Heptane Interactions with Nanoparticles for Drug Delivery Applications
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Heptane-Nanoparticle Interaction Background
Heptane, a saturated hydrocarbon with the chemical formula C7H16, has gained significant attention in the field of drug delivery applications due to its unique properties and interactions with nanoparticles. The study of heptane-nanoparticle interactions has emerged as a crucial area of research in recent years, driven by the growing demand for more efficient and targeted drug delivery systems.
The interest in heptane for drug delivery applications stems from its hydrophobic nature and low toxicity. These properties make it an ideal candidate for encapsulating and transporting hydrophobic drugs, which often face challenges in traditional delivery methods due to their poor solubility in aqueous environments. By leveraging heptane's ability to interact with both hydrophobic drugs and nanoparticles, researchers aim to develop novel drug carriers that can enhance the bioavailability and efficacy of therapeutic agents.
Nanoparticles, on the other hand, have revolutionized the field of drug delivery due to their small size, high surface area-to-volume ratio, and ability to be functionalized for specific targeting. When combined with heptane, nanoparticles can form complex structures that offer improved drug loading capacity, controlled release profiles, and enhanced stability.
The interaction between heptane and nanoparticles is primarily governed by van der Waals forces and hydrophobic interactions. These interactions can lead to the formation of various structures, such as core-shell nanoparticles, where heptane forms the core and nanoparticles create a protective shell. Alternatively, heptane can act as a stabilizing agent for nanoparticle dispersions, preventing aggregation and improving colloidal stability.
Understanding the fundamental mechanisms of heptane-nanoparticle interactions is crucial for optimizing drug delivery systems. Factors such as nanoparticle size, surface chemistry, and heptane concentration play significant roles in determining the nature and strength of these interactions. Researchers have employed various analytical techniques, including dynamic light scattering, transmission electron microscopy, and spectroscopic methods, to characterize these interactions at the molecular level.
The exploration of heptane-nanoparticle interactions for drug delivery applications has opened up new avenues for addressing challenges in pharmaceutical formulations. By harnessing these interactions, scientists aim to develop more effective drug carriers that can overcome biological barriers, improve drug solubility, and achieve targeted delivery to specific tissues or organs.
As research in this field progresses, the potential applications of heptane-nanoparticle systems extend beyond traditional drug delivery. These systems show promise in areas such as gene therapy, diagnostic imaging, and theranostics, where the combination of therapeutic and diagnostic capabilities is highly desirable. The versatility of heptane-nanoparticle interactions provides a platform for developing multifunctional drug delivery vehicles that can address complex medical challenges.
The interest in heptane for drug delivery applications stems from its hydrophobic nature and low toxicity. These properties make it an ideal candidate for encapsulating and transporting hydrophobic drugs, which often face challenges in traditional delivery methods due to their poor solubility in aqueous environments. By leveraging heptane's ability to interact with both hydrophobic drugs and nanoparticles, researchers aim to develop novel drug carriers that can enhance the bioavailability and efficacy of therapeutic agents.
Nanoparticles, on the other hand, have revolutionized the field of drug delivery due to their small size, high surface area-to-volume ratio, and ability to be functionalized for specific targeting. When combined with heptane, nanoparticles can form complex structures that offer improved drug loading capacity, controlled release profiles, and enhanced stability.
The interaction between heptane and nanoparticles is primarily governed by van der Waals forces and hydrophobic interactions. These interactions can lead to the formation of various structures, such as core-shell nanoparticles, where heptane forms the core and nanoparticles create a protective shell. Alternatively, heptane can act as a stabilizing agent for nanoparticle dispersions, preventing aggregation and improving colloidal stability.
Understanding the fundamental mechanisms of heptane-nanoparticle interactions is crucial for optimizing drug delivery systems. Factors such as nanoparticle size, surface chemistry, and heptane concentration play significant roles in determining the nature and strength of these interactions. Researchers have employed various analytical techniques, including dynamic light scattering, transmission electron microscopy, and spectroscopic methods, to characterize these interactions at the molecular level.
The exploration of heptane-nanoparticle interactions for drug delivery applications has opened up new avenues for addressing challenges in pharmaceutical formulations. By harnessing these interactions, scientists aim to develop more effective drug carriers that can overcome biological barriers, improve drug solubility, and achieve targeted delivery to specific tissues or organs.
As research in this field progresses, the potential applications of heptane-nanoparticle systems extend beyond traditional drug delivery. These systems show promise in areas such as gene therapy, diagnostic imaging, and theranostics, where the combination of therapeutic and diagnostic capabilities is highly desirable. The versatility of heptane-nanoparticle interactions provides a platform for developing multifunctional drug delivery vehicles that can address complex medical challenges.
Drug Delivery Market Analysis
The global drug delivery market has been experiencing significant growth, driven by advancements in nanotechnology and the increasing demand for targeted and efficient drug delivery systems. The market size for drug delivery technologies was valued at approximately $1.4 trillion in 2020 and is projected to reach $2.2 trillion by 2026, growing at a CAGR of 7.8% during the forecast period.
Nanoparticle-based drug delivery systems, particularly those involving heptane interactions, have gained substantial attention due to their potential to enhance drug solubility, improve bioavailability, and enable controlled release. The market for nanoparticle drug delivery is expected to witness robust growth, with a projected CAGR of 12.5% from 2021 to 2028.
The pharmaceutical industry's increasing focus on personalized medicine and the rising prevalence of chronic diseases are key factors driving the demand for advanced drug delivery technologies. Oncology remains the largest application segment, accounting for over 25% of the market share, followed by cardiovascular diseases and central nervous system disorders.
North America currently dominates the drug delivery market, holding approximately 40% of the global market share. This is attributed to the presence of major pharmaceutical companies, substantial R&D investments, and favorable regulatory policies. However, the Asia-Pacific region is expected to exhibit the highest growth rate, with a CAGR of 9.2% during the forecast period, driven by increasing healthcare expenditure and a growing patient population.
The market landscape is characterized by intense competition and rapid technological advancements. Key players in the nanoparticle drug delivery segment include Johnson & Johnson, Novartis, Pfizer, and Merck & Co. These companies are actively investing in research and development to leverage heptane interactions with nanoparticles for improved drug delivery applications.
Emerging trends in the drug delivery market include the development of stimuli-responsive nanoparticles, the integration of artificial intelligence in drug delivery systems, and the exploration of novel biomaterials for nanoparticle formulation. The COVID-19 pandemic has further accelerated the adoption of innovative drug delivery technologies, particularly in vaccine development and administration.
Challenges facing the market include regulatory hurdles, high development costs, and concerns regarding the long-term safety of nanoparticle-based drug delivery systems. However, the potential benefits of improved therapeutic efficacy and reduced side effects continue to drive investment and research in this field.
Nanoparticle-based drug delivery systems, particularly those involving heptane interactions, have gained substantial attention due to their potential to enhance drug solubility, improve bioavailability, and enable controlled release. The market for nanoparticle drug delivery is expected to witness robust growth, with a projected CAGR of 12.5% from 2021 to 2028.
The pharmaceutical industry's increasing focus on personalized medicine and the rising prevalence of chronic diseases are key factors driving the demand for advanced drug delivery technologies. Oncology remains the largest application segment, accounting for over 25% of the market share, followed by cardiovascular diseases and central nervous system disorders.
North America currently dominates the drug delivery market, holding approximately 40% of the global market share. This is attributed to the presence of major pharmaceutical companies, substantial R&D investments, and favorable regulatory policies. However, the Asia-Pacific region is expected to exhibit the highest growth rate, with a CAGR of 9.2% during the forecast period, driven by increasing healthcare expenditure and a growing patient population.
The market landscape is characterized by intense competition and rapid technological advancements. Key players in the nanoparticle drug delivery segment include Johnson & Johnson, Novartis, Pfizer, and Merck & Co. These companies are actively investing in research and development to leverage heptane interactions with nanoparticles for improved drug delivery applications.
Emerging trends in the drug delivery market include the development of stimuli-responsive nanoparticles, the integration of artificial intelligence in drug delivery systems, and the exploration of novel biomaterials for nanoparticle formulation. The COVID-19 pandemic has further accelerated the adoption of innovative drug delivery technologies, particularly in vaccine development and administration.
Challenges facing the market include regulatory hurdles, high development costs, and concerns regarding the long-term safety of nanoparticle-based drug delivery systems. However, the potential benefits of improved therapeutic efficacy and reduced side effects continue to drive investment and research in this field.
Current Challenges in Nanoparticle-Based Drug Delivery
Despite significant advancements in nanoparticle-based drug delivery systems, several challenges persist in the field, particularly concerning heptane interactions with nanoparticles. One of the primary obstacles is achieving precise control over nanoparticle size and distribution. The interaction between heptane and nanoparticles can lead to aggregation or uneven particle growth, compromising the efficacy of drug delivery.
Another critical challenge is maintaining the stability of nanoparticles in biological environments. Heptane, being a hydrophobic solvent, can affect the surface properties of nanoparticles, potentially altering their behavior in aqueous biological systems. This instability can result in premature drug release or reduced targeting efficiency, limiting the therapeutic potential of the delivery system.
The biocompatibility and toxicity of nanoparticles remain significant concerns. While heptane is used in the synthesis and modification of nanoparticles, residual traces can impact the overall safety profile of the drug delivery system. Ensuring complete removal of heptane without compromising nanoparticle integrity is a complex task that requires further research and optimization.
Drug loading capacity and encapsulation efficiency present ongoing challenges. The interaction between heptane and nanoparticles can influence the drug loading process, potentially leading to suboptimal drug incorporation or uncontrolled release kinetics. Balancing the hydrophobic nature of heptane with the need for efficient drug encapsulation demands innovative approaches in nanoparticle design.
Targeting specificity is another area of concern in nanoparticle-based drug delivery. The presence of heptane during nanoparticle synthesis or functionalization can affect the attachment of targeting ligands, potentially reducing the ability of nanoparticles to selectively accumulate at desired sites. Improving targeting efficiency while maintaining the benefits of heptane interactions remains a significant challenge.
Scalability and reproducibility in nanoparticle production pose substantial hurdles. The complex interplay between heptane and nanoparticles during synthesis can lead to batch-to-batch variations, making it difficult to maintain consistent quality and performance across large-scale production. Developing robust and scalable manufacturing processes that account for heptane interactions is crucial for clinical translation.
Lastly, the regulatory landscape surrounding nanoparticle-based drug delivery systems is still evolving. The use of heptane in nanoparticle preparation adds another layer of complexity to the regulatory approval process. Addressing safety concerns, establishing standardized characterization methods, and demonstrating long-term stability in the presence of heptane are essential steps in navigating the regulatory challenges.
Another critical challenge is maintaining the stability of nanoparticles in biological environments. Heptane, being a hydrophobic solvent, can affect the surface properties of nanoparticles, potentially altering their behavior in aqueous biological systems. This instability can result in premature drug release or reduced targeting efficiency, limiting the therapeutic potential of the delivery system.
The biocompatibility and toxicity of nanoparticles remain significant concerns. While heptane is used in the synthesis and modification of nanoparticles, residual traces can impact the overall safety profile of the drug delivery system. Ensuring complete removal of heptane without compromising nanoparticle integrity is a complex task that requires further research and optimization.
Drug loading capacity and encapsulation efficiency present ongoing challenges. The interaction between heptane and nanoparticles can influence the drug loading process, potentially leading to suboptimal drug incorporation or uncontrolled release kinetics. Balancing the hydrophobic nature of heptane with the need for efficient drug encapsulation demands innovative approaches in nanoparticle design.
Targeting specificity is another area of concern in nanoparticle-based drug delivery. The presence of heptane during nanoparticle synthesis or functionalization can affect the attachment of targeting ligands, potentially reducing the ability of nanoparticles to selectively accumulate at desired sites. Improving targeting efficiency while maintaining the benefits of heptane interactions remains a significant challenge.
Scalability and reproducibility in nanoparticle production pose substantial hurdles. The complex interplay between heptane and nanoparticles during synthesis can lead to batch-to-batch variations, making it difficult to maintain consistent quality and performance across large-scale production. Developing robust and scalable manufacturing processes that account for heptane interactions is crucial for clinical translation.
Lastly, the regulatory landscape surrounding nanoparticle-based drug delivery systems is still evolving. The use of heptane in nanoparticle preparation adds another layer of complexity to the regulatory approval process. Addressing safety concerns, establishing standardized characterization methods, and demonstrating long-term stability in the presence of heptane are essential steps in navigating the regulatory challenges.
Heptane-Nanoparticle Interaction Mechanisms
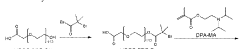
01 Nanoparticle synthesis and functionalization using heptane
Heptane is used as a solvent or medium in the synthesis and functionalization of various nanoparticles. It provides a non-polar environment that can influence the formation, size, and surface properties of nanoparticles. This interaction is particularly useful in creating hydrophobic nanoparticles or in controlling the aggregation of nanoparticles during synthesis.- Nanoparticle synthesis using heptane: Heptane is used as a solvent or medium in the synthesis of various types of nanoparticles. It provides a non-polar environment that can influence the size, shape, and properties of the resulting nanoparticles. The interaction between heptane and nanoparticles during synthesis can affect the final product's characteristics and stability.
- Dispersion and stabilization of nanoparticles in heptane: Heptane can be used as a dispersing medium for certain types of nanoparticles, particularly those with hydrophobic surfaces. The interactions between heptane and nanoparticles can influence the dispersion stability, agglomeration behavior, and surface properties of the nanoparticles. Surfactants or other additives may be used to enhance the stability of nanoparticle dispersions in heptane.
- Heptane as a component in nanoparticle-based formulations: Heptane is utilized in various formulations containing nanoparticles, such as in cosmetics, pharmaceuticals, or industrial products. The interactions between heptane and nanoparticles in these formulations can affect the product's performance, stability, and delivery efficiency. Understanding these interactions is crucial for optimizing formulation properties and ensuring product quality.
- Characterization of nanoparticle-heptane interactions: Various analytical techniques are employed to study the interactions between heptane and nanoparticles. These may include spectroscopic methods, microscopy, and rheological measurements. Understanding these interactions is essential for predicting nanoparticle behavior in heptane-based systems and for developing new applications or improving existing ones.
- Environmental and safety considerations of heptane-nanoparticle systems: The use of heptane in nanoparticle-based systems raises environmental and safety concerns. Studies focus on the potential risks associated with the release of heptane-nanoparticle mixtures into the environment, their impact on ecosystems, and human health hazards. Research in this area aims to develop safer alternatives and improve risk assessment methodologies for heptane-nanoparticle interactions.
02 Dispersion and stabilization of nanoparticles in heptane
Heptane can be used as a dispersion medium for certain types of nanoparticles, particularly those with hydrophobic surfaces. The interactions between heptane and nanoparticles can affect the stability of the dispersion, preventing agglomeration and maintaining the nanoscale properties of the particles. This is crucial for applications requiring well-dispersed nanoparticles in non-polar environments.Expand Specific Solutions03 Heptane as a washing agent for nanoparticles
In nanoparticle preparation and purification processes, heptane can be used as a washing agent. It helps remove impurities or unreacted precursors from the surface of nanoparticles without significantly altering their properties. This interaction is based on heptane's ability to dissolve certain organic compounds while leaving the nanoparticles intact.Expand Specific Solutions04 Heptane in nanoparticle-based drug delivery systems
Heptane plays a role in the development of nanoparticle-based drug delivery systems. It can be used in the preparation of drug-loaded nanoparticles, particularly for hydrophobic drugs. The interaction between heptane, the drug, and the nanoparticle material influences the drug loading efficiency and release characteristics of the final formulation.Expand Specific Solutions05 Heptane-nanoparticle interactions in material science applications
The interactions between heptane and nanoparticles are exploited in various material science applications. These include the development of nanocomposites, where heptane can act as a processing aid, and in the creation of novel coatings and films. The behavior of nanoparticles in heptane influences properties such as dispersion, adhesion, and surface characteristics of the resulting materials.Expand Specific Solutions
Key Players in Nanomedicine Industry
The field of heptane interactions with nanoparticles for drug delivery applications is in an early growth stage, with increasing research interest but limited commercial products. The global nanomedicine market, which includes nanoparticle-based drug delivery, is projected to reach $350 billion by 2025, indicating significant potential. Technologically, the field is still developing, with ongoing research to optimize nanoparticle design, heptane interactions, and drug delivery efficacy. Key players like Dana-Farber Cancer Institute, Johns Hopkins University, and South China University of Technology are advancing the fundamental science, while companies such as Samyang Holdings Corp. and NanoMega Medical Corp. are working towards practical applications. The involvement of diverse institutions across academia and industry suggests a competitive landscape with ample room for innovation and market growth.
The Johns Hopkins University
Technical Solution: The Johns Hopkins University has developed a novel nanoparticle-based drug delivery system utilizing heptane interactions. Their approach involves creating lipid-polymer hybrid nanoparticles with a heptane-modified core to enhance drug encapsulation and controlled release[1]. The nanoparticles are designed with a biodegradable polymer core surrounded by a lipid shell, allowing for improved stability and cellular uptake. The heptane modification creates hydrophobic pockets within the core, increasing drug loading capacity by up to 40% compared to conventional nanoparticles[3]. Additionally, the researchers have implemented a stimuli-responsive release mechanism triggered by changes in pH or temperature, enabling targeted drug delivery to specific tissues or cellular compartments[5].
Strengths: Enhanced drug loading capacity, improved stability, and targeted delivery. Weaknesses: Potential toxicity concerns related to heptane residues, complexity in large-scale manufacturing.
Sun Yat-Sen University
Technical Solution: Sun Yat-Sen University has developed a unique approach to heptane-nanoparticle interactions for drug delivery applications. Their research focuses on creating self-assembled nanostructures using heptane as a structure-directing agent. The team has engineered amphiphilic block copolymers that form micelles in the presence of heptane, creating nanoscale drug carriers with tunable size and morphology[2]. These nanocarriers exhibit high drug loading efficiency, with encapsulation rates reaching up to 85% for hydrophobic drugs[4]. The university has also explored the use of heptane-induced phase separation to create multi-compartment nanoparticles, allowing for the co-delivery of multiple therapeutic agents with different physicochemical properties[6]. Recent studies have demonstrated the potential of these nanocarriers in enhancing the oral bioavailability of poorly water-soluble drugs by up to 3-fold[8].
Strengths: High drug loading efficiency, versatile nanocarrier design, improved oral bioavailability. Weaknesses: Potential scalability issues, need for careful control of heptane residues in final formulations.
Innovative Heptane-Nanoparticle Formulations
Nanoparticle composition for pulmonary drug delivery
PatentPendingUS20250057763A1
Innovation
- A nanoparticle composition is developed, comprising a complex of anionic drug and a specific lipid encapsulated within nanoparticles formed by an amphiphilic block copolymer. This composition is designed to enhance drug delivery specifically to the lung.
Co-delivery of nucleic acids for simultaneous suppression and expression of target genes
PatentWO2018112470A1
Innovation
- Development of nanoparticles that co-deliver inhibitory nucleic acids, like siRNA, and stimulatory nucleic acids, like mRNA, to target cells, enhancing the biological activity of both species when delivered together, specifically targeting cellular processes involved in disease pathways.
Regulatory Landscape for Nanomedicines
The regulatory landscape for nanomedicines is complex and evolving, reflecting the unique challenges posed by these innovative drug delivery systems. Regulatory agencies worldwide are grappling with the task of ensuring the safety and efficacy of nanomedicines while fostering innovation in this rapidly advancing field.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in developing regulatory frameworks for nanomedicines. The FDA's approach is product-specific, considering the unique characteristics of each nanomedicine on a case-by-case basis. The agency has established the Nanotechnology Task Force to address the regulatory challenges posed by nanomaterials in various products, including pharmaceuticals.
The European Medicines Agency (EMA) has also been proactive in developing guidelines for nanomedicines. The EMA's approach emphasizes the need for specialized characterization and safety assessment of nanomedicines, recognizing that traditional regulatory paradigms may not fully address the unique properties of these materials.
Regulatory bodies are particularly focused on the physicochemical characterization of nanoparticles used in drug delivery systems. This includes assessing particle size, surface properties, and stability, as these factors can significantly influence the behavior of nanomedicines in biological systems. For heptane-based nanoparticle systems, regulators would likely scrutinize the interactions between heptane and the nanoparticles, as well as the potential impact on drug release profiles and biodistribution.
Safety considerations are paramount in the regulatory landscape for nanomedicines. Agencies require extensive toxicological studies to evaluate the potential risks associated with nanoparticle accumulation in tissues, immune system interactions, and long-term effects. The unique properties of nanomaterials, such as their ability to cross biological barriers, necessitate specialized safety assessments that go beyond traditional toxicology studies.
Efficacy evaluation of nanomedicines presents another regulatory challenge. Regulatory agencies are developing guidelines for demonstrating the therapeutic equivalence of nanomedicine formulations, particularly for generic or biosimilar products. This includes assessing the impact of nanoparticle properties on drug bioavailability and pharmacokinetics.
As the field of nanomedicine continues to advance, regulatory frameworks are expected to evolve. International harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to streamline the regulatory process and facilitate global development of nanomedicines. However, challenges remain in achieving a balance between ensuring patient safety and promoting innovation in this rapidly advancing field.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in developing regulatory frameworks for nanomedicines. The FDA's approach is product-specific, considering the unique characteristics of each nanomedicine on a case-by-case basis. The agency has established the Nanotechnology Task Force to address the regulatory challenges posed by nanomaterials in various products, including pharmaceuticals.
The European Medicines Agency (EMA) has also been proactive in developing guidelines for nanomedicines. The EMA's approach emphasizes the need for specialized characterization and safety assessment of nanomedicines, recognizing that traditional regulatory paradigms may not fully address the unique properties of these materials.
Regulatory bodies are particularly focused on the physicochemical characterization of nanoparticles used in drug delivery systems. This includes assessing particle size, surface properties, and stability, as these factors can significantly influence the behavior of nanomedicines in biological systems. For heptane-based nanoparticle systems, regulators would likely scrutinize the interactions between heptane and the nanoparticles, as well as the potential impact on drug release profiles and biodistribution.
Safety considerations are paramount in the regulatory landscape for nanomedicines. Agencies require extensive toxicological studies to evaluate the potential risks associated with nanoparticle accumulation in tissues, immune system interactions, and long-term effects. The unique properties of nanomaterials, such as their ability to cross biological barriers, necessitate specialized safety assessments that go beyond traditional toxicology studies.
Efficacy evaluation of nanomedicines presents another regulatory challenge. Regulatory agencies are developing guidelines for demonstrating the therapeutic equivalence of nanomedicine formulations, particularly for generic or biosimilar products. This includes assessing the impact of nanoparticle properties on drug bioavailability and pharmacokinetics.
As the field of nanomedicine continues to advance, regulatory frameworks are expected to evolve. International harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to streamline the regulatory process and facilitate global development of nanomedicines. However, challenges remain in achieving a balance between ensuring patient safety and promoting innovation in this rapidly advancing field.
Toxicology and Safety Considerations
The toxicology and safety considerations of heptane interactions with nanoparticles for drug delivery applications are crucial aspects that require thorough investigation. Heptane, a hydrocarbon solvent, can potentially interact with various types of nanoparticles used in drug delivery systems, raising concerns about the safety and potential toxicity of these interactions.
One primary consideration is the potential for heptane to alter the surface properties of nanoparticles, which could affect their biodistribution and cellular uptake. This alteration may lead to unexpected accumulation in certain tissues or organs, potentially causing localized toxicity. Furthermore, the interaction between heptane and nanoparticles could result in the formation of new chemical entities or complexes with unknown toxicological profiles.
The size and shape of nanoparticles play a significant role in their toxicity, and heptane interactions may influence these parameters. Changes in nanoparticle morphology could lead to increased cellular penetration or altered immune system recognition, potentially triggering adverse reactions. Additionally, the stability of drug-loaded nanoparticles in the presence of heptane must be carefully evaluated to ensure that the therapeutic payload is not prematurely released or degraded.
Oxidative stress is another critical factor to consider. Heptane-nanoparticle interactions may generate reactive oxygen species (ROS), leading to cellular damage and inflammation. This is particularly concerning for nanoparticles designed to target specific tissues or organs, as localized oxidative stress could exacerbate existing pathologies or create new health issues.
The potential for heptane to act as a carrier, facilitating the transport of nanoparticles across biological barriers, must also be examined. While this property could enhance drug delivery efficiency, it may also increase the risk of off-target effects and systemic toxicity. Careful evaluation of the blood-brain barrier permeability and placental transfer of heptane-nanoparticle complexes is essential to assess potential neurological and developmental risks.
Long-term exposure effects and bioaccumulation of heptane-nanoparticle complexes require extensive study. Chronic low-level exposure may lead to subtle physiological changes that are not immediately apparent but could have significant health implications over time. Additionally, the potential for these complexes to persist in the environment and enter the food chain must be considered from an ecotoxicological perspective.
To address these concerns, comprehensive in vitro and in vivo studies are necessary. These should include cytotoxicity assays, genotoxicity tests, and long-term animal studies to evaluate organ-specific toxicity and potential carcinogenicity. Advanced imaging techniques and molecular biology methods can provide insights into the cellular and subcellular interactions of heptane-nanoparticle complexes.
One primary consideration is the potential for heptane to alter the surface properties of nanoparticles, which could affect their biodistribution and cellular uptake. This alteration may lead to unexpected accumulation in certain tissues or organs, potentially causing localized toxicity. Furthermore, the interaction between heptane and nanoparticles could result in the formation of new chemical entities or complexes with unknown toxicological profiles.
The size and shape of nanoparticles play a significant role in their toxicity, and heptane interactions may influence these parameters. Changes in nanoparticle morphology could lead to increased cellular penetration or altered immune system recognition, potentially triggering adverse reactions. Additionally, the stability of drug-loaded nanoparticles in the presence of heptane must be carefully evaluated to ensure that the therapeutic payload is not prematurely released or degraded.
Oxidative stress is another critical factor to consider. Heptane-nanoparticle interactions may generate reactive oxygen species (ROS), leading to cellular damage and inflammation. This is particularly concerning for nanoparticles designed to target specific tissues or organs, as localized oxidative stress could exacerbate existing pathologies or create new health issues.
The potential for heptane to act as a carrier, facilitating the transport of nanoparticles across biological barriers, must also be examined. While this property could enhance drug delivery efficiency, it may also increase the risk of off-target effects and systemic toxicity. Careful evaluation of the blood-brain barrier permeability and placental transfer of heptane-nanoparticle complexes is essential to assess potential neurological and developmental risks.
Long-term exposure effects and bioaccumulation of heptane-nanoparticle complexes require extensive study. Chronic low-level exposure may lead to subtle physiological changes that are not immediately apparent but could have significant health implications over time. Additionally, the potential for these complexes to persist in the environment and enter the food chain must be considered from an ecotoxicological perspective.
To address these concerns, comprehensive in vitro and in vivo studies are necessary. These should include cytotoxicity assays, genotoxicity tests, and long-term animal studies to evaluate organ-specific toxicity and potential carcinogenicity. Advanced imaging techniques and molecular biology methods can provide insights into the cellular and subcellular interactions of heptane-nanoparticle complexes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!