GC-MS vs UV/VIS: Derivative Compounds Detection
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Analytical Detection Background and Objectives
Analytical techniques for compound detection have evolved significantly over the past century, with Gas Chromatography-Mass Spectrometry (GC-MS) and Ultraviolet-Visible Spectroscopy (UV/VIS) emerging as cornerstone methodologies in chemical analysis. The development of these technologies has revolutionized our ability to identify, quantify, and characterize compounds across diverse fields including pharmaceuticals, environmental monitoring, forensics, and food safety.
GC-MS technology originated in the 1950s when the first successful coupling of gas chromatography with mass spectrometry was achieved. This integration created a powerful analytical tool capable of separating complex mixtures and providing structural information about individual components. The technique has since undergone continuous refinement, with improvements in column technology, ionization methods, and detector sensitivity dramatically enhancing its capabilities.
UV/VIS spectroscopy has an even longer history, dating back to the early 20th century. This technique leverages the interaction between electromagnetic radiation and matter to identify compounds based on their absorption patterns. While initially limited by instrumentation, modern UV/VIS systems offer exceptional sensitivity and reproducibility, particularly for compounds with chromophores.
The detection of derivative compounds presents unique challenges that have driven technological innovation in both platforms. Derivatives—compounds formed by slight modifications to a parent structure—often share similar physical and chemical properties, making their differentiation particularly challenging. This has spurred the development of specialized techniques such as derivatization methods that enhance detection specificity and sensitivity.
The primary objective of this technical research is to comprehensively evaluate the comparative strengths, limitations, and applications of GC-MS and UV/VIS technologies specifically for derivative compound detection. We aim to establish clear parameters for technology selection based on specific analytical needs, sample types, and operational constraints.
Additionally, this research seeks to identify emerging trends in both technologies, including advancements in hyphenated techniques, miniaturization efforts, automation capabilities, and integration with artificial intelligence for data interpretation. Understanding these trajectories is crucial for strategic technology investment and development planning.
The ultimate goal is to provide a framework for optimizing analytical workflows by leveraging the complementary nature of these technologies, recognizing that in many modern laboratories, the question is not which technology to choose exclusively, but rather how to strategically implement both for maximum analytical power and efficiency.
GC-MS technology originated in the 1950s when the first successful coupling of gas chromatography with mass spectrometry was achieved. This integration created a powerful analytical tool capable of separating complex mixtures and providing structural information about individual components. The technique has since undergone continuous refinement, with improvements in column technology, ionization methods, and detector sensitivity dramatically enhancing its capabilities.
UV/VIS spectroscopy has an even longer history, dating back to the early 20th century. This technique leverages the interaction between electromagnetic radiation and matter to identify compounds based on their absorption patterns. While initially limited by instrumentation, modern UV/VIS systems offer exceptional sensitivity and reproducibility, particularly for compounds with chromophores.
The detection of derivative compounds presents unique challenges that have driven technological innovation in both platforms. Derivatives—compounds formed by slight modifications to a parent structure—often share similar physical and chemical properties, making their differentiation particularly challenging. This has spurred the development of specialized techniques such as derivatization methods that enhance detection specificity and sensitivity.
The primary objective of this technical research is to comprehensively evaluate the comparative strengths, limitations, and applications of GC-MS and UV/VIS technologies specifically for derivative compound detection. We aim to establish clear parameters for technology selection based on specific analytical needs, sample types, and operational constraints.
Additionally, this research seeks to identify emerging trends in both technologies, including advancements in hyphenated techniques, miniaturization efforts, automation capabilities, and integration with artificial intelligence for data interpretation. Understanding these trajectories is crucial for strategic technology investment and development planning.
The ultimate goal is to provide a framework for optimizing analytical workflows by leveraging the complementary nature of these technologies, recognizing that in many modern laboratories, the question is not which technology to choose exclusively, but rather how to strategically implement both for maximum analytical power and efficiency.
Market Applications for Derivative Compounds Analysis
The derivative compounds analysis market spans multiple sectors with significant growth potential. Pharmaceutical industry represents the largest application segment, where derivative analysis is crucial for drug development, quality control, and metabolite identification. GC-MS and UV/VIS spectroscopy serve complementary roles in this sector, with GC-MS primarily used for complex mixture analysis and UV/VIS for routine quality control and high-throughput screening.
Clinical diagnostics constitutes another major market, where derivative compound detection enables disease biomarker identification and therapeutic drug monitoring. The precision of GC-MS makes it valuable for detecting trace metabolites in biological samples, while UV/VIS offers rapid screening capabilities in point-of-care settings.
Environmental monitoring represents a rapidly expanding application area, with regulatory agencies worldwide mandating testing for derivative compounds in water, soil, and air. GC-MS dominates this sector due to its superior sensitivity for detecting trace contaminants, though UV/VIS finds application in field-portable devices for preliminary screening.
The food and beverage industry increasingly relies on derivative compound analysis for quality control, authenticity verification, and contaminant detection. GC-MS provides detailed compositional analysis of flavor compounds and adulterants, while UV/VIS offers cost-effective solutions for routine quality parameters.
Forensic toxicology represents a specialized but critical market segment where derivative compound detection helps identify substances in biological specimens. GC-MS serves as the gold standard for confirmatory testing, while UV/VIS provides preliminary screening capabilities.
Academic and research institutions form a substantial market for both technologies, driving innovation in methodology and applications. The complementary nature of GC-MS and UV/VIS has led to integrated workflow solutions that leverage the strengths of both techniques.
Emerging applications include personalized medicine, where metabolite profiling enables tailored therapeutic approaches, and industrial quality control in sectors ranging from cosmetics to petrochemicals. The market is witnessing a trend toward miniaturization and automation, with portable GC-MS systems and smartphone-integrated UV/VIS devices expanding accessibility beyond traditional laboratory settings.
Clinical diagnostics constitutes another major market, where derivative compound detection enables disease biomarker identification and therapeutic drug monitoring. The precision of GC-MS makes it valuable for detecting trace metabolites in biological samples, while UV/VIS offers rapid screening capabilities in point-of-care settings.
Environmental monitoring represents a rapidly expanding application area, with regulatory agencies worldwide mandating testing for derivative compounds in water, soil, and air. GC-MS dominates this sector due to its superior sensitivity for detecting trace contaminants, though UV/VIS finds application in field-portable devices for preliminary screening.
The food and beverage industry increasingly relies on derivative compound analysis for quality control, authenticity verification, and contaminant detection. GC-MS provides detailed compositional analysis of flavor compounds and adulterants, while UV/VIS offers cost-effective solutions for routine quality parameters.
Forensic toxicology represents a specialized but critical market segment where derivative compound detection helps identify substances in biological specimens. GC-MS serves as the gold standard for confirmatory testing, while UV/VIS provides preliminary screening capabilities.
Academic and research institutions form a substantial market for both technologies, driving innovation in methodology and applications. The complementary nature of GC-MS and UV/VIS has led to integrated workflow solutions that leverage the strengths of both techniques.
Emerging applications include personalized medicine, where metabolite profiling enables tailored therapeutic approaches, and industrial quality control in sectors ranging from cosmetics to petrochemicals. The market is witnessing a trend toward miniaturization and automation, with portable GC-MS systems and smartphone-integrated UV/VIS devices expanding accessibility beyond traditional laboratory settings.
GC-MS and UV/VIS Technical Limitations
Gas Chromatography-Mass Spectrometry (GC-MS) and Ultraviolet-Visible Spectroscopy (UV/VIS) represent two distinct analytical approaches for detecting derivative compounds, each with inherent technical limitations that impact their application scope and effectiveness.
GC-MS systems face significant challenges with thermally labile compounds, as the high temperatures required during the gas chromatography process can cause decomposition of heat-sensitive derivatives before detection occurs. This thermal degradation introduces analytical inaccuracies and potentially misleading results, particularly when working with complex biological samples or pharmaceuticals containing unstable functional groups.
Sample preparation for GC-MS presents another substantial limitation, requiring derivatization procedures for non-volatile compounds to enhance their volatility. These additional preparation steps not only extend analysis time but also introduce potential sources of error and contamination, reducing overall method reliability. The derivatization process itself may alter the chemical structure of target compounds, complicating data interpretation.
UV/VIS spectroscopy, while more straightforward in operation, suffers from limited specificity when analyzing complex mixtures. The broad absorption bands characteristic of UV/VIS spectra often result in peak overlapping, making it difficult to distinguish between structurally similar derivatives in multi-component samples. This limitation becomes particularly problematic in environmental monitoring and pharmaceutical quality control applications.
Sensitivity constraints represent another critical limitation of UV/VIS technology. The detection limits for many derivative compounds fall in the mid to high ppm range, significantly higher than those achievable with GC-MS. This sensitivity gap restricts UV/VIS applications in trace analysis scenarios, such as detecting low-concentration metabolites or environmental contaminants.
Matrix effects pose challenges for both technologies but manifest differently. In GC-MS, matrix components can cause ion suppression or enhancement in the mass spectrometer, while in UV/VIS, they may contribute to background interference and baseline shifts. These effects compromise quantitative accuracy and method robustness, particularly when analyzing derivatives in complex biological fluids or environmental samples.
Instrument complexity and expertise requirements create operational barriers for GC-MS implementation. The sophisticated instrumentation demands specialized training for operation and maintenance, with data interpretation requiring advanced knowledge of mass spectral patterns and fragmentation pathways. Conversely, while UV/VIS offers simpler operation, its limited discriminatory power necessitates complementary techniques for definitive compound identification.
Cost considerations further differentiate these technologies, with GC-MS systems requiring substantial capital investment and ongoing maintenance expenses. This economic barrier restricts access to GC-MS capabilities, particularly for smaller laboratories or resource-limited settings, where UV/VIS may represent the only feasible option despite its technical limitations.
GC-MS systems face significant challenges with thermally labile compounds, as the high temperatures required during the gas chromatography process can cause decomposition of heat-sensitive derivatives before detection occurs. This thermal degradation introduces analytical inaccuracies and potentially misleading results, particularly when working with complex biological samples or pharmaceuticals containing unstable functional groups.
Sample preparation for GC-MS presents another substantial limitation, requiring derivatization procedures for non-volatile compounds to enhance their volatility. These additional preparation steps not only extend analysis time but also introduce potential sources of error and contamination, reducing overall method reliability. The derivatization process itself may alter the chemical structure of target compounds, complicating data interpretation.
UV/VIS spectroscopy, while more straightforward in operation, suffers from limited specificity when analyzing complex mixtures. The broad absorption bands characteristic of UV/VIS spectra often result in peak overlapping, making it difficult to distinguish between structurally similar derivatives in multi-component samples. This limitation becomes particularly problematic in environmental monitoring and pharmaceutical quality control applications.
Sensitivity constraints represent another critical limitation of UV/VIS technology. The detection limits for many derivative compounds fall in the mid to high ppm range, significantly higher than those achievable with GC-MS. This sensitivity gap restricts UV/VIS applications in trace analysis scenarios, such as detecting low-concentration metabolites or environmental contaminants.
Matrix effects pose challenges for both technologies but manifest differently. In GC-MS, matrix components can cause ion suppression or enhancement in the mass spectrometer, while in UV/VIS, they may contribute to background interference and baseline shifts. These effects compromise quantitative accuracy and method robustness, particularly when analyzing derivatives in complex biological fluids or environmental samples.
Instrument complexity and expertise requirements create operational barriers for GC-MS implementation. The sophisticated instrumentation demands specialized training for operation and maintenance, with data interpretation requiring advanced knowledge of mass spectral patterns and fragmentation pathways. Conversely, while UV/VIS offers simpler operation, its limited discriminatory power necessitates complementary techniques for definitive compound identification.
Cost considerations further differentiate these technologies, with GC-MS systems requiring substantial capital investment and ongoing maintenance expenses. This economic barrier restricts access to GC-MS capabilities, particularly for smaller laboratories or resource-limited settings, where UV/VIS may represent the only feasible option despite its technical limitations.
Current Analytical Approaches Comparison
01 GC-MS detection capabilities for chemical analysis
Gas Chromatography-Mass Spectrometry (GC-MS) offers high sensitivity and specificity for detecting and identifying volatile and semi-volatile compounds. This technique combines the separation capabilities of gas chromatography with the identification power of mass spectrometry, allowing for detection of trace amounts of compounds in complex mixtures. The technology enables quantitative analysis with low detection limits and can be optimized for various sample types through different ionization methods and detector configurations.- GC-MS detection capabilities for chemical analysis: Gas Chromatography-Mass Spectrometry (GC-MS) provides high sensitivity and specificity for detecting and identifying volatile and semi-volatile compounds. This technique combines the separation capabilities of gas chromatography with the identification power of mass spectrometry, allowing for detection of trace amounts of substances in complex mixtures. The technology enables quantitative analysis with low detection limits and can be optimized for various sample types through different ionization methods and detector configurations.
- UV/VIS spectroscopy detection methods and applications: UV/VIS spectroscopy offers detection capabilities based on the absorption of ultraviolet and visible light by molecules. This technique provides quantitative and qualitative analysis of compounds that absorb in the UV-visible spectrum range. The detection sensitivity depends on the molar absorptivity of the analyte, with detection limits typically in the ppm to ppb range. UV/VIS spectroscopy is particularly useful for analyzing conjugated systems, aromatic compounds, and molecules with chromophores, enabling rapid screening and quantification in various applications.
- Combined GC-MS and UV/VIS spectroscopy systems: Integration of GC-MS and UV/VIS spectroscopy technologies creates comprehensive analytical platforms with enhanced detection capabilities. These combined systems allow for multi-dimensional analysis, providing both structural information from mass spectrometry and electronic transition data from UV/VIS spectroscopy. The complementary nature of these techniques improves compound identification confidence and expands the range of detectable substances. Such integrated approaches are particularly valuable for complex sample analysis where multiple detection methods provide verification and additional characterization information.
- Detection limit improvements and sensitivity enhancements: Various technological advancements have improved the detection limits and sensitivity of both GC-MS and UV/VIS spectroscopy. These include novel sample preparation techniques, improved ionization methods, advanced detector designs, and signal processing algorithms. Preconcentration methods, derivatization techniques, and specialized sample introduction systems can significantly lower detection limits. Additionally, modern instruments incorporate noise reduction technologies, enhanced optical components, and advanced data processing algorithms to improve signal-to-noise ratios and detection capabilities for trace analysis.
- Specialized applications and sample-specific detection methods: Specialized adaptations of GC-MS and UV/VIS spectroscopy have been developed for specific sample types and applications. These include modifications for environmental monitoring, pharmaceutical analysis, food safety testing, and forensic applications. Custom sample preparation protocols, specialized columns or separation methods, and application-specific detection parameters enhance the performance for particular analytes or matrices. These specialized approaches optimize detection capabilities for challenging samples or specific compound classes, enabling targeted analysis with improved sensitivity and selectivity.
02 UV/VIS spectroscopy detection methods and sensitivity
UV/VIS spectroscopy provides detection capabilities based on the absorption of ultraviolet and visible light by molecules. This technique offers advantages in analyzing compounds with chromophores, allowing for quantitative determination of concentration through Beer-Lambert law relationships. Modern UV/VIS systems can achieve detection limits in the nanogram to picogram range depending on the analyte and matrix. The technique is particularly valuable for rapid screening and can be coupled with separation techniques for enhanced analytical performance.Expand Specific Solutions03 Combined analytical approaches using GC-MS and UV/VIS
Integrating GC-MS and UV/VIS spectroscopy creates complementary analytical systems that overcome the limitations of individual techniques. This combined approach allows for comprehensive sample characterization, with GC-MS providing molecular identification and UV/VIS offering rapid quantification. The dual-method strategy enhances detection capabilities across a wider range of compounds and concentrations, improving analytical reliability through orthogonal confirmation. Such integrated systems are particularly valuable for complex environmental, pharmaceutical, and forensic analyses.Expand Specific Solutions04 Detection limit improvements and calibration techniques
Advanced calibration methods and signal processing techniques can significantly improve detection limits for both GC-MS and UV/VIS spectroscopy. These include internal standardization, matrix-matched calibration, standard addition methods, and chemometric approaches. Signal enhancement strategies such as derivative spectroscopy for UV/VIS and selected ion monitoring for GC-MS can further lower detection thresholds. Proper calibration ensures accurate quantification across the linear dynamic range while minimizing matrix effects and instrumental drift.Expand Specific Solutions05 Sample preparation and preconcentration for enhanced detection
Effective sample preparation techniques significantly enhance detection capabilities of both GC-MS and UV/VIS spectroscopy. Methods such as solid-phase extraction, liquid-liquid extraction, and headspace sampling can concentrate analytes while removing interfering matrix components. Derivatization strategies can improve volatility for GC-MS analysis or introduce chromophores for UV/VIS detection. These pretreatment approaches can lower detection limits by orders of magnitude and improve selectivity, enabling the analysis of trace compounds in complex matrices.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The GC-MS vs UV/VIS derivative compounds detection market is currently in a growth phase, with increasing applications across pharmaceutical, environmental, and industrial sectors. The global analytical instrumentation market is estimated to exceed $85 billion, with spectroscopy and chromatography segments showing robust annual growth rates of 5-7%. Leading players include established analytical instrument manufacturers like Agilent Technologies, Shimadzu Corporation, and LECO Corp, who have developed advanced GC-MS systems with enhanced sensitivity and specificity for derivative compound detection. Research institutions such as California Institute of Technology and specialized companies like Spectra Analysis Instruments are driving innovation through novel detection methodologies. The technology landscape shows varying maturity levels, with GC-MS representing a more mature but continuously evolving technology compared to newer UV/VIS applications for derivative compound analysis.
Shimadzu Corp.
Technical Solution: Shimadzu has pioneered a dual-technology approach combining their GCMS-TQ8050 NX triple quadrupole system with their UV-1900i spectrophotometer for comprehensive derivative compound analysis. Their technology utilizes automated sample preparation with pre-column derivatization capabilities, allowing for improved detection of non-volatile compounds. Shimadzu's LabSolutions software platform integrates data from both technologies, providing unified analysis workflows. Their GC-MS systems feature a unique ion source design that minimizes contamination from derivatization reagents, extending maintenance intervals by approximately 30%. For UV/VIS applications, they've developed specialized derivative spectroscopy techniques that enhance sensitivity through mathematical manipulation of spectral data, improving detection limits by up to 40% compared to conventional methods[2][4]. Their systems also incorporate automated method development tools that optimize separation parameters for derivative compounds.
Strengths: Excellent integration between GC-MS and UV/VIS platforms, robust ion source design resistant to derivatization reagents, and comprehensive software for unified data analysis. Weaknesses: Complex system configuration requires significant laboratory space, higher power consumption compared to standalone systems, and requires specialized training for optimal utilization.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed integrated GC-MS and UV/VIS solutions specifically optimized for derivative compound detection. Their flagship 8890 GC system coupled with their 5977 MS detector offers industry-leading sensitivity with detection limits in the femtogram range for derivatized compounds. Their approach combines chemical derivatization techniques with advanced mass spectral libraries containing over 750,000 compounds for improved identification accuracy. Agilent's MassHunter software incorporates specialized algorithms for derivative peak deconvolution and quantification, allowing automated processing of complex samples with multiple derivatives. Their technology enables simultaneous analysis of both derivatized and non-derivatized compounds in a single run, with retention time locking technology ensuring consistent results across different instruments and laboratories[1][3].
Strengths: Superior sensitivity for trace analysis, comprehensive spectral libraries specifically for derivatives, and advanced software integration. Weaknesses: Higher initial investment cost compared to standalone UV/VIS systems, requires more specialized training for operators, and has higher operational costs for consumables and maintenance.
Key Technological Innovations in Detection Sensitivity
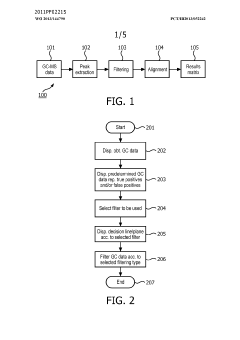
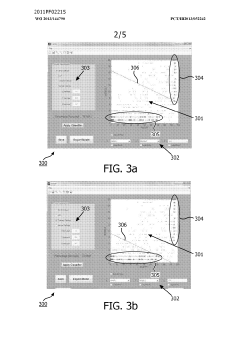
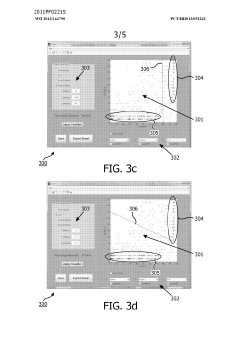
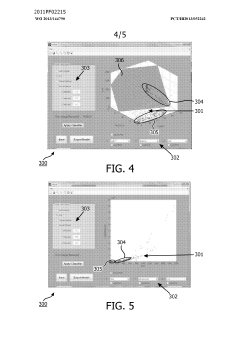
Method and system for filtering gas chromatography-mass spectrometry data
PatentWO2013144790A1
Innovation
- A method and system for filtering GC-MS data that distinguishes between true and false positives, allowing users to visually select filtering methods based on predetermined data structures and decision lines or planes, reducing data noise and improving processing efficiency.
Hydrocarbon markers
PatentInactiveIE20130364A1
Innovation
- The use of alkoxy naphthalene derivatives, specifically 2-methoxynaphthalene and its analogs, which are highly soluble, stable, and resistant to chemical laundering, allowing for specific detection and measurement in complex matrices, and are compatible with combustion processes.
Method Validation and Standardization Protocols
Method validation is a critical component in analytical chemistry, particularly when comparing sophisticated techniques like GC-MS and UV/VIS for derivative compounds detection. Standardized validation protocols ensure that analytical methods produce reliable, reproducible, and accurate results across different laboratories and testing conditions.
For GC-MS analysis of derivative compounds, validation protocols typically include assessment of specificity, linearity, accuracy, precision, limit of detection (LOD), limit of quantification (LOQ), and robustness. The International Conference on Harmonization (ICH) guidelines recommend a minimum of five concentration levels for calibration curves when establishing linearity. System suitability tests must be performed before each analytical run to verify chromatographic parameters such as resolution, tailing factor, and theoretical plate count.
UV/VIS spectroscopy validation follows similar principles but requires additional considerations for derivative compounds. Validation protocols must address potential interference from matrix components and verify that derivatization reactions proceed to completion consistently. Stability of derivatized compounds under analysis conditions must be established through forced degradation studies, with acceptance criteria typically set at ≥98% recovery after predetermined time intervals.
Interlaboratory comparison studies represent a crucial aspect of method standardization for both techniques. These studies should include at least six participating laboratories analyzing identical samples to establish reproducibility limits. Statistical evaluation using ANOVA or Youden plots helps identify systematic errors and determine method transferability across different instrument configurations.
Uncertainty measurement calculations must be incorporated into validation protocols, following ISO/IEC 17025 requirements. For derivative compound analysis, this includes accounting for uncertainties in derivatization efficiency, which can significantly impact final results. Measurement uncertainty budgets should document all potential error sources, with expanded uncertainty typically reported at 95% confidence level (k=2).
Revalidation triggers must be clearly defined in standardization protocols. These include changes to critical reagents, instrument components, or software versions. Partial revalidation may be sufficient for minor changes, while major modifications require comprehensive revalidation. Documentation standards should specify minimum requirements for validation reports, including raw data retention policies and electronic data integrity measures compliant with 21 CFR Part 11 or equivalent regulations.
For GC-MS analysis of derivative compounds, validation protocols typically include assessment of specificity, linearity, accuracy, precision, limit of detection (LOD), limit of quantification (LOQ), and robustness. The International Conference on Harmonization (ICH) guidelines recommend a minimum of five concentration levels for calibration curves when establishing linearity. System suitability tests must be performed before each analytical run to verify chromatographic parameters such as resolution, tailing factor, and theoretical plate count.
UV/VIS spectroscopy validation follows similar principles but requires additional considerations for derivative compounds. Validation protocols must address potential interference from matrix components and verify that derivatization reactions proceed to completion consistently. Stability of derivatized compounds under analysis conditions must be established through forced degradation studies, with acceptance criteria typically set at ≥98% recovery after predetermined time intervals.
Interlaboratory comparison studies represent a crucial aspect of method standardization for both techniques. These studies should include at least six participating laboratories analyzing identical samples to establish reproducibility limits. Statistical evaluation using ANOVA or Youden plots helps identify systematic errors and determine method transferability across different instrument configurations.
Uncertainty measurement calculations must be incorporated into validation protocols, following ISO/IEC 17025 requirements. For derivative compound analysis, this includes accounting for uncertainties in derivatization efficiency, which can significantly impact final results. Measurement uncertainty budgets should document all potential error sources, with expanded uncertainty typically reported at 95% confidence level (k=2).
Revalidation triggers must be clearly defined in standardization protocols. These include changes to critical reagents, instrument components, or software versions. Partial revalidation may be sufficient for minor changes, while major modifications require comprehensive revalidation. Documentation standards should specify minimum requirements for validation reports, including raw data retention policies and electronic data integrity measures compliant with 21 CFR Part 11 or equivalent regulations.
Environmental and Safety Considerations
The environmental and safety considerations of analytical techniques are increasingly important in modern laboratory practices. When comparing GC-MS and UV/VIS spectroscopy for derivative compounds detection, several key environmental and safety aspects must be evaluated. GC-MS systems typically require significant energy consumption due to their high operating temperatures and vacuum systems, resulting in a larger carbon footprint compared to UV/VIS spectrophotometers which operate at ambient conditions with lower power requirements.
Solvent usage represents a major environmental concern for both techniques. GC-MS often requires organic solvents like hexane, dichloromethane, and acetone for sample preparation and analysis, many of which are classified as hazardous waste. UV/VIS spectroscopy generally uses less toxic solvents such as water, ethanol, or methanol, though certain applications may still require more problematic solvents. The volume of waste generated by GC-MS is typically higher due to the extensive sample preparation procedures often required.
Gas consumption is another environmental factor specific to GC-MS, which requires carrier gases such as helium, hydrogen, or nitrogen. Helium, commonly used in GC-MS, is a non-renewable resource facing global supply challenges. UV/VIS spectroscopy does not require these gases, giving it an advantage in this aspect.
From a safety perspective, GC-MS presents more significant hazards. The high temperatures (up to 350°C) in the GC oven and potential for gas leaks, particularly when using hydrogen as a carrier gas, create fire and explosion risks. Mass spectrometers also operate under vacuum conditions and utilize high voltages, requiring careful handling and maintenance. UV/VIS instruments generally pose fewer safety risks, though proper handling of UV radiation sources is necessary to prevent exposure.
Chemical exposure risks exist for both techniques but differ in nature. GC-MS often involves more hazardous chemicals during sample derivatization processes, which may include toxic reagents like BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide) or MTBSTFA. UV/VIS sample preparation typically involves less hazardous reagents, though this depends on the specific application and derivative formation methods employed.
Laboratory waste management protocols must be more stringent for GC-MS operations due to the higher volume and toxicity of waste generated. This includes proper disposal of used columns, septa, liners, and spent solvents. Both techniques require adherence to local environmental regulations and waste disposal guidelines, with GC-MS typically demanding more comprehensive waste management systems.
Solvent usage represents a major environmental concern for both techniques. GC-MS often requires organic solvents like hexane, dichloromethane, and acetone for sample preparation and analysis, many of which are classified as hazardous waste. UV/VIS spectroscopy generally uses less toxic solvents such as water, ethanol, or methanol, though certain applications may still require more problematic solvents. The volume of waste generated by GC-MS is typically higher due to the extensive sample preparation procedures often required.
Gas consumption is another environmental factor specific to GC-MS, which requires carrier gases such as helium, hydrogen, or nitrogen. Helium, commonly used in GC-MS, is a non-renewable resource facing global supply challenges. UV/VIS spectroscopy does not require these gases, giving it an advantage in this aspect.
From a safety perspective, GC-MS presents more significant hazards. The high temperatures (up to 350°C) in the GC oven and potential for gas leaks, particularly when using hydrogen as a carrier gas, create fire and explosion risks. Mass spectrometers also operate under vacuum conditions and utilize high voltages, requiring careful handling and maintenance. UV/VIS instruments generally pose fewer safety risks, though proper handling of UV radiation sources is necessary to prevent exposure.
Chemical exposure risks exist for both techniques but differ in nature. GC-MS often involves more hazardous chemicals during sample derivatization processes, which may include toxic reagents like BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide) or MTBSTFA. UV/VIS sample preparation typically involves less hazardous reagents, though this depends on the specific application and derivative formation methods employed.
Laboratory waste management protocols must be more stringent for GC-MS operations due to the higher volume and toxicity of waste generated. This includes proper disposal of used columns, septa, liners, and spent solvents. Both techniques require adherence to local environmental regulations and waste disposal guidelines, with GC-MS typically demanding more comprehensive waste management systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!