Heptane's Contribution to Gasoline's Reid Vapor Pressure
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Heptane and RVP Background
Heptane, a hydrocarbon compound with the chemical formula C7H16, plays a significant role in determining the Reid Vapor Pressure (RVP) of gasoline. RVP is a crucial parameter used to measure the volatility of gasoline and other petroleum products. This measurement is essential for understanding fuel performance, emissions, and safety characteristics in various environmental conditions.
The concept of RVP originated in the early 20th century when the need for a standardized method to measure fuel volatility became apparent. Reid Vapor Pressure, named after its developer E.W. Reid, was established as a standard test method by the American Society for Testing and Materials (ASTM) in 1930. The test involves measuring the vapor pressure of a fuel sample at 100°F (37.8°C), which closely approximates the maximum temperature experienced by fuel in vehicle tanks during summer months.
Heptane, as a component of gasoline, contributes significantly to its RVP due to its relatively high vapor pressure compared to other hydrocarbons found in the fuel mixture. The presence of heptane in gasoline formulations affects the fuel's evaporation characteristics, which in turn influences engine performance, cold-start behavior, and emissions.
The relationship between heptane and RVP is complex and depends on various factors, including the concentration of heptane in the gasoline blend, the presence of other volatile compounds, and environmental conditions. Generally, an increase in the heptane content of gasoline tends to raise its RVP, making the fuel more volatile and prone to evaporation.
Understanding the impact of heptane on RVP is crucial for refiners and fuel blenders who must balance the need for adequate volatility with regulatory requirements and environmental concerns. In many regions, RVP limits are seasonally adjusted to control evaporative emissions, particularly during warmer months when fuel evaporation is more pronounced.
The historical development of RVP regulations has been driven by environmental concerns and the need to reduce smog-forming emissions. As a result, the petroleum industry has had to adapt its refining and blending processes to meet increasingly stringent RVP standards while maintaining fuel performance and efficiency.
In recent years, the focus on reducing greenhouse gas emissions and improving air quality has led to further scrutiny of fuel volatility and composition. This has prompted ongoing research into alternative fuel formulations and additives that can help manage RVP while meeting performance and environmental objectives.
The concept of RVP originated in the early 20th century when the need for a standardized method to measure fuel volatility became apparent. Reid Vapor Pressure, named after its developer E.W. Reid, was established as a standard test method by the American Society for Testing and Materials (ASTM) in 1930. The test involves measuring the vapor pressure of a fuel sample at 100°F (37.8°C), which closely approximates the maximum temperature experienced by fuel in vehicle tanks during summer months.
Heptane, as a component of gasoline, contributes significantly to its RVP due to its relatively high vapor pressure compared to other hydrocarbons found in the fuel mixture. The presence of heptane in gasoline formulations affects the fuel's evaporation characteristics, which in turn influences engine performance, cold-start behavior, and emissions.
The relationship between heptane and RVP is complex and depends on various factors, including the concentration of heptane in the gasoline blend, the presence of other volatile compounds, and environmental conditions. Generally, an increase in the heptane content of gasoline tends to raise its RVP, making the fuel more volatile and prone to evaporation.
Understanding the impact of heptane on RVP is crucial for refiners and fuel blenders who must balance the need for adequate volatility with regulatory requirements and environmental concerns. In many regions, RVP limits are seasonally adjusted to control evaporative emissions, particularly during warmer months when fuel evaporation is more pronounced.
The historical development of RVP regulations has been driven by environmental concerns and the need to reduce smog-forming emissions. As a result, the petroleum industry has had to adapt its refining and blending processes to meet increasingly stringent RVP standards while maintaining fuel performance and efficiency.
In recent years, the focus on reducing greenhouse gas emissions and improving air quality has led to further scrutiny of fuel volatility and composition. This has prompted ongoing research into alternative fuel formulations and additives that can help manage RVP while meeting performance and environmental objectives.
Gasoline Market Dynamics
The gasoline market is characterized by dynamic fluctuations influenced by various factors, including global crude oil prices, geopolitical events, seasonal demand patterns, and regulatory changes. As a key component of the transportation sector, gasoline consumption trends closely mirror economic growth and consumer behavior. In recent years, the market has witnessed significant shifts due to the increasing adoption of electric vehicles and the implementation of stricter environmental regulations.
Global gasoline demand has shown steady growth over the past decade, primarily driven by emerging economies in Asia and Africa. However, mature markets such as North America and Europe have experienced stagnant or declining consumption due to improved fuel efficiency standards and the gradual transition to alternative energy sources. The COVID-19 pandemic caused a temporary disruption in demand patterns, but the market has since rebounded to pre-pandemic levels in many regions.
Supply-side dynamics play a crucial role in shaping the gasoline market. Refinery capacity utilization, maintenance schedules, and unexpected outages can significantly impact gasoline availability and pricing. The United States, China, and Russia are among the top gasoline-producing countries, with their refining capacities and export policies influencing global market trends.
Pricing mechanisms in the gasoline market are complex, involving a combination of crude oil costs, refining margins, transportation expenses, and taxes. Regional price disparities are common due to variations in local supply and demand conditions, as well as differences in taxation and regulatory frameworks. Futures markets and speculative trading activities also contribute to short-term price volatility.
Environmental concerns and regulatory pressures have led to the development and adoption of cleaner-burning gasoline formulations. The introduction of reformulated gasoline (RFG) and the phasing out of lead-based additives have significantly impacted market dynamics. Additionally, the blending of biofuels, such as ethanol, into gasoline has become increasingly prevalent, driven by renewable fuel mandates in many countries.
The gasoline market's future outlook is shaped by several key trends, including the ongoing energy transition, technological advancements in vehicle efficiency, and evolving consumer preferences. While gasoline is expected to remain a dominant transportation fuel in the near term, the long-term trajectory points towards a gradual decline in market share as alternative energy sources gain traction.
Global gasoline demand has shown steady growth over the past decade, primarily driven by emerging economies in Asia and Africa. However, mature markets such as North America and Europe have experienced stagnant or declining consumption due to improved fuel efficiency standards and the gradual transition to alternative energy sources. The COVID-19 pandemic caused a temporary disruption in demand patterns, but the market has since rebounded to pre-pandemic levels in many regions.
Supply-side dynamics play a crucial role in shaping the gasoline market. Refinery capacity utilization, maintenance schedules, and unexpected outages can significantly impact gasoline availability and pricing. The United States, China, and Russia are among the top gasoline-producing countries, with their refining capacities and export policies influencing global market trends.
Pricing mechanisms in the gasoline market are complex, involving a combination of crude oil costs, refining margins, transportation expenses, and taxes. Regional price disparities are common due to variations in local supply and demand conditions, as well as differences in taxation and regulatory frameworks. Futures markets and speculative trading activities also contribute to short-term price volatility.
Environmental concerns and regulatory pressures have led to the development and adoption of cleaner-burning gasoline formulations. The introduction of reformulated gasoline (RFG) and the phasing out of lead-based additives have significantly impacted market dynamics. Additionally, the blending of biofuels, such as ethanol, into gasoline has become increasingly prevalent, driven by renewable fuel mandates in many countries.
The gasoline market's future outlook is shaped by several key trends, including the ongoing energy transition, technological advancements in vehicle efficiency, and evolving consumer preferences. While gasoline is expected to remain a dominant transportation fuel in the near term, the long-term trajectory points towards a gradual decline in market share as alternative energy sources gain traction.
RVP Measurement Challenges
Measuring Reid Vapor Pressure (RVP) accurately presents several challenges, particularly when considering heptane's contribution to gasoline's RVP. The complexity of gasoline composition, including various hydrocarbons like heptane, makes precise RVP determination difficult. Temperature fluctuations during measurement can significantly affect results, as vapor pressure is highly temperature-dependent.
The standard ASTM D323 method for RVP measurement, while widely used, has inherent limitations. It requires careful sample handling and preparation to avoid volatile component loss, which can skew results. The method's reliance on manual operations introduces potential human error, affecting reproducibility across different laboratories or operators.
Heptane's presence in gasoline further complicates RVP measurement. As a relatively volatile component, heptane can evaporate quickly during sample transfer or preparation, leading to underestimation of RVP. Its low boiling point (98.4°C) means it readily contributes to vapor pressure, but its exact contribution can vary based on gasoline composition and environmental conditions.
Modern automated RVP analyzers aim to address some of these challenges, offering improved precision and reduced operator dependency. However, they still face issues with sample integrity and representation, especially for complex fuel blends containing heptane and other volatile components.
The heterogeneity of gasoline samples poses another challenge. Stratification of components can occur, leading to non-representative sampling. This is particularly relevant for heptane, which may not be evenly distributed throughout the fuel sample due to its volatility.
Calibration and standardization of RVP measurement equipment present ongoing challenges. Regular calibration is crucial to ensure accuracy, but the process can be time-consuming and requires specialized reference materials. The presence of heptane and other volatile components necessitates frequent recalibration to maintain measurement integrity.
Environmental factors, such as atmospheric pressure and humidity, can influence RVP measurements. These variables are particularly significant when considering heptane's contribution, as its vapor pressure is sensitive to atmospheric conditions. Controlling these factors in a laboratory setting adds complexity to the measurement process.
In conclusion, while RVP measurement is critical for assessing gasoline quality and environmental compliance, it faces numerous challenges. The presence of volatile components like heptane exacerbates these difficulties, requiring careful consideration in measurement protocols and interpretation of results. Ongoing research and technological advancements are essential to improve the accuracy and reliability of RVP measurements, particularly in complex fuel compositions.
The standard ASTM D323 method for RVP measurement, while widely used, has inherent limitations. It requires careful sample handling and preparation to avoid volatile component loss, which can skew results. The method's reliance on manual operations introduces potential human error, affecting reproducibility across different laboratories or operators.
Heptane's presence in gasoline further complicates RVP measurement. As a relatively volatile component, heptane can evaporate quickly during sample transfer or preparation, leading to underestimation of RVP. Its low boiling point (98.4°C) means it readily contributes to vapor pressure, but its exact contribution can vary based on gasoline composition and environmental conditions.
Modern automated RVP analyzers aim to address some of these challenges, offering improved precision and reduced operator dependency. However, they still face issues with sample integrity and representation, especially for complex fuel blends containing heptane and other volatile components.
The heterogeneity of gasoline samples poses another challenge. Stratification of components can occur, leading to non-representative sampling. This is particularly relevant for heptane, which may not be evenly distributed throughout the fuel sample due to its volatility.
Calibration and standardization of RVP measurement equipment present ongoing challenges. Regular calibration is crucial to ensure accuracy, but the process can be time-consuming and requires specialized reference materials. The presence of heptane and other volatile components necessitates frequent recalibration to maintain measurement integrity.
Environmental factors, such as atmospheric pressure and humidity, can influence RVP measurements. These variables are particularly significant when considering heptane's contribution, as its vapor pressure is sensitive to atmospheric conditions. Controlling these factors in a laboratory setting adds complexity to the measurement process.
In conclusion, while RVP measurement is critical for assessing gasoline quality and environmental compliance, it faces numerous challenges. The presence of volatile components like heptane exacerbates these difficulties, requiring careful consideration in measurement protocols and interpretation of results. Ongoing research and technological advancements are essential to improve the accuracy and reliability of RVP measurements, particularly in complex fuel compositions.
Current RVP Control Methods
01 Measurement and analysis of Reid vapor pressure
Various methods and apparatus are used to measure and analyze the Reid vapor pressure of heptane and other hydrocarbons. These techniques involve specialized equipment and procedures to accurately determine vapor pressure, which is crucial for understanding fuel properties and environmental impacts.- Measurement of Reid Vapor Pressure for heptane: Reid Vapor Pressure (RVP) is a common method for measuring the vapor pressure of volatile petroleum products, including heptane. The test involves heating a liquid sample to 100°F (37.8°C) in a sealed container and measuring the resulting pressure. This method is particularly useful for determining the volatility of heptane and its blends in various applications.
- Heptane as a component in fuel blends: Heptane is often used as a component in fuel blends, particularly in gasoline formulations. Its Reid Vapor Pressure characteristics play a crucial role in determining the overall volatility and performance of the fuel mixture. The vapor pressure of heptane-containing blends is carefully controlled to meet environmental regulations and ensure optimal engine performance.
- Environmental impact and regulations related to heptane vapor pressure: The vapor pressure of heptane and its blends is subject to environmental regulations due to its impact on air quality and emissions. Regulatory bodies often set limits on the Reid Vapor Pressure of fuels containing heptane to control volatile organic compound (VOC) emissions. This has led to the development of new formulations and testing methods to ensure compliance with these environmental standards.
- Applications of heptane in chemical processes: Heptane's Reid Vapor Pressure characteristics are important in various chemical processes and industries. It is used as a solvent, a calibration standard for vapor pressure measurements, and in the production of various chemicals. Understanding and controlling the vapor pressure of heptane is crucial in these applications to ensure safety, efficiency, and product quality.
- Innovative methods for measuring and controlling heptane vapor pressure: New technologies and methods are being developed to more accurately measure and control the Reid Vapor Pressure of heptane and its mixtures. These innovations include advanced sensors, real-time monitoring systems, and novel testing procedures. Such advancements aim to improve the precision of vapor pressure measurements and provide better control in various applications involving heptane.
02 Fuel composition and blending
Heptane is often used as a component in fuel blends. The Reid vapor pressure of heptane and its impact on overall fuel properties are considered when formulating fuel compositions. This is important for optimizing fuel performance and meeting regulatory standards.Expand Specific Solutions03 Environmental and safety considerations
The Reid vapor pressure of heptane is an important factor in assessing environmental impact and safety risks. This includes considerations for emissions, storage, transportation, and handling of heptane-containing products. Proper understanding and management of vapor pressure are crucial for compliance with environmental regulations.Expand Specific Solutions04 Industrial applications and processes
Heptane's Reid vapor pressure is relevant in various industrial applications and processes. This includes its use as a solvent, in chemical reactions, and in manufacturing processes where vapor pressure characteristics are important for process control and product quality.Expand Specific Solutions05 Analytical techniques and instrumentation
Advanced analytical techniques and specialized instrumentation are employed to accurately measure and analyze the Reid vapor pressure of heptane. This includes the development of new methods and equipment to improve precision, efficiency, and reliability of vapor pressure measurements.Expand Specific Solutions
Key Players in Fuel Industry
The competitive landscape for "Heptane's Contribution to Gasoline's Reid Vapor Pressure" is characterized by a mature industry with established players. The market is dominated by major oil and gas companies such as China Petroleum & Chemical Corp., Chevron U.S.A., Inc., and BP Corporation North America, Inc. These companies, along with research institutions like UOP LLC and Sinopec Research Institute of Petroleum Processing, are at the forefront of developing and optimizing fuel formulations. The technology is well-established, with ongoing research focused on improving efficiency and meeting evolving environmental regulations. Market size is significant, given the global demand for gasoline, but growth is moderate due to the industry's maturity and increasing focus on alternative fuels.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced blending techniques to optimize heptane's contribution to gasoline's Reid Vapor Pressure (RVP). Their approach involves precise control of heptane content in gasoline blends, typically maintaining it between 1-3% by volume[1]. This allows for fine-tuning of the RVP to meet seasonal and regional regulatory requirements. Sinopec's method incorporates real-time analysis of blend components, using near-infrared spectroscopy to adjust heptane levels dynamically during the blending process[3]. This ensures consistent RVP across batches while maximizing fuel economy and minimizing emissions.
Strengths: Precise control over RVP, adaptability to varying regulations, and improved fuel quality. Weaknesses: Potentially higher production costs and complexity in blending operations.
UOP LLC
Technical Solution: UOP LLC, a Honeywell company, has pioneered a novel approach to managing heptane's impact on gasoline RVP through their advanced isomerization technology. Their UOP Penex™ process specifically targets the conversion of normal heptane to its branched isomers, which have lower vapor pressures[2]. This process allows refineries to maintain the desired octane number while reducing the overall RVP contribution from heptane. UOP's technology can achieve up to 98% conversion of normal heptane[4], significantly altering the gasoline blend's vapor pressure characteristics. The process also incorporates a proprietary catalyst that enhances selectivity towards branched heptane isomers, minimizing unwanted side reactions.
Strengths: High conversion efficiency, maintenance of octane number, and flexibility in gasoline blending. Weaknesses: High initial investment costs and potential catalyst sensitivity to feed impurities.
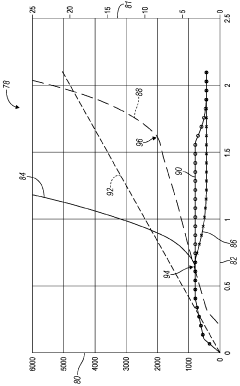
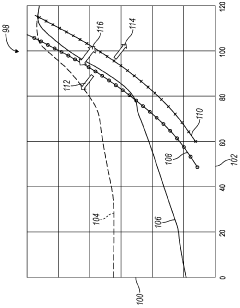
Heptane-RVP Relationship Analysis
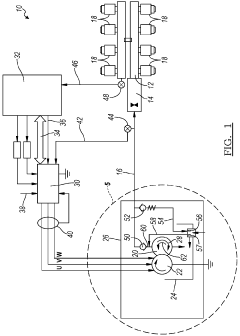
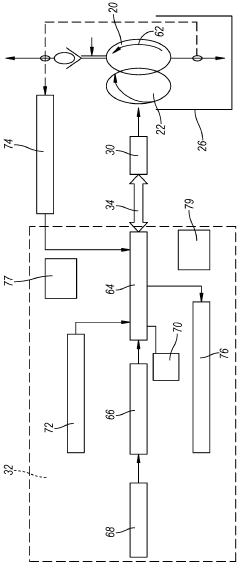
Reid vapor pressure control process
PatentInactiveUS20150329443A1
Innovation
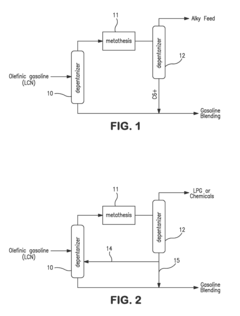
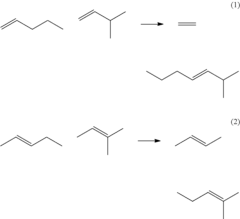
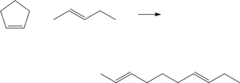
- The process involves ring opening metathesis (ROM) of C5 olefins in the presence of a catalyst to convert cyclopentene into higher molecular weight hydrocarbons, reducing ASO formation and allowing for increased C5 olefin utilization in the isoparaffin-olefin alkylation process without increasing vapor pressure.
reid vapor pressure sensing of fuel with brushless fuel pump
PatentInactiveDE102018120866A1
Innovation
- A fuel RVP sensing system using a brushless fuel pump with a controller that reverses its rotation direction to create sub-atmospheric pressure, allowing for rapid vaporization and measurement of fuel RVP, correlated with engine speed and current changes, and utilizing an engine control module for precise RVP determination.
Environmental Regulations Impact
Environmental regulations have significantly impacted the use of heptane in gasoline formulations, particularly concerning Reid Vapor Pressure (RVP) standards. The Clean Air Act Amendments of 1990 established stringent requirements for gasoline volatility, aiming to reduce emissions of volatile organic compounds (VOCs) that contribute to ground-level ozone formation. These regulations have led to the implementation of seasonal RVP limits in many regions, especially during summer months when ozone formation is more prevalent.
The Environmental Protection Agency (EPA) has set specific RVP limits for gasoline, which vary depending on the time of year and geographical location. In most areas, the summer RVP limit is 9.0 psi, while some regions with more severe air quality issues have even stricter limits of 7.8 psi. These regulations have forced refineries to carefully manage the composition of their gasoline blends, including the amount of heptane and other light hydrocarbons used.
As heptane is a significant contributor to gasoline's RVP, its use has been closely scrutinized and often limited in summer-grade gasoline formulations. Refineries have had to develop sophisticated blending strategies to meet RVP requirements while maintaining other essential fuel properties. This has led to increased use of alternative components and blending agents that can help reduce overall vapor pressure without compromising fuel performance.
The impact of these regulations extends beyond just the formulation of gasoline. It has driven innovation in fuel production technologies, catalyzed the development of more advanced refining processes, and spurred research into alternative fuel additives. Additionally, it has influenced the automotive industry, encouraging the development of vehicles with enhanced evaporative emission control systems.
Compliance with RVP regulations has also necessitated significant investments in infrastructure and testing equipment at refineries and distribution terminals. Regular monitoring and reporting of gasoline RVP levels have become standard practice, with substantial penalties for non-compliance. This has created a new market for RVP testing equipment and services, as well as consulting expertise in gasoline blending strategies.
The regulatory landscape continues to evolve, with ongoing discussions about potential further reductions in RVP limits and the expansion of regulated areas. These potential changes keep refineries and fuel producers in a state of constant adaptation, balancing the need for environmental compliance with the demands for high-performance, cost-effective fuel products.
The Environmental Protection Agency (EPA) has set specific RVP limits for gasoline, which vary depending on the time of year and geographical location. In most areas, the summer RVP limit is 9.0 psi, while some regions with more severe air quality issues have even stricter limits of 7.8 psi. These regulations have forced refineries to carefully manage the composition of their gasoline blends, including the amount of heptane and other light hydrocarbons used.
As heptane is a significant contributor to gasoline's RVP, its use has been closely scrutinized and often limited in summer-grade gasoline formulations. Refineries have had to develop sophisticated blending strategies to meet RVP requirements while maintaining other essential fuel properties. This has led to increased use of alternative components and blending agents that can help reduce overall vapor pressure without compromising fuel performance.
The impact of these regulations extends beyond just the formulation of gasoline. It has driven innovation in fuel production technologies, catalyzed the development of more advanced refining processes, and spurred research into alternative fuel additives. Additionally, it has influenced the automotive industry, encouraging the development of vehicles with enhanced evaporative emission control systems.
Compliance with RVP regulations has also necessitated significant investments in infrastructure and testing equipment at refineries and distribution terminals. Regular monitoring and reporting of gasoline RVP levels have become standard practice, with substantial penalties for non-compliance. This has created a new market for RVP testing equipment and services, as well as consulting expertise in gasoline blending strategies.
The regulatory landscape continues to evolve, with ongoing discussions about potential further reductions in RVP limits and the expansion of regulated areas. These potential changes keep refineries and fuel producers in a state of constant adaptation, balancing the need for environmental compliance with the demands for high-performance, cost-effective fuel products.
Fuel Blending Optimization
Fuel blending optimization plays a crucial role in the production of gasoline that meets specific Reid Vapor Pressure (RVP) requirements. The contribution of heptane to gasoline's RVP is a key consideration in this process. Refineries must carefully balance the composition of various hydrocarbon components to achieve the desired RVP while maintaining other essential fuel properties.
The optimization process involves sophisticated mathematical models and algorithms that account for the complex interactions between different fuel components. These models consider the non-linear blending characteristics of various hydrocarbons, including heptane, and their impact on the final product's RVP. Advanced software tools are employed to simulate and predict the behavior of fuel blends, allowing refiners to make informed decisions about component ratios.
One of the primary challenges in fuel blending optimization is the variability of feedstocks and the need to adapt to changing market demands. Refineries must be able to adjust their blending strategies quickly to accommodate fluctuations in crude oil quality, seasonal RVP requirements, and shifts in consumer preferences. This necessitates a flexible and responsive optimization approach that can handle real-time adjustments to blending recipes.
The optimization process also takes into account economic factors, such as the cost of different blend components and the market value of the final product. Refiners aim to minimize production costs while meeting regulatory standards and customer specifications. This often involves a delicate balance between using lower-cost components and maintaining the required fuel quality, including the appropriate RVP level influenced by heptane and other light hydrocarbons.
Advanced control systems and online analyzers are increasingly being integrated into the blending process to enable real-time optimization. These systems continuously monitor key parameters, including RVP, and make automatic adjustments to the blend composition. This dynamic approach ensures that the final product consistently meets specifications while maximizing efficiency and minimizing waste.
Furthermore, the optimization of fuel blending extends beyond the refinery to the entire supply chain. Considerations such as storage stability, transportation logistics, and regional regulatory requirements all factor into the overall optimization strategy. Refiners must anticipate how the blended fuel will perform under various conditions and ensure that the RVP remains within acceptable limits throughout the distribution process.
As environmental regulations become more stringent, fuel blending optimization is also evolving to address emissions concerns. The inclusion of renewable components, such as ethanol, introduces additional complexity to the blending process and its impact on RVP. Optimizing these blends requires a comprehensive understanding of how biofuel components interact with traditional petroleum-based hydrocarbons, including heptane, to affect the final product's vapor pressure characteristics.
The optimization process involves sophisticated mathematical models and algorithms that account for the complex interactions between different fuel components. These models consider the non-linear blending characteristics of various hydrocarbons, including heptane, and their impact on the final product's RVP. Advanced software tools are employed to simulate and predict the behavior of fuel blends, allowing refiners to make informed decisions about component ratios.
One of the primary challenges in fuel blending optimization is the variability of feedstocks and the need to adapt to changing market demands. Refineries must be able to adjust their blending strategies quickly to accommodate fluctuations in crude oil quality, seasonal RVP requirements, and shifts in consumer preferences. This necessitates a flexible and responsive optimization approach that can handle real-time adjustments to blending recipes.
The optimization process also takes into account economic factors, such as the cost of different blend components and the market value of the final product. Refiners aim to minimize production costs while meeting regulatory standards and customer specifications. This often involves a delicate balance between using lower-cost components and maintaining the required fuel quality, including the appropriate RVP level influenced by heptane and other light hydrocarbons.
Advanced control systems and online analyzers are increasingly being integrated into the blending process to enable real-time optimization. These systems continuously monitor key parameters, including RVP, and make automatic adjustments to the blend composition. This dynamic approach ensures that the final product consistently meets specifications while maximizing efficiency and minimizing waste.
Furthermore, the optimization of fuel blending extends beyond the refinery to the entire supply chain. Considerations such as storage stability, transportation logistics, and regional regulatory requirements all factor into the overall optimization strategy. Refiners must anticipate how the blended fuel will perform under various conditions and ensure that the RVP remains within acceptable limits throughout the distribution process.
As environmental regulations become more stringent, fuel blending optimization is also evolving to address emissions concerns. The inclusion of renewable components, such as ethanol, introduces additional complexity to the blending process and its impact on RVP. Optimizing these blends requires a comprehensive understanding of how biofuel components interact with traditional petroleum-based hydrocarbons, including heptane, to affect the final product's vapor pressure characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!