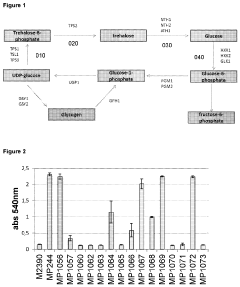
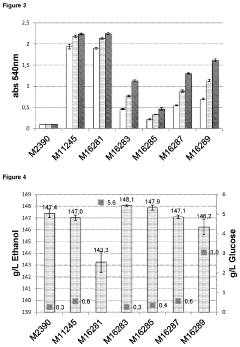
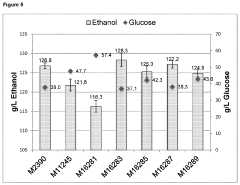
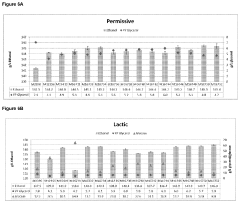
Glycogenolysis Pathway: Control and Regulation
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Pathway Background and Objectives
Glycogenolysis, the biochemical pathway responsible for the breakdown of glycogen into glucose-1-phosphate and glucose, represents a critical metabolic process in maintaining blood glucose homeostasis. This pathway has evolved over millions of years as a fundamental survival mechanism, allowing organisms to access stored energy during periods of metabolic demand. The historical development of our understanding of glycogenolysis dates back to the early 20th century, with significant contributions from scientists like Carl and Gerty Cori, who elucidated the cyclical nature of glycogen metabolism.
The technical evolution of glycogenolysis research has progressed from basic biochemical characterization to sophisticated molecular and structural analyses. Early studies focused on identifying the enzymes involved, while recent advances have revealed intricate regulatory networks that control this pathway at multiple levels. The integration of systems biology approaches has further enhanced our understanding of how glycogenolysis responds to various physiological and pathological conditions.
Current trends in glycogenolysis research are moving toward precision medicine applications, particularly in metabolic disorders like glycogen storage diseases, diabetes, and certain cancers where abnormal glycogen metabolism plays a role. The emergence of computational modeling and artificial intelligence tools has accelerated our ability to predict pathway behaviors under various conditions, opening new avenues for therapeutic interventions.
The primary technical objectives of this investigation include: comprehensively mapping the regulatory mechanisms controlling glycogenolysis across different tissues; identifying novel molecular targets for therapeutic intervention; developing innovative analytical methods for real-time monitoring of glycogenolysis in vivo; and establishing predictive models that integrate glycogenolysis with other metabolic pathways.
Additionally, we aim to explore the evolutionary conservation of glycogenolysis regulation across species, which may reveal fundamental principles of metabolic control. Understanding tissue-specific variations in glycogenolysis regulation presents another critical objective, as these differences underlie the diverse metabolic responses observed in liver, muscle, and brain tissues under various physiological states.
The long-term goal is to translate this enhanced understanding into practical applications, including novel diagnostic tools for metabolic disorders, targeted therapeutics that modulate specific aspects of the pathway, and personalized nutritional strategies that optimize glycogen utilization based on individual metabolic profiles.
The technical evolution of glycogenolysis research has progressed from basic biochemical characterization to sophisticated molecular and structural analyses. Early studies focused on identifying the enzymes involved, while recent advances have revealed intricate regulatory networks that control this pathway at multiple levels. The integration of systems biology approaches has further enhanced our understanding of how glycogenolysis responds to various physiological and pathological conditions.
Current trends in glycogenolysis research are moving toward precision medicine applications, particularly in metabolic disorders like glycogen storage diseases, diabetes, and certain cancers where abnormal glycogen metabolism plays a role. The emergence of computational modeling and artificial intelligence tools has accelerated our ability to predict pathway behaviors under various conditions, opening new avenues for therapeutic interventions.
The primary technical objectives of this investigation include: comprehensively mapping the regulatory mechanisms controlling glycogenolysis across different tissues; identifying novel molecular targets for therapeutic intervention; developing innovative analytical methods for real-time monitoring of glycogenolysis in vivo; and establishing predictive models that integrate glycogenolysis with other metabolic pathways.
Additionally, we aim to explore the evolutionary conservation of glycogenolysis regulation across species, which may reveal fundamental principles of metabolic control. Understanding tissue-specific variations in glycogenolysis regulation presents another critical objective, as these differences underlie the diverse metabolic responses observed in liver, muscle, and brain tissues under various physiological states.
The long-term goal is to translate this enhanced understanding into practical applications, including novel diagnostic tools for metabolic disorders, targeted therapeutics that modulate specific aspects of the pathway, and personalized nutritional strategies that optimize glycogen utilization based on individual metabolic profiles.
Market Applications and Therapeutic Demand Analysis
The glycogenolysis pathway represents a significant therapeutic target across multiple disease areas, with substantial market potential in diabetes management, rare metabolic disorders, and exercise performance enhancement. The global diabetes therapeutics market, where glycogenolysis regulation plays a crucial role, was valued at approximately $58 billion in 2022 and is projected to reach $78 billion by 2027, growing at a CAGR of 6.1%. This growth is primarily driven by the increasing prevalence of diabetes worldwide, with over 537 million adults currently living with the condition.
Glycogen storage diseases (GSDs), though rare, present a specialized market segment with high unmet needs. The global orphan drug market for these conditions is expanding rapidly, with treatments for disorders like von Gierke disease (GSD type I) and Pompe disease (GSD type II) commanding premium pricing due to their specialized nature and limited patient populations. The market for GSD therapeutics is estimated to be growing at 8-10% annually, reflecting increased diagnosis rates and improved treatment options.
Sports nutrition and performance enhancement represent an emerging application area for glycogenolysis pathway modulation. The global sports nutrition market exceeded $15 billion in 2022, with products targeting glycogen metabolism for improved endurance and recovery gaining significant traction among both professional athletes and fitness enthusiasts. Compounds that can safely optimize glycogen utilization during exercise are particularly sought after in this segment.
From a pharmaceutical development perspective, several major companies have active research programs targeting glycogenolysis regulation, including Pfizer, Novo Nordisk, and Sanofi. These efforts focus primarily on developing selective enzyme inhibitors and activators that can modulate the pathway with minimal off-target effects. The pipeline for such therapeutics includes both small molecule approaches and emerging biological therapies.
Healthcare providers increasingly recognize the importance of glycogen metabolism in managing conditions beyond diabetes, including cardiovascular disease and certain neurological disorders. This expanding therapeutic scope has created new market opportunities for diagnostic tools that can assess glycogenolysis pathway function, with the global market for metabolic testing estimated to be growing at 7-8% annually.
Patient advocacy groups for rare metabolic disorders have also become influential stakeholders, driving both research funding and regulatory accommodations for novel therapeutics targeting glycogenolysis. Their involvement has helped accelerate clinical development timelines and create specialized market access pathways for treatments addressing these conditions.
Glycogen storage diseases (GSDs), though rare, present a specialized market segment with high unmet needs. The global orphan drug market for these conditions is expanding rapidly, with treatments for disorders like von Gierke disease (GSD type I) and Pompe disease (GSD type II) commanding premium pricing due to their specialized nature and limited patient populations. The market for GSD therapeutics is estimated to be growing at 8-10% annually, reflecting increased diagnosis rates and improved treatment options.
Sports nutrition and performance enhancement represent an emerging application area for glycogenolysis pathway modulation. The global sports nutrition market exceeded $15 billion in 2022, with products targeting glycogen metabolism for improved endurance and recovery gaining significant traction among both professional athletes and fitness enthusiasts. Compounds that can safely optimize glycogen utilization during exercise are particularly sought after in this segment.
From a pharmaceutical development perspective, several major companies have active research programs targeting glycogenolysis regulation, including Pfizer, Novo Nordisk, and Sanofi. These efforts focus primarily on developing selective enzyme inhibitors and activators that can modulate the pathway with minimal off-target effects. The pipeline for such therapeutics includes both small molecule approaches and emerging biological therapies.
Healthcare providers increasingly recognize the importance of glycogen metabolism in managing conditions beyond diabetes, including cardiovascular disease and certain neurological disorders. This expanding therapeutic scope has created new market opportunities for diagnostic tools that can assess glycogenolysis pathway function, with the global market for metabolic testing estimated to be growing at 7-8% annually.
Patient advocacy groups for rare metabolic disorders have also become influential stakeholders, driving both research funding and regulatory accommodations for novel therapeutics targeting glycogenolysis. Their involvement has helped accelerate clinical development timelines and create specialized market access pathways for treatments addressing these conditions.
Current Understanding and Technical Challenges
The glycogenolysis pathway represents a critical metabolic process for energy mobilization in various tissues, particularly in liver and muscle. Current scientific understanding has established that this pathway involves the sequential breakdown of glycogen to glucose-1-phosphate through the action of glycogen phosphorylase, followed by conversion to glucose-6-phosphate. In liver tissue, glucose-6-phosphatase subsequently converts this intermediate to free glucose for release into the bloodstream, while muscle tissue lacks this enzyme and utilizes the glucose-6-phosphate directly for glycolysis.
Recent advances in structural biology have elucidated the allosteric regulation mechanisms of glycogen phosphorylase, revealing distinct T (tense, less active) and R (relaxed, more active) conformational states. These conformational changes are influenced by various metabolic signals including AMP, ATP, glucose, and glucose-6-phosphate, allowing for precise control of glycogenolysis in response to cellular energy status.
Despite these advances, significant technical challenges persist in fully understanding the integrated regulation of glycogenolysis. One major challenge involves the real-time monitoring of pathway flux in living tissues, as current methodologies often rely on static measurements or require tissue disruption. Innovative approaches using metabolic tracers and advanced imaging techniques are being developed but remain limited in spatial and temporal resolution.
Another substantial challenge lies in understanding tissue-specific regulation patterns. While liver and muscle glycogenolysis have been extensively studied, the regulation in other tissues such as brain, kidney, and adipose tissue remains poorly characterized. The interplay between hormonal signals (insulin, glucagon, epinephrine) and local metabolic factors creates a complex regulatory network that varies significantly between tissues and physiological states.
The integration of glycogenolysis with other metabolic pathways presents additional complexity. Cross-talk between glycogenolysis, glycolysis, gluconeogenesis, and the pentose phosphate pathway involves numerous feedback loops and shared intermediates, making it difficult to isolate and study glycogenolysis in isolation. Systems biology approaches are being applied to address this challenge, but computational models still struggle to accurately predict pathway behavior under diverse physiological conditions.
Technical limitations in studying post-translational modifications of regulatory enzymes also hinder comprehensive understanding. Phosphorylation, acetylation, and other modifications can rapidly alter enzyme activity, but detecting these changes in real-time remains technically challenging. Emerging proteomic technologies show promise but require further refinement for studying dynamic regulatory processes.
Recent advances in structural biology have elucidated the allosteric regulation mechanisms of glycogen phosphorylase, revealing distinct T (tense, less active) and R (relaxed, more active) conformational states. These conformational changes are influenced by various metabolic signals including AMP, ATP, glucose, and glucose-6-phosphate, allowing for precise control of glycogenolysis in response to cellular energy status.
Despite these advances, significant technical challenges persist in fully understanding the integrated regulation of glycogenolysis. One major challenge involves the real-time monitoring of pathway flux in living tissues, as current methodologies often rely on static measurements or require tissue disruption. Innovative approaches using metabolic tracers and advanced imaging techniques are being developed but remain limited in spatial and temporal resolution.
Another substantial challenge lies in understanding tissue-specific regulation patterns. While liver and muscle glycogenolysis have been extensively studied, the regulation in other tissues such as brain, kidney, and adipose tissue remains poorly characterized. The interplay between hormonal signals (insulin, glucagon, epinephrine) and local metabolic factors creates a complex regulatory network that varies significantly between tissues and physiological states.
The integration of glycogenolysis with other metabolic pathways presents additional complexity. Cross-talk between glycogenolysis, glycolysis, gluconeogenesis, and the pentose phosphate pathway involves numerous feedback loops and shared intermediates, making it difficult to isolate and study glycogenolysis in isolation. Systems biology approaches are being applied to address this challenge, but computational models still struggle to accurately predict pathway behavior under diverse physiological conditions.
Technical limitations in studying post-translational modifications of regulatory enzymes also hinder comprehensive understanding. Phosphorylation, acetylation, and other modifications can rapidly alter enzyme activity, but detecting these changes in real-time remains technically challenging. Emerging proteomic technologies show promise but require further refinement for studying dynamic regulatory processes.
Established Regulatory Mechanisms and Pathways
01 Hormonal regulation of glycogenolysis
Hormones play a crucial role in regulating glycogenolysis, the breakdown of glycogen to glucose. Hormones such as glucagon, epinephrine, and cortisol can activate glycogenolysis during periods of fasting or stress. These hormones bind to specific receptors on cell surfaces, triggering signaling cascades that ultimately activate glycogen phosphorylase, the key enzyme in glycogenolysis. This hormonal control ensures glucose homeostasis by releasing glucose into the bloodstream when needed.- Hormonal regulation of glycogenolysis: Hormones play a crucial role in regulating glycogenolysis, the breakdown of glycogen to glucose. Hormones such as glucagon, epinephrine, and norepinephrine activate signaling cascades that lead to the phosphorylation and activation of glycogen phosphorylase, the key enzyme in glycogenolysis. These hormonal pathways involve G-protein coupled receptors, adenylyl cyclase activation, and protein kinase A-mediated phosphorylation events that ultimately increase glucose production during periods of metabolic need.
- Enzyme-mediated control of glycogen breakdown: The glycogenolysis pathway is tightly controlled by several enzymes, with glycogen phosphorylase being the rate-limiting enzyme. This enzyme exists in active (phosphorylated) and inactive (dephosphorylated) forms. Other regulatory enzymes include phosphorylase kinase, which activates glycogen phosphorylase, and protein phosphatase-1, which inactivates it. Allosteric regulation by metabolites such as AMP, ATP, and glucose-6-phosphate further fine-tunes the pathway according to cellular energy status.
- Metabolic disorders related to glycogenolysis regulation: Dysregulation of the glycogenolysis pathway is associated with various metabolic disorders. Glycogen storage diseases result from genetic defects in enzymes involved in glycogen metabolism, leading to abnormal glycogen accumulation or utilization. Diabetes mellitus involves inappropriate activation of glycogenolysis, contributing to hyperglycemia. Understanding the regulatory mechanisms of glycogenolysis has led to the development of therapeutic approaches targeting specific control points in the pathway to treat these disorders.
- Computational models for glycogenolysis pathway analysis: Advanced computational models have been developed to analyze and predict the behavior of the glycogenolysis pathway under various conditions. These models integrate data on enzyme kinetics, metabolite concentrations, and regulatory interactions to simulate pathway dynamics. Machine learning algorithms and systems biology approaches are being used to identify key control points and predict responses to perturbations, facilitating drug discovery and personalized medicine approaches for disorders involving glycogen metabolism.
- Novel therapeutic targets in glycogenolysis regulation: Research has identified novel targets for therapeutic intervention in the glycogenolysis pathway. These include specific phosphorylation sites on regulatory proteins, allosteric binding sites on enzymes, and components of signaling cascades that control glycogen breakdown. Small molecule inhibitors, peptide-based drugs, and gene therapy approaches are being developed to modulate these targets, offering potential treatments for diabetes, hypoglycemia, and glycogen storage diseases by precisely controlling the rate of glycogen breakdown.
02 Enzymatic control mechanisms in glycogenolysis
The glycogenolysis pathway is tightly regulated by various enzymes, with glycogen phosphorylase being the rate-limiting enzyme. This enzyme exists in active (phosphorylated) and inactive (dephosphorylated) forms. Phosphorylation by protein kinases activates glycogen phosphorylase, while dephosphorylation by protein phosphatases inactivates it. Additionally, allosteric regulators such as AMP, ATP, and glucose-6-phosphate can modulate enzyme activity, providing fine-tuned control of glycogen breakdown based on cellular energy status.Expand Specific Solutions03 Metabolic signaling pathways in glycogenolysis regulation
Glycogenolysis is regulated through complex signaling pathways that integrate various metabolic signals. The cAMP-dependent protein kinase A (PKA) pathway is activated by hormones and leads to phosphorylation of glycogen phosphorylase. Calcium-dependent pathways, activated by neural stimulation, also play a role in glycogenolysis regulation, particularly in muscle tissue. These signaling cascades allow for rapid response to changing energy demands and coordinate glycogenolysis with other metabolic processes.Expand Specific Solutions04 Pathological alterations in glycogenolysis control
Dysregulation of glycogenolysis control mechanisms can lead to various pathological conditions. Glycogen storage diseases result from genetic defects in enzymes involved in glycogen metabolism, leading to abnormal glycogen accumulation or utilization. Diabetes mellitus involves impaired glycogenolysis regulation due to insulin resistance or deficiency. Understanding these pathological alterations provides insights into potential therapeutic targets for metabolic disorders and helps in developing diagnostic methods for early detection.Expand Specific Solutions05 Computational models and analytical methods for glycogenolysis pathway analysis
Advanced computational models and analytical methods have been developed to study the complex regulation of the glycogenolysis pathway. These include systems biology approaches that integrate multi-omics data, machine learning algorithms for predicting pathway behavior, and mathematical modeling of enzyme kinetics. These computational tools help in understanding the dynamic regulation of glycogenolysis under various physiological conditions and can predict responses to perturbations, facilitating drug discovery and development of personalized therapeutic strategies.Expand Specific Solutions
Key Research Institutions and Pharmaceutical Players
The glycogenolysis pathway regulation market is currently in a growth phase, with increasing research focus on metabolic disorders and diabetes management. The global market size for related therapeutics is expanding, driven by rising prevalence of metabolic diseases. Leading pharmaceutical companies like Novo Nordisk, Janssen Pharmaceutica, and F. Hoffmann-La Roche are at the forefront of technology development, with varying degrees of maturity in their glycogenolysis-targeting approaches. Academic institutions including MIT and National University of Singapore are contributing fundamental research, while companies like AbbVie and Merck are advancing clinical applications. The technology shows promising maturity in diabetes applications but remains emerging in other therapeutic areas, with competition intensifying as understanding of pathway control mechanisms deepens.
AbbVie, Inc.
Technical Solution: AbbVie has developed a novel approach to glycogenolysis regulation focusing on the intersection between inflammatory pathways and metabolic control. Their technology platform targets the JAK/STAT signaling pathway, which they have shown plays a previously underappreciated role in modulating glycogen phosphorylase activity through post-translational modifications. AbbVie's proprietary small molecule inhibitors selectively block specific JAK isoforms that influence glycogenolysis without broadly suppressing inflammatory responses. Their research has demonstrated that this approach can reduce hepatic glucose output while simultaneously addressing the inflammatory component of metabolic disorders. Additionally, AbbVie has explored combination therapies that pair their JAK inhibitors with direct glycogen phosphorylase modulators, creating a multi-modal approach to glycogenolysis regulation. This strategy has shown promise in preclinical models of both type 2 diabetes and non-alcoholic steatohepatitis (NASH), where dysregulated glycogenolysis contributes to disease pathology.
Strengths: Innovative approach targeting the inflammation-metabolism interface provides unique mechanism of action; potential for addressing multiple aspects of metabolic diseases simultaneously. Weaknesses: Complex interplay between inflammatory and metabolic pathways may lead to unpredictable effects; relatively newer approach with less established clinical validation compared to direct enzyme inhibition.
Hoffmann-La Roche, Inc.
Technical Solution: Hoffmann-La Roche has developed innovative approaches to modulate glycogenolysis through targeting the allosteric regulation of glycogen phosphorylase. Their technology platform includes small molecule inhibitors that bind to the AMP allosteric site, the indole inhibitor site, and the purine nucleoside site of glycogen phosphorylase. These compounds prevent the conformational changes necessary for enzyme activation, thereby reducing glycogen breakdown and hepatic glucose output. Roche's research has demonstrated that their lead compounds can reduce blood glucose levels by up to 40% in preclinical models of type 2 diabetes. Additionally, they have explored the use of antisense oligonucleotides to reduce glycogen phosphorylase expression at the genetic level, providing a complementary approach to their small molecule inhibitors. Their dual-targeting strategy aims to provide more comprehensive control over glycogenolysis in conditions of metabolic dysregulation, with potential applications in diabetes, glycogen storage diseases, and certain cancers where glycogen metabolism is altered.
Strengths: Comprehensive approach targeting both enzyme activity and expression provides multiple mechanisms for pathway regulation; extensive experience in developing small molecule therapeutics facilitates clinical translation. Weaknesses: Potential for off-target effects due to structural similarities between glycogen phosphorylase isoforms; challenges in achieving sufficient tissue penetration for antisense therapeutics.
Critical Enzymes and Signaling Molecules Analysis
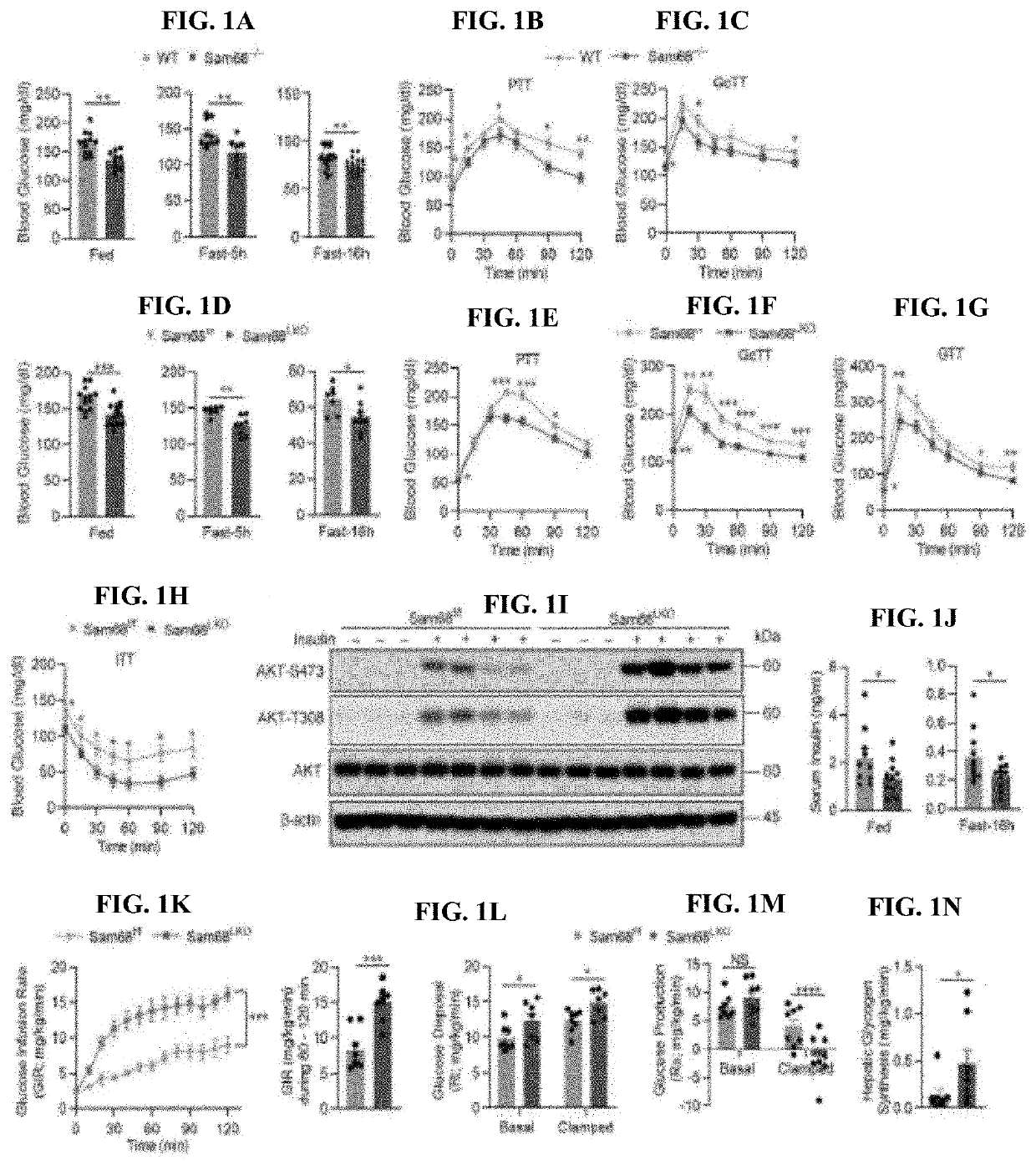
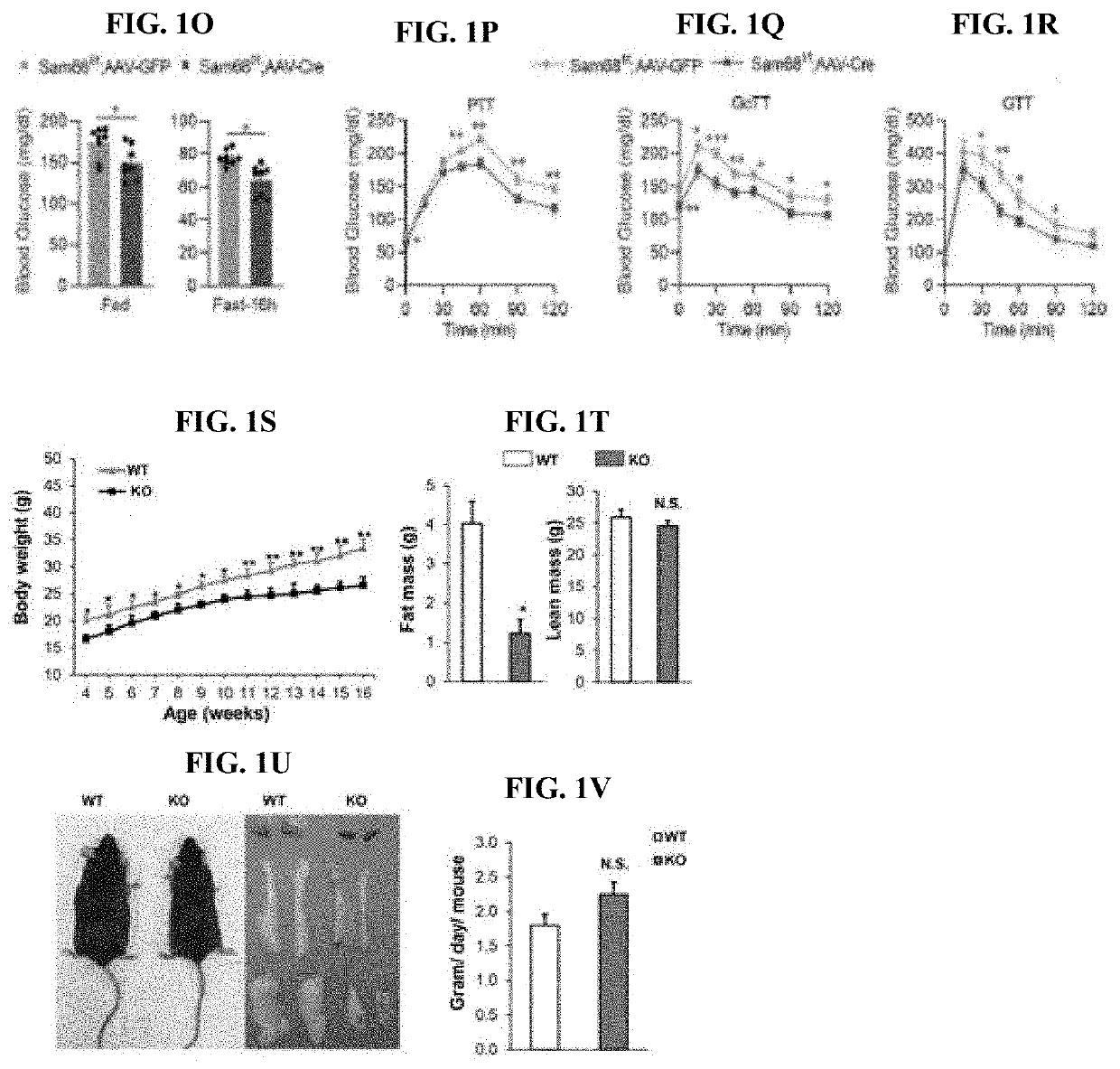
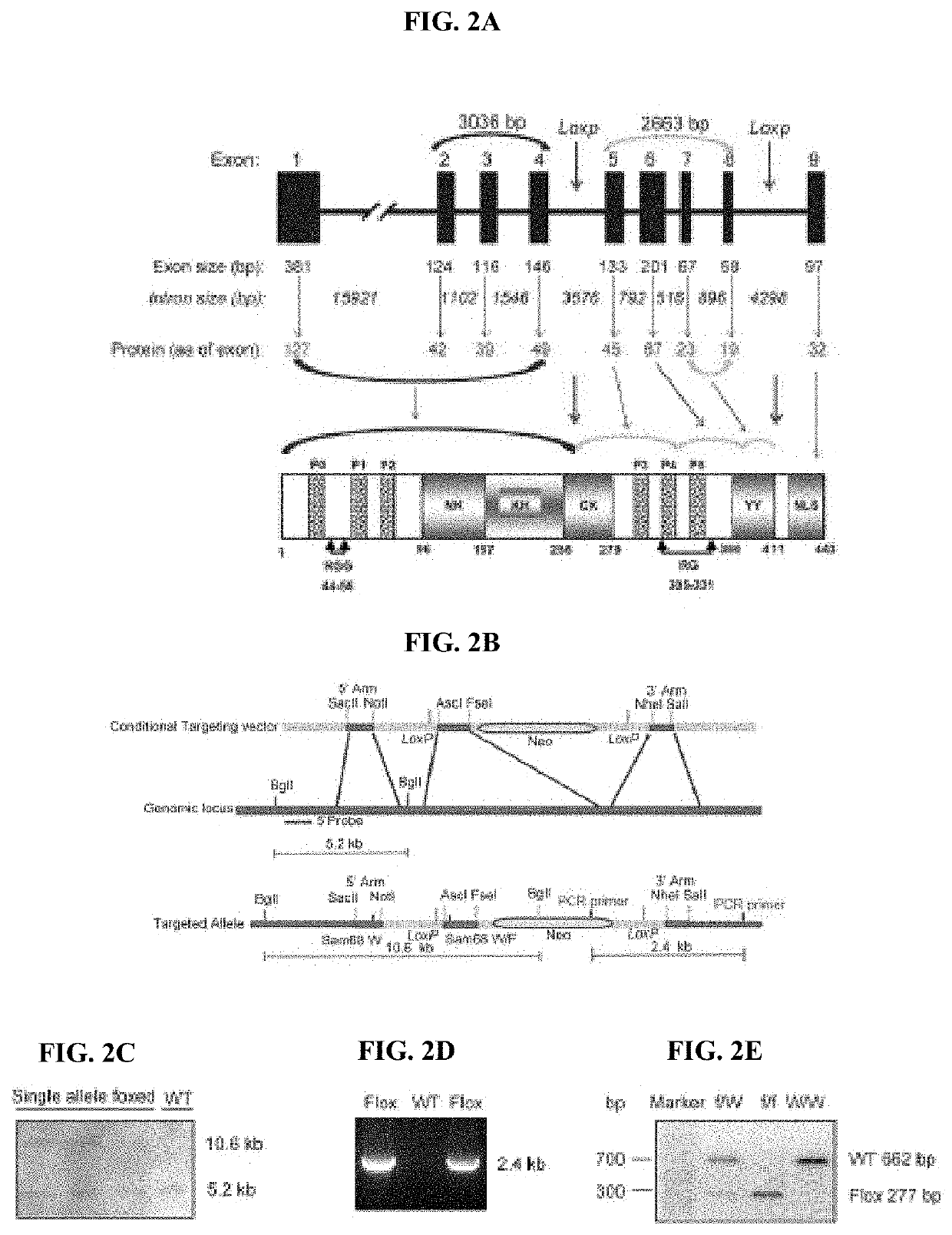
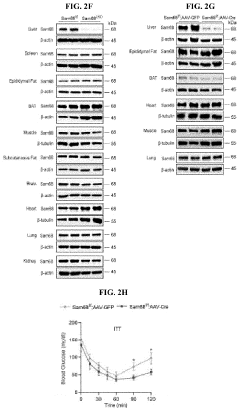
Compositions and methods for treatment of diabetes, obesity, hyper-cholesterolemia, and atherosclerosis by inhibition of sam68
PatentInactiveUS20220119511A1
Innovation
- Targeting Sam68, an RNA-binding adaptor protein, through inhibitors such as small molecules, peptides, antibodies, or RNA molecules like shRNA and siRNA to reduce hepatic gluconeogenesis by decreasing CRTC2 protein stability and gluconeogenic gene transcription, thereby lowering blood glucose levels and improving insulin sensitivity.
Combined expression of trehalose producing and trehalose degrading enzymes
PatentInactiveUS20220220487A1
Innovation
- A recombinant yeast host cell is developed with two genetic modifications: one for expressing a heterologous trehalase to degrade trehalose outside the cell and another for increasing trehalose production inside the cell, using enzymes like TPS1 and TPS2, to enhance fermentation yield and maintain robustness.
Metabolic Disorders and Clinical Implications
Disruptions in the glycogenolysis pathway are implicated in several metabolic disorders with significant clinical consequences. Glycogen Storage Diseases (GSDs) represent the most direct manifestation of glycogenolysis dysfunction, with at least 15 different types identified, each resulting from specific enzyme deficiencies within the pathway. Type Ia (von Gierke disease), caused by glucose-6-phosphatase deficiency, presents with severe hypoglycemia, hepatomegaly, growth retardation, and lactic acidosis, requiring careful dietary management with frequent feeding and cornstarch supplementation.
Type III (Cori disease), resulting from debranching enzyme deficiency, affects both liver and muscle tissues, leading to hepatomegaly, hypoglycemia, and progressive myopathy. Type V (McArdle disease) specifically impacts skeletal muscle due to myophosphorylase deficiency, causing exercise intolerance, premature fatigue, and the characteristic "second wind" phenomenon where patients experience improved exercise capacity after initial symptoms.
Beyond classical GSDs, dysregulated glycogenolysis contributes to broader metabolic conditions. In diabetes mellitus, particularly type 2, excessive hepatic glucose production via enhanced glycogenolysis and gluconeogenesis exacerbates hyperglycemia, creating a vicious cycle of insulin resistance and metabolic dysfunction. Current therapeutic approaches increasingly target these pathways, with medications like metformin partially acting through modulation of hepatic glucose output.
Hypoglycemic disorders represent another clinical manifestation where glycogenolysis plays a crucial role. Conditions such as hyperinsulinism, hormone deficiencies (cortisol, growth hormone), or enzymatic defects can impair the normal glycogenolytic response to falling blood glucose levels, resulting in dangerous hypoglycemic episodes. This is particularly concerning in pediatric populations and during prolonged fasting states.
Emerging research has identified connections between glycogenolysis dysregulation and neurodegenerative disorders. The brain, while primarily dependent on glucose, maintains small glycogen reserves predominantly in astrocytes. Disruptions in this cerebral energy metabolism may contribute to conditions like Alzheimer's disease, where altered brain energy utilization precedes clinical symptoms.
Clinical management of glycogenolysis-related disorders requires a multidisciplinary approach. Diagnosis typically involves enzyme assays, genetic testing, and tissue biopsies. Treatment strategies range from dietary interventions (frequent meals, uncooked cornstarch) to enzyme replacement therapies and emerging gene therapy approaches. Recent advances in CRISPR-Cas9 technology offer promising avenues for correcting the underlying genetic defects in glycogen metabolism disorders, potentially transforming treatment paradigms in the coming decade.
Type III (Cori disease), resulting from debranching enzyme deficiency, affects both liver and muscle tissues, leading to hepatomegaly, hypoglycemia, and progressive myopathy. Type V (McArdle disease) specifically impacts skeletal muscle due to myophosphorylase deficiency, causing exercise intolerance, premature fatigue, and the characteristic "second wind" phenomenon where patients experience improved exercise capacity after initial symptoms.
Beyond classical GSDs, dysregulated glycogenolysis contributes to broader metabolic conditions. In diabetes mellitus, particularly type 2, excessive hepatic glucose production via enhanced glycogenolysis and gluconeogenesis exacerbates hyperglycemia, creating a vicious cycle of insulin resistance and metabolic dysfunction. Current therapeutic approaches increasingly target these pathways, with medications like metformin partially acting through modulation of hepatic glucose output.
Hypoglycemic disorders represent another clinical manifestation where glycogenolysis plays a crucial role. Conditions such as hyperinsulinism, hormone deficiencies (cortisol, growth hormone), or enzymatic defects can impair the normal glycogenolytic response to falling blood glucose levels, resulting in dangerous hypoglycemic episodes. This is particularly concerning in pediatric populations and during prolonged fasting states.
Emerging research has identified connections between glycogenolysis dysregulation and neurodegenerative disorders. The brain, while primarily dependent on glucose, maintains small glycogen reserves predominantly in astrocytes. Disruptions in this cerebral energy metabolism may contribute to conditions like Alzheimer's disease, where altered brain energy utilization precedes clinical symptoms.
Clinical management of glycogenolysis-related disorders requires a multidisciplinary approach. Diagnosis typically involves enzyme assays, genetic testing, and tissue biopsies. Treatment strategies range from dietary interventions (frequent meals, uncooked cornstarch) to enzyme replacement therapies and emerging gene therapy approaches. Recent advances in CRISPR-Cas9 technology offer promising avenues for correcting the underlying genetic defects in glycogen metabolism disorders, potentially transforming treatment paradigms in the coming decade.
Computational Modeling and Simulation Approaches
Computational modeling and simulation approaches have revolutionized our understanding of the glycogenolysis pathway's control and regulation mechanisms. These in silico methods provide powerful tools for investigating complex biochemical processes that would be challenging to study through traditional experimental techniques alone. Various computational approaches have been developed to model the intricate dynamics of glycogenolysis at different scales, from molecular interactions to whole-cell metabolism.
Kinetic modeling represents a fundamental approach for simulating glycogenolysis regulation. These models incorporate rate equations for each enzymatic reaction in the pathway, accounting for substrate concentrations, enzyme kinetics, and regulatory effects of allosteric modulators. Software platforms such as COPASI and CellDesigner enable researchers to construct detailed kinetic models that can predict how changes in enzyme activities or metabolite concentrations affect glycogen breakdown rates under various physiological conditions.
Molecular dynamics (MD) simulations offer atomic-level insights into the structural dynamics of key regulatory enzymes in the glycogenolysis pathway. By simulating the physical movements of atoms and molecules, MD approaches reveal how conformational changes in enzymes like glycogen phosphorylase are influenced by regulatory molecules such as AMP, ATP, and glucose-6-phosphate. These simulations typically require high-performance computing resources but provide valuable mechanistic understanding of allosteric regulation.
Systems biology approaches integrate multiple data types to create comprehensive models of glycogenolysis within the broader context of cellular metabolism. Constraint-based modeling techniques, particularly flux balance analysis (FBA), have been applied to predict metabolic flux distributions during glycogenolysis under different physiological states. These models incorporate genome-scale metabolic networks and can simulate how glycogenolysis responds to various nutritional and hormonal signals.
Machine learning algorithms are increasingly being applied to analyze large datasets related to glycogenolysis regulation. Neural networks and other AI approaches can identify patterns in multi-omics data that reveal novel regulatory mechanisms or predict metabolic responses to perturbations. These data-driven models complement traditional mechanistic approaches by uncovering unexpected relationships between variables that might not be apparent from first principles.
Agent-based modeling represents an emerging approach for simulating spatial aspects of glycogenolysis regulation within cellular compartments. These models treat enzymes and metabolites as individual agents with defined behaviors, allowing researchers to investigate how subcellular localization and diffusion limitations influence pathway dynamics. Such spatial considerations are particularly relevant for understanding glycogenolysis regulation in specialized cells like hepatocytes and myocytes.
Kinetic modeling represents a fundamental approach for simulating glycogenolysis regulation. These models incorporate rate equations for each enzymatic reaction in the pathway, accounting for substrate concentrations, enzyme kinetics, and regulatory effects of allosteric modulators. Software platforms such as COPASI and CellDesigner enable researchers to construct detailed kinetic models that can predict how changes in enzyme activities or metabolite concentrations affect glycogen breakdown rates under various physiological conditions.
Molecular dynamics (MD) simulations offer atomic-level insights into the structural dynamics of key regulatory enzymes in the glycogenolysis pathway. By simulating the physical movements of atoms and molecules, MD approaches reveal how conformational changes in enzymes like glycogen phosphorylase are influenced by regulatory molecules such as AMP, ATP, and glucose-6-phosphate. These simulations typically require high-performance computing resources but provide valuable mechanistic understanding of allosteric regulation.
Systems biology approaches integrate multiple data types to create comprehensive models of glycogenolysis within the broader context of cellular metabolism. Constraint-based modeling techniques, particularly flux balance analysis (FBA), have been applied to predict metabolic flux distributions during glycogenolysis under different physiological states. These models incorporate genome-scale metabolic networks and can simulate how glycogenolysis responds to various nutritional and hormonal signals.
Machine learning algorithms are increasingly being applied to analyze large datasets related to glycogenolysis regulation. Neural networks and other AI approaches can identify patterns in multi-omics data that reveal novel regulatory mechanisms or predict metabolic responses to perturbations. These data-driven models complement traditional mechanistic approaches by uncovering unexpected relationships between variables that might not be apparent from first principles.
Agent-based modeling represents an emerging approach for simulating spatial aspects of glycogenolysis regulation within cellular compartments. These models treat enzymes and metabolites as individual agents with defined behaviors, allowing researchers to investigate how subcellular localization and diffusion limitations influence pathway dynamics. Such spatial considerations are particularly relevant for understanding glycogenolysis regulation in specialized cells like hepatocytes and myocytes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!