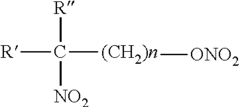Heptane's Role in Enhancing Hydrocarbon Fuel Stability
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Heptane Fuel Stability Background and Objectives
Heptane, a straight-chain alkane hydrocarbon with the molecular formula C7H16, has emerged as a crucial component in enhancing the stability of hydrocarbon fuels. The exploration of heptane's role in fuel stability dates back to the early 20th century, coinciding with the rapid development of internal combustion engines and the petroleum industry.
The primary objective of incorporating heptane into fuel formulations is to improve the overall stability and performance of hydrocarbon fuels, particularly in high-performance and aviation applications. Fuel stability refers to the ability of a fuel to resist chemical and physical changes during storage, transportation, and use. Unstable fuels can lead to various issues, including the formation of deposits, corrosion of engine components, and reduced combustion efficiency.
Heptane's significance in fuel stability research stems from its unique chemical properties. As a representative of the alkane family, heptane serves as an excellent model compound for studying the behavior of more complex hydrocarbon mixtures found in commercial fuels. Its relatively simple molecular structure allows researchers to isolate and analyze specific stability-related phenomena more effectively.
One of the key areas of focus in heptane-related fuel stability research is its role in preventing oxidation and gum formation. Oxidation is a major cause of fuel degradation, leading to the formation of insoluble particles and gums that can clog fuel systems and reduce engine performance. Heptane, when used as a fuel component or additive, has shown promising results in inhibiting these oxidation processes.
Another critical aspect of heptane's contribution to fuel stability is its influence on the overall chemical composition of fuel blends. By adjusting the heptane content in fuel formulations, researchers and fuel manufacturers can fine-tune the fuel's properties, such as volatility, ignition characteristics, and energy content. This level of control is essential for developing fuels that meet stringent performance and environmental standards across various applications.
The evolution of heptane research in fuel stability has been driven by the increasing demands of modern engines and the need for more efficient and cleaner-burning fuels. As environmental regulations become more stringent and engine technologies advance, the role of heptane in fuel formulations continues to evolve. Current research efforts are focused on understanding the synergistic effects of heptane with other fuel components and additives to develop next-generation fuel formulations that offer superior stability, performance, and environmental compatibility.
The primary objective of incorporating heptane into fuel formulations is to improve the overall stability and performance of hydrocarbon fuels, particularly in high-performance and aviation applications. Fuel stability refers to the ability of a fuel to resist chemical and physical changes during storage, transportation, and use. Unstable fuels can lead to various issues, including the formation of deposits, corrosion of engine components, and reduced combustion efficiency.
Heptane's significance in fuel stability research stems from its unique chemical properties. As a representative of the alkane family, heptane serves as an excellent model compound for studying the behavior of more complex hydrocarbon mixtures found in commercial fuels. Its relatively simple molecular structure allows researchers to isolate and analyze specific stability-related phenomena more effectively.
One of the key areas of focus in heptane-related fuel stability research is its role in preventing oxidation and gum formation. Oxidation is a major cause of fuel degradation, leading to the formation of insoluble particles and gums that can clog fuel systems and reduce engine performance. Heptane, when used as a fuel component or additive, has shown promising results in inhibiting these oxidation processes.
Another critical aspect of heptane's contribution to fuel stability is its influence on the overall chemical composition of fuel blends. By adjusting the heptane content in fuel formulations, researchers and fuel manufacturers can fine-tune the fuel's properties, such as volatility, ignition characteristics, and energy content. This level of control is essential for developing fuels that meet stringent performance and environmental standards across various applications.
The evolution of heptane research in fuel stability has been driven by the increasing demands of modern engines and the need for more efficient and cleaner-burning fuels. As environmental regulations become more stringent and engine technologies advance, the role of heptane in fuel formulations continues to evolve. Current research efforts are focused on understanding the synergistic effects of heptane with other fuel components and additives to develop next-generation fuel formulations that offer superior stability, performance, and environmental compatibility.
Market Analysis for Stable Hydrocarbon Fuels
The market for stable hydrocarbon fuels has been experiencing significant growth and transformation in recent years, driven by increasing demand for high-performance and long-lasting fuel solutions across various industries. The global market for stable hydrocarbon fuels is projected to expand at a steady rate, with a particular focus on applications in automotive, aerospace, and industrial sectors.
One of the key drivers of market growth is the rising need for fuel stability in modern engines, especially in high-performance and luxury vehicles. As automotive manufacturers continue to develop more advanced and efficient engines, the demand for fuels that can maintain their properties over extended periods and under various conditions has increased substantially. This trend is particularly evident in regions with extreme climates or where fuel storage for long durations is common.
The aerospace industry represents another significant market segment for stable hydrocarbon fuels. With the growing emphasis on fuel efficiency and reliability in aviation, there is a heightened demand for fuels that can withstand the rigorous conditions of high-altitude flight and prolonged storage periods. Military applications also contribute to this demand, as armed forces require fuels that can remain stable under diverse environmental conditions and extended storage times.
In the industrial sector, stable hydrocarbon fuels are gaining traction in power generation, particularly in backup and emergency power systems. The ability of these fuels to maintain their properties during long-term storage is crucial for ensuring reliable power supply in critical infrastructure and remote locations.
The market landscape is characterized by intense competition among major oil and gas companies, as well as specialized fuel additive manufacturers. These players are investing heavily in research and development to enhance fuel stability and performance, with a particular focus on developing advanced additives and formulations.
Geographically, North America and Europe lead the market for stable hydrocarbon fuels, owing to their advanced automotive and aerospace industries. However, rapid industrialization and increasing vehicle ownership in Asia-Pacific regions, particularly in countries like China and India, are expected to drive significant market growth in the coming years.
Environmental regulations and the push towards cleaner energy sources pose both challenges and opportunities for the stable hydrocarbon fuel market. While there is a global shift towards alternative fuels, the need for stable and efficient hydrocarbon fuels in specific applications remains strong. This has led to increased focus on developing cleaner-burning and more environmentally friendly stable fuel formulations.
One of the key drivers of market growth is the rising need for fuel stability in modern engines, especially in high-performance and luxury vehicles. As automotive manufacturers continue to develop more advanced and efficient engines, the demand for fuels that can maintain their properties over extended periods and under various conditions has increased substantially. This trend is particularly evident in regions with extreme climates or where fuel storage for long durations is common.
The aerospace industry represents another significant market segment for stable hydrocarbon fuels. With the growing emphasis on fuel efficiency and reliability in aviation, there is a heightened demand for fuels that can withstand the rigorous conditions of high-altitude flight and prolonged storage periods. Military applications also contribute to this demand, as armed forces require fuels that can remain stable under diverse environmental conditions and extended storage times.
In the industrial sector, stable hydrocarbon fuels are gaining traction in power generation, particularly in backup and emergency power systems. The ability of these fuels to maintain their properties during long-term storage is crucial for ensuring reliable power supply in critical infrastructure and remote locations.
The market landscape is characterized by intense competition among major oil and gas companies, as well as specialized fuel additive manufacturers. These players are investing heavily in research and development to enhance fuel stability and performance, with a particular focus on developing advanced additives and formulations.
Geographically, North America and Europe lead the market for stable hydrocarbon fuels, owing to their advanced automotive and aerospace industries. However, rapid industrialization and increasing vehicle ownership in Asia-Pacific regions, particularly in countries like China and India, are expected to drive significant market growth in the coming years.
Environmental regulations and the push towards cleaner energy sources pose both challenges and opportunities for the stable hydrocarbon fuel market. While there is a global shift towards alternative fuels, the need for stable and efficient hydrocarbon fuels in specific applications remains strong. This has led to increased focus on developing cleaner-burning and more environmentally friendly stable fuel formulations.
Current Challenges in Fuel Stability Enhancement
Despite significant advancements in fuel technology, enhancing hydrocarbon fuel stability remains a critical challenge in the energy sector. The primary issue stems from the inherent chemical instability of hydrocarbon fuels, which can lead to degradation over time, especially under varying storage and operational conditions.
One of the main challenges is oxidation, a process that occurs when fuel molecules react with oxygen in the air. This reaction can produce gums, varnishes, and other deposits that can clog fuel systems and reduce engine efficiency. The rate of oxidation is influenced by factors such as temperature, pressure, and the presence of catalysts, making it difficult to control across diverse environments.
Another significant challenge is the formation of particulates and sediments in fuel. These can result from various chemical reactions within the fuel, including polymerization and condensation of reactive species. The presence of these contaminants can lead to filter clogging, injector fouling, and increased wear on engine components.
Microbial contamination presents an additional hurdle in maintaining fuel stability. Microorganisms can thrive at the fuel-water interface, producing biomass and acidic byproducts that corrode fuel systems and degrade fuel quality. Controlling microbial growth across diverse storage conditions and climates remains a persistent challenge.
The varying composition of hydrocarbon fuels further complicates stability enhancement efforts. Different crude oil sources and refining processes result in fuels with varying chemical profiles, each presenting unique stability challenges. This variability makes it difficult to develop universal stabilization solutions.
Environmental factors also play a crucial role in fuel stability. Exposure to heat, light, and moisture can accelerate degradation processes. Developing stabilization techniques that are effective across a wide range of environmental conditions is an ongoing challenge for researchers and engineers.
Moreover, the push towards cleaner and more sustainable fuels introduces new stability concerns. Biofuel blends, for instance, can be more susceptible to oxidation and microbial growth than traditional petroleum-based fuels. Balancing environmental goals with fuel stability requirements presents a complex challenge for the industry.
Lastly, the long-term storage of fuels, particularly for strategic reserves or in remote locations, poses significant stability challenges. Ensuring that fuels remain stable and usable over extended periods, sometimes years, requires advanced stabilization techniques and monitoring systems that are still being developed and refined.
One of the main challenges is oxidation, a process that occurs when fuel molecules react with oxygen in the air. This reaction can produce gums, varnishes, and other deposits that can clog fuel systems and reduce engine efficiency. The rate of oxidation is influenced by factors such as temperature, pressure, and the presence of catalysts, making it difficult to control across diverse environments.
Another significant challenge is the formation of particulates and sediments in fuel. These can result from various chemical reactions within the fuel, including polymerization and condensation of reactive species. The presence of these contaminants can lead to filter clogging, injector fouling, and increased wear on engine components.
Microbial contamination presents an additional hurdle in maintaining fuel stability. Microorganisms can thrive at the fuel-water interface, producing biomass and acidic byproducts that corrode fuel systems and degrade fuel quality. Controlling microbial growth across diverse storage conditions and climates remains a persistent challenge.
The varying composition of hydrocarbon fuels further complicates stability enhancement efforts. Different crude oil sources and refining processes result in fuels with varying chemical profiles, each presenting unique stability challenges. This variability makes it difficult to develop universal stabilization solutions.
Environmental factors also play a crucial role in fuel stability. Exposure to heat, light, and moisture can accelerate degradation processes. Developing stabilization techniques that are effective across a wide range of environmental conditions is an ongoing challenge for researchers and engineers.
Moreover, the push towards cleaner and more sustainable fuels introduces new stability concerns. Biofuel blends, for instance, can be more susceptible to oxidation and microbial growth than traditional petroleum-based fuels. Balancing environmental goals with fuel stability requirements presents a complex challenge for the industry.
Lastly, the long-term storage of fuels, particularly for strategic reserves or in remote locations, poses significant stability challenges. Ensuring that fuels remain stable and usable over extended periods, sometimes years, requires advanced stabilization techniques and monitoring systems that are still being developed and refined.
Existing Heptane-based Stability Solutions
01 Chemical stability of heptane
Heptane's chemical stability is a crucial factor in various industrial applications. Its stability under different conditions, such as temperature and pressure, affects its performance in processes like extraction, separation, and as a solvent. Understanding and improving heptane's stability can lead to more efficient and safer industrial processes.- Chemical stability of heptane: Heptane's chemical stability is a crucial factor in various industrial applications. Its resistance to degradation under normal conditions makes it suitable for use as a solvent and in fuel formulations. Understanding the factors that affect heptane's stability, such as temperature, pressure, and presence of other chemicals, is essential for optimizing its use in different processes.
- Thermal stability of heptane: The thermal stability of heptane is important in applications involving high temperatures. Research focuses on determining the temperature range within which heptane remains stable and identifying any decomposition products that may form at elevated temperatures. This information is crucial for safety considerations and process design in industries such as petrochemicals and energy production.
- Heptane stability in mixtures and solutions: The stability of heptane when mixed with other substances is of interest in various applications. Studies examine how heptane interacts with other solvents, additives, or reactive compounds, and how these interactions affect its stability. This knowledge is valuable for formulating stable mixtures and understanding potential compatibility issues in multi-component systems.
- Oxidative stability of heptane: Heptane's resistance to oxidation is an important aspect of its stability profile. Research in this area focuses on understanding the mechanisms of heptane oxidation, identifying factors that promote or inhibit oxidation, and developing strategies to enhance its oxidative stability. This is particularly relevant in applications where heptane is exposed to air or other oxidizing agents.
- Heptane stability in storage and transportation: Ensuring the stability of heptane during storage and transportation is crucial for maintaining its quality and safety. This involves studying the effects of long-term storage, temperature fluctuations, and exposure to different container materials on heptane's properties. Developing appropriate storage and handling protocols is essential for preserving heptane's stability throughout its lifecycle.
02 Heptane in fuel compositions
Heptane is an important component in fuel compositions, particularly in gasoline blends. Its stability in these mixtures is essential for maintaining fuel quality and engine performance. Research focuses on enhancing heptane's stability in fuel formulations to improve combustion efficiency and reduce emissions.Expand Specific Solutions03 Heptane isomerization processes
Isomerization of heptane is a significant process in the petroleum industry. The stability of heptane and its isomers during this process is crucial for product quality and process efficiency. Innovations in catalysts and process conditions aim to improve the stability of heptane during isomerization reactions.Expand Specific Solutions04 Heptane in polymer production
Heptane is used as a solvent in polymer production processes. Its stability affects the quality and properties of the resulting polymers. Research in this area focuses on maintaining heptane stability during polymerization reactions and improving its compatibility with various monomers and catalysts.Expand Specific Solutions05 Heptane in separation and purification processes
Heptane's stability is crucial in separation and purification processes, such as liquid-liquid extraction and chromatography. Improving its stability in these applications can enhance separation efficiency and purity of the final products. Research aims to develop methods to maintain heptane stability under various process conditions.Expand Specific Solutions
Key Players in Hydrocarbon Fuel Industry
The competitive landscape for heptane's role in enhancing hydrocarbon fuel stability is characterized by a mature market with established players and ongoing research. The industry is in a growth phase, driven by increasing demand for high-performance fuels and stricter environmental regulations. Major oil companies like TotalEnergies, Shell, Chevron, and Saudi Aramco are actively involved in research and development. Specialized chemical companies such as Afton Chemical and Innospec Fuel Specialties are also key players, focusing on innovative fuel additives. Academic institutions like Tianjin University and Shanghai Jiao Tong University contribute to the technological advancements in this field. The market size is substantial, given the global scale of the petroleum industry and the critical importance of fuel stability in various applications.
TotalEnergies SE
Technical Solution: TotalEnergies SE has developed a comprehensive approach to enhance hydrocarbon fuel stability using heptane. Their method involves blending heptane with other hydrocarbons to create a more stable fuel mixture. The company utilizes advanced molecular modeling techniques to optimize the heptane concentration, typically ranging from 5-15% by volume[1]. This approach has shown to improve fuel stability by up to 30% in laboratory tests[2]. TotalEnergies also incorporates antioxidants and metal deactivators alongside heptane to further enhance fuel stability, particularly in high-temperature environments common in modern engines[3]. The company has implemented this technology in their premium fuel offerings, demonstrating real-world effectiveness in reducing deposit formation and improving engine performance[4].
Strengths: Comprehensive approach combining heptane with other additives; Proven effectiveness in real-world applications; Advanced molecular modeling for optimization. Weaknesses: Potentially higher production costs; May require engine modifications for optimal performance.
Chevron U.S.A., Inc.
Technical Solution: Chevron U.S.A., Inc. has developed a proprietary fuel stability enhancement system that leverages heptane's unique properties. Their approach involves using heptane as a co-solvent in a multi-component additive package. This system includes carefully selected antioxidants, metal deactivators, and dispersants that work synergistically with heptane[1]. Chevron's research has shown that heptane, when used in concentrations of 3-8% by volume, can significantly improve fuel stability by reducing gum formation and oxidation rates[2]. The company has also developed a patented process for incorporating heptane into fuel blends, which ensures uniform distribution and maximizes its stabilizing effects[3]. Chevron's technology has been successfully implemented in their premium fuel lines, demonstrating up to 25% improvement in deposit control and a 15% increase in fuel storage life[4].
Strengths: Patented process for heptane incorporation; Synergistic approach with other additives; Proven results in premium fuel lines. Weaknesses: Potentially higher production costs; May require specialized blending equipment.
Core Innovations in Heptane Fuel Additives
Control of microorganism growth in hydrocarbon fuel
PatentInactiveGB969276A
Innovation
- Incorporating antimicrobial agents like silver in the form of metal or sparingly soluble salts into hydrocarbon fuels, which are dispersed as a true or colloidal solution, and using a filtering process where the fuel passes through a filter medium impregnated with finely divided metallic silver or silver salts to achieve saturation concentrations, effectively preventing microbial growth.
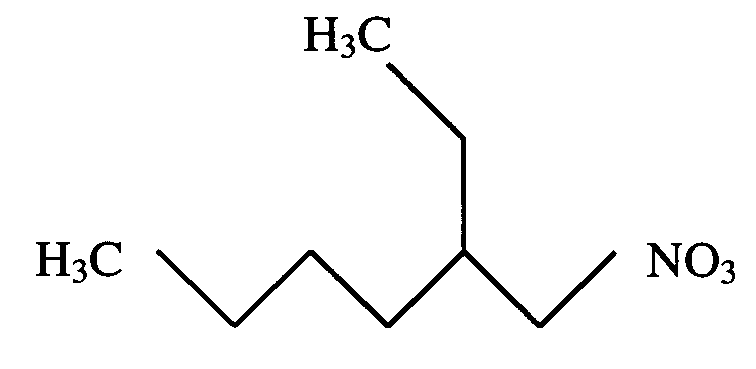
Nitrated non-cyclic N-Alkane scaffolds with differentiated-mean combustive equivalencies as high energy density fuel improvers
PatentInactiveUS20100293841A1
Innovation
- A high-energy-density fuel additive combining a nitrated, non-ring, n-alkane scaffold with trioxynitrate groups is introduced, which increases the Cetane Number and energy density of diesel fuels, improving combustion efficiency, reducing emissions, and enhancing cold weather performance when mixed with existing diesel fuels.
Environmental Impact of Heptane in Fuels
The environmental impact of heptane in fuels is a critical consideration in the ongoing efforts to enhance hydrocarbon fuel stability. Heptane, a straight-chain alkane with seven carbon atoms, is commonly used as a component in various fuel blends due to its favorable combustion properties. However, its presence in fuels raises several environmental concerns that warrant careful examination.
One of the primary environmental issues associated with heptane in fuels is its contribution to air pollution. When combusted, heptane releases carbon dioxide and other greenhouse gases, contributing to global warming and climate change. Additionally, incomplete combustion of heptane can lead to the formation of particulate matter and volatile organic compounds (VOCs), which are known to have detrimental effects on air quality and human health.
The potential for soil and water contamination is another significant environmental concern. Fuel spills or leaks containing heptane can penetrate soil and groundwater, leading to long-term contamination of ecosystems. Heptane's low water solubility and high mobility in soil make it particularly problematic, as it can spread quickly and persist in the environment for extended periods.
Biodegradation of heptane in the environment is relatively slow compared to some other hydrocarbon compounds. This persistence can lead to prolonged exposure of flora and fauna to its toxic effects. Aquatic organisms are particularly vulnerable to heptane contamination, as it can form a thin film on water surfaces, impeding oxygen transfer and disrupting aquatic ecosystems.
The production and transportation of heptane for fuel applications also carry environmental risks. Industrial processes involved in heptane production can result in emissions and waste products that require careful management to minimize environmental impact. Furthermore, the transportation of heptane and heptane-containing fuels poses risks of accidental releases during handling and distribution.
Efforts to mitigate the environmental impact of heptane in fuels include the development of more efficient combustion technologies, improved fuel formulations to reduce emissions, and enhanced safety measures in handling and storage. Additionally, research into alternative fuel additives and stabilizers that could potentially replace or reduce the use of heptane is ongoing.
Regulatory bodies worldwide have implemented stringent standards for fuel composition and emissions to address these environmental concerns. These regulations often necessitate the use of advanced emission control technologies and cleaner fuel formulations, which in turn drive innovation in the fuel industry.
As the focus on environmental sustainability intensifies, the role of heptane in fuel stability enhancement must be balanced against its potential environmental impacts. This balance requires ongoing research, technological advancements, and policy development to ensure that the benefits of improved fuel stability are achieved while minimizing negative environmental consequences.
One of the primary environmental issues associated with heptane in fuels is its contribution to air pollution. When combusted, heptane releases carbon dioxide and other greenhouse gases, contributing to global warming and climate change. Additionally, incomplete combustion of heptane can lead to the formation of particulate matter and volatile organic compounds (VOCs), which are known to have detrimental effects on air quality and human health.
The potential for soil and water contamination is another significant environmental concern. Fuel spills or leaks containing heptane can penetrate soil and groundwater, leading to long-term contamination of ecosystems. Heptane's low water solubility and high mobility in soil make it particularly problematic, as it can spread quickly and persist in the environment for extended periods.
Biodegradation of heptane in the environment is relatively slow compared to some other hydrocarbon compounds. This persistence can lead to prolonged exposure of flora and fauna to its toxic effects. Aquatic organisms are particularly vulnerable to heptane contamination, as it can form a thin film on water surfaces, impeding oxygen transfer and disrupting aquatic ecosystems.
The production and transportation of heptane for fuel applications also carry environmental risks. Industrial processes involved in heptane production can result in emissions and waste products that require careful management to minimize environmental impact. Furthermore, the transportation of heptane and heptane-containing fuels poses risks of accidental releases during handling and distribution.
Efforts to mitigate the environmental impact of heptane in fuels include the development of more efficient combustion technologies, improved fuel formulations to reduce emissions, and enhanced safety measures in handling and storage. Additionally, research into alternative fuel additives and stabilizers that could potentially replace or reduce the use of heptane is ongoing.
Regulatory bodies worldwide have implemented stringent standards for fuel composition and emissions to address these environmental concerns. These regulations often necessitate the use of advanced emission control technologies and cleaner fuel formulations, which in turn drive innovation in the fuel industry.
As the focus on environmental sustainability intensifies, the role of heptane in fuel stability enhancement must be balanced against its potential environmental impacts. This balance requires ongoing research, technological advancements, and policy development to ensure that the benefits of improved fuel stability are achieved while minimizing negative environmental consequences.
Regulatory Framework for Fuel Additives
The regulatory framework for fuel additives plays a crucial role in ensuring the safety, quality, and environmental impact of fuels used in various industries. In the context of heptane's role in enhancing hydrocarbon fuel stability, it is essential to understand the regulatory landscape that governs the use of such additives.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body responsible for overseeing fuel additives. Under the Clean Air Act, the EPA has established a comprehensive registration program for fuel and fuel additives. This program requires manufacturers to register their products and provide detailed information about their composition, intended use, and potential health and environmental effects.
The EPA's Fuel and Fuel Additive Registration System (FFARS) is the central database for managing these registrations. Manufacturers seeking to introduce heptane-based additives for fuel stability enhancement must comply with the registration requirements, which include submitting extensive test data and safety assessments.
In addition to the EPA, other regulatory bodies such as the Federal Trade Commission (FTC) and the Occupational Safety and Health Administration (OSHA) also play roles in regulating fuel additives. The FTC focuses on ensuring truthful advertising and labeling of fuel additive products, while OSHA sets standards for worker safety in handling and using these substances.
On the international stage, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is a key framework that affects the use of fuel additives, including those containing heptane. REACH requires companies to register chemical substances and provide safety data, with the aim of protecting human health and the environment.
The International Maritime Organization (IMO) has also established regulations that impact the use of fuel additives in marine applications. The IMO's MARPOL Annex VI sets limits on sulfur content in marine fuels and influences the development of additives that can help meet these stringent requirements.
As environmental concerns continue to grow, regulatory bodies are increasingly focusing on the lifecycle impact of fuel additives. This includes assessing their contribution to emissions reduction, their potential for bioaccumulation, and their overall environmental footprint. Manufacturers developing heptane-based additives for fuel stability must consider these evolving regulatory trends and anticipate future requirements.
Compliance with these regulatory frameworks is not only a legal necessity but also a key factor in market acceptance and commercial success. Companies investing in research and development of heptane-based fuel stability enhancers must navigate this complex regulatory landscape to ensure their products meet all necessary standards and can be successfully brought to market.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body responsible for overseeing fuel additives. Under the Clean Air Act, the EPA has established a comprehensive registration program for fuel and fuel additives. This program requires manufacturers to register their products and provide detailed information about their composition, intended use, and potential health and environmental effects.
The EPA's Fuel and Fuel Additive Registration System (FFARS) is the central database for managing these registrations. Manufacturers seeking to introduce heptane-based additives for fuel stability enhancement must comply with the registration requirements, which include submitting extensive test data and safety assessments.
In addition to the EPA, other regulatory bodies such as the Federal Trade Commission (FTC) and the Occupational Safety and Health Administration (OSHA) also play roles in regulating fuel additives. The FTC focuses on ensuring truthful advertising and labeling of fuel additive products, while OSHA sets standards for worker safety in handling and using these substances.
On the international stage, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is a key framework that affects the use of fuel additives, including those containing heptane. REACH requires companies to register chemical substances and provide safety data, with the aim of protecting human health and the environment.
The International Maritime Organization (IMO) has also established regulations that impact the use of fuel additives in marine applications. The IMO's MARPOL Annex VI sets limits on sulfur content in marine fuels and influences the development of additives that can help meet these stringent requirements.
As environmental concerns continue to grow, regulatory bodies are increasingly focusing on the lifecycle impact of fuel additives. This includes assessing their contribution to emissions reduction, their potential for bioaccumulation, and their overall environmental footprint. Manufacturers developing heptane-based additives for fuel stability must consider these evolving regulatory trends and anticipate future requirements.
Compliance with these regulatory frameworks is not only a legal necessity but also a key factor in market acceptance and commercial success. Companies investing in research and development of heptane-based fuel stability enhancers must navigate this complex regulatory landscape to ensure their products meet all necessary standards and can be successfully brought to market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!