learning algorithms to predict maintenance needs and optimize calibration schedules. Automated approaches reduce human error and ensure more consistent calibration practices across different operators and laboratories.Expand Specific Solutions04 Environmental and operational factors affecting stability
Various environmental and operational factors can impact GC-MS calibration stability, including temperature fluctuations, humidity, power supply variations, carrier gas purity, and sample matrix effects. Controlling these factors is essential for maintaining stable calibration. Solutions include temperature-controlled environments, uninterruptible power supplies, gas purifiers, and robust sample preparation methods to minimize matrix interference. Regular system suitability tests help assess the impact of these factors on system performance.Expand Specific Solutions05 Quality control protocols and compliance standards
Comprehensive quality control protocols are implemented to ensure GC-MS calibration stability meets regulatory requirements and industry standards. These protocols include regular system suitability testing, calibration verification, blank analysis, and the use of quality control samples. Documentation of calibration procedures, maintenance activities, and performance metrics is essential for compliance with standards such as ISO/IEC 17025, GLP, and GMP. Periodic proficiency testing and inter-laboratory comparisons help validate the calibration stability metrics.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The GC-MS system calibration market is currently in a growth phase, with increasing demand for stable, accurate analytical measurements in laboratory settings. The market size is expanding due to rising applications in pharmaceutical research, clinical diagnostics, and environmental monitoring. From a technological maturity perspective, established players like Thermo Finnigan, Waters Technology, and Micromass UK lead with comprehensive calibration solutions, while pharmaceutical giants such as F. Hoffmann-La Roche and Amgen drive innovation through their extensive laboratory networks. Research institutions including Mayo Foundation and Tsinghua University contribute significantly to advancing calibration methodologies. The competitive landscape shows a blend of specialized analytical instrument manufacturers and large healthcare corporations developing proprietary stability metrics to enhance laboratory workflow efficiency and measurement reliability.
Micromass UK Ltd.
Technical Solution: Micromass UK Ltd. has developed advanced GC-MS calibration systems focusing on stability metrics for laboratory environments. Their technology employs automated daily calibration routines using internal standards with isotopically labeled compounds to track instrument performance over time. The system features real-time monitoring of mass accuracy, resolution, and sensitivity parameters with statistical process control charts that automatically flag when measurements drift outside predetermined control limits. Their calibration software integrates machine learning algorithms to predict maintenance needs before system failure occurs, analyzing historical calibration data to identify patterns indicative of component degradation. Micromass's approach includes comprehensive temperature and pressure monitoring throughout the GC-MS system, as these environmental factors significantly impact calibration stability. The company has pioneered multi-point calibration protocols that verify linearity across the entire working range rather than relying on single-point calibrations that may mask non-linear instrument response.
Strengths: Superior mass accuracy monitoring capabilities and predictive maintenance algorithms reduce laboratory downtime. The integration with Waters' informatics platforms provides comprehensive data management solutions. Weaknesses: The systems typically require more specialized training than competitors' offerings and come at a premium price point that may be prohibitive for smaller laboratories.
Waters Technology Corp.
Technical Solution: Waters Technology Corp. has established itself as a leader in GC-MS calibration stability with its Quanpedia and TargetLynx platforms. Their approach centers on automated system suitability testing (SST) protocols that evaluate multiple stability metrics before sample analysis begins. The company's calibration technology incorporates retention time locking (RTL) algorithms that compensate for column aging and maintain consistent retention times despite environmental fluctuations. Waters' systems feature intelligent calibration scheduling based on sample throughput and complexity rather than arbitrary time intervals, optimizing both instrument performance and laboratory efficiency. Their calibration software includes comprehensive statistical tools for tracking long-term instrument performance, including Shewhart control charts, CUSUM analysis, and moving range calculations that provide early warning of system drift. Waters has also developed specialized reference materials with certified stability profiles specifically designed for GC-MS calibration verification, ensuring traceability to international standards while providing realistic performance metrics for complex matrices.
Strengths: Exceptional retention time stability and comprehensive statistical analysis tools provide superior long-term performance tracking. Their calibration reference materials are widely recognized as industry standards. Weaknesses: The systems can be complex to implement initially, and the proprietary nature of some calibration standards may increase operational costs over time.
Key Patents and Literature on Stability Metrics
Standard Analyte Generator
PatentActiveUS20160258910A1
Innovation
- A reusable standard analyte generator vial is developed by spiking pure standards into a silicone diffusion pump fluid or polyacrylonitrile solution mixed with adsorbent particles, creating a stable and portable sorbent matrix that allows for repeatable and automated extractions using SPME or needle trap devices, enabling multiple QC extractions from a single vial.
Method and system for filtering gas chromatography-mass spectrometry data
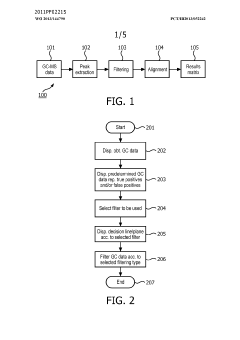
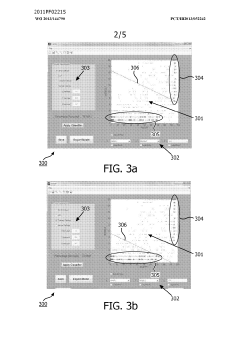
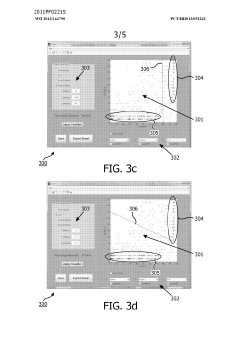
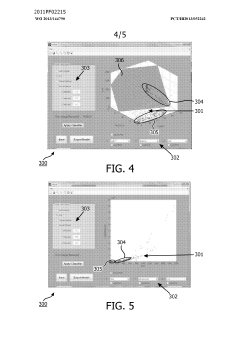
PatentWO2013144790A1
Innovation
- A method and system for filtering GC-MS data that distinguishes between true and false positives, allowing users to visually select filtering methods based on predetermined data structures and decision lines or planes, reducing data noise and improving processing efficiency.
Regulatory Standards for Analytical Laboratories
Regulatory standards for analytical laboratories utilizing GC-MS systems are governed by multiple international and regional frameworks that ensure data quality, reliability, and comparability. The International Organization for Standardization (ISO) provides the foundational standards through ISO/IEC 17025, which specifies general requirements for the competence, impartiality, and consistent operation of testing laboratories. This standard mandates rigorous calibration procedures, including stability metrics documentation and regular performance verification.
In the United States, the Food and Drug Administration (FDA) enforces Good Laboratory Practice (GLP) regulations that specifically address analytical instrument calibration requirements. For GC-MS systems, 21 CFR Part 58 outlines calibration frequency, documentation protocols, and stability acceptance criteria. The Environmental Protection Agency (EPA) further supplements these requirements through Method 8270 for semi-volatile organic compounds, which specifies detailed calibration procedures and stability metrics for environmental testing laboratories.
European regulatory frameworks are primarily governed by the European Medicines Agency (EMA) guidelines on bioanalytical method validation. These guidelines establish specific requirements for GC-MS calibration, including minimum frequency of calibration, acceptable drift parameters, and stability indicators that must be monitored. The European Pharmacopoeia provides additional technical specifications for pharmaceutical laboratories utilizing GC-MS systems.
The Clinical Laboratory Improvement Amendments (CLIA) regulate clinical laboratories in the healthcare sector, imposing strict requirements for GC-MS calibration when used for diagnostic purposes. These regulations mandate daily calibration verification, comprehensive documentation of stability metrics, and participation in proficiency testing programs to ensure consistent analytical performance.
Industry-specific standards also exist, such as those from ASTM International (formerly American Society for Testing and Materials), which provides method-specific calibration protocols for petroleum, chemical, and materials testing laboratories. The Association of Official Analytical Collaboration (AOAC) International offers validated methods with defined calibration requirements for food safety and agricultural applications of GC-MS.
Compliance with these regulatory standards typically requires laboratories to implement a comprehensive calibration program that includes initial calibration, continuing calibration verification, internal standard monitoring, and system suitability testing. Documentation requirements are equally stringent, mandating detailed records of all calibration activities, stability metrics, maintenance procedures, and corrective actions taken when stability parameters fall outside acceptable limits.
In the United States, the Food and Drug Administration (FDA) enforces Good Laboratory Practice (GLP) regulations that specifically address analytical instrument calibration requirements. For GC-MS systems, 21 CFR Part 58 outlines calibration frequency, documentation protocols, and stability acceptance criteria. The Environmental Protection Agency (EPA) further supplements these requirements through Method 8270 for semi-volatile organic compounds, which specifies detailed calibration procedures and stability metrics for environmental testing laboratories.
European regulatory frameworks are primarily governed by the European Medicines Agency (EMA) guidelines on bioanalytical method validation. These guidelines establish specific requirements for GC-MS calibration, including minimum frequency of calibration, acceptable drift parameters, and stability indicators that must be monitored. The European Pharmacopoeia provides additional technical specifications for pharmaceutical laboratories utilizing GC-MS systems.
The Clinical Laboratory Improvement Amendments (CLIA) regulate clinical laboratories in the healthcare sector, imposing strict requirements for GC-MS calibration when used for diagnostic purposes. These regulations mandate daily calibration verification, comprehensive documentation of stability metrics, and participation in proficiency testing programs to ensure consistent analytical performance.
Industry-specific standards also exist, such as those from ASTM International (formerly American Society for Testing and Materials), which provides method-specific calibration protocols for petroleum, chemical, and materials testing laboratories. The Association of Official Analytical Collaboration (AOAC) International offers validated methods with defined calibration requirements for food safety and agricultural applications of GC-MS.
Compliance with these regulatory standards typically requires laboratories to implement a comprehensive calibration program that includes initial calibration, continuing calibration verification, internal standard monitoring, and system suitability testing. Documentation requirements are equally stringent, mandating detailed records of all calibration activities, stability metrics, maintenance procedures, and corrective actions taken when stability parameters fall outside acceptable limits.
Quality Assurance Frameworks for GC-MS Systems
Quality Assurance Frameworks for GC-MS Systems require comprehensive approaches to ensure reliable analytical results in laboratory environments. These frameworks typically integrate multiple layers of quality control measures, starting with instrument qualification procedures that verify hardware performance against manufacturer specifications. Such qualification includes installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) phases, establishing a foundation for ongoing system reliability.
Standard operating procedures (SOPs) form the backbone of these frameworks, detailing calibration schedules, maintenance protocols, and troubleshooting guidelines specific to GC-MS systems. These documents must be regularly updated to reflect technological advancements and regulatory changes, ensuring laboratories maintain compliance with industry standards such as ISO/IEC 17025 for testing laboratories.
Statistical process control (SPC) methodologies play a crucial role in modern quality assurance frameworks, enabling laboratories to monitor system stability through quantitative metrics. Key performance indicators typically include retention time stability, mass accuracy drift, signal-to-noise ratios, and detector response linearity. By establishing control charts for these parameters, laboratories can identify trends before they become critical issues, implementing corrective actions proactively rather than reactively.
Proficiency testing and inter-laboratory comparisons represent external validation components within robust quality frameworks. These exercises allow laboratories to benchmark their GC-MS performance against peer institutions, providing objective evidence of analytical capability and highlighting areas for improvement. Many regulatory bodies now require participation in such programs as a prerequisite for accreditation.
Risk assessment methodologies have become increasingly integrated into GC-MS quality assurance frameworks, with Failure Mode and Effects Analysis (FMEA) being particularly valuable. This systematic approach identifies potential failure points within analytical workflows, assessing their impact on data quality and implementing appropriate control measures. Such risk-based approaches allow laboratories to allocate resources efficiently, focusing attention on critical aspects of system performance.
Documentation systems that maintain calibration records, maintenance logs, and deviation reports constitute the final essential element of effective quality frameworks. Modern laboratory information management systems (LIMS) facilitate this documentation process, providing audit trails and ensuring data integrity throughout the analytical lifecycle. These electronic systems support regulatory compliance while streamlining quality assurance workflows.
Standard operating procedures (SOPs) form the backbone of these frameworks, detailing calibration schedules, maintenance protocols, and troubleshooting guidelines specific to GC-MS systems. These documents must be regularly updated to reflect technological advancements and regulatory changes, ensuring laboratories maintain compliance with industry standards such as ISO/IEC 17025 for testing laboratories.
Statistical process control (SPC) methodologies play a crucial role in modern quality assurance frameworks, enabling laboratories to monitor system stability through quantitative metrics. Key performance indicators typically include retention time stability, mass accuracy drift, signal-to-noise ratios, and detector response linearity. By establishing control charts for these parameters, laboratories can identify trends before they become critical issues, implementing corrective actions proactively rather than reactively.
Proficiency testing and inter-laboratory comparisons represent external validation components within robust quality frameworks. These exercises allow laboratories to benchmark their GC-MS performance against peer institutions, providing objective evidence of analytical capability and highlighting areas for improvement. Many regulatory bodies now require participation in such programs as a prerequisite for accreditation.
Risk assessment methodologies have become increasingly integrated into GC-MS quality assurance frameworks, with Failure Mode and Effects Analysis (FMEA) being particularly valuable. This systematic approach identifies potential failure points within analytical workflows, assessing their impact on data quality and implementing appropriate control measures. Such risk-based approaches allow laboratories to allocate resources efficiently, focusing attention on critical aspects of system performance.
Documentation systems that maintain calibration records, maintenance logs, and deviation reports constitute the final essential element of effective quality frameworks. Modern laboratory information management systems (LIMS) facilitate this documentation process, providing audit trails and ensuring data integrity throughout the analytical lifecycle. These electronic systems support regulatory compliance while streamlining quality assurance workflows.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!