Heptane's Role in Nanotube Synthesis by Chemical Vapor Deposition
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CNT Synthesis Background
Carbon nanotubes (CNTs) have emerged as a revolutionary material in the field of nanotechnology since their discovery in 1991. These cylindrical structures of carbon atoms exhibit exceptional mechanical, electrical, and thermal properties, making them highly attractive for various applications in electronics, materials science, and energy storage.
The synthesis of CNTs has been a subject of intense research over the past three decades. Among the various methods developed, Chemical Vapor Deposition (CVD) has gained prominence due to its scalability, controllability, and ability to produce high-quality nanotubes. CVD involves the decomposition of carbon-containing precursors over catalytic surfaces at elevated temperatures, resulting in the growth of CNTs.
Heptane, a hydrocarbon with the molecular formula C7H16, has emerged as a significant precursor in CNT synthesis via CVD. Its role in the process is multifaceted, contributing to both the carbon source and the reaction environment. As a carbon source, heptane undergoes thermal decomposition, releasing carbon atoms that serve as building blocks for CNT growth.
The use of heptane in CNT synthesis offers several advantages. Its relatively low boiling point (98.4°C) allows for easy vaporization and control of precursor delivery. Additionally, heptane's chemical structure provides a balanced carbon-to-hydrogen ratio, which can influence the growth kinetics and morphology of the resulting nanotubes.
Research has shown that the decomposition of heptane during CVD can lead to the formation of various intermediate species, including smaller hydrocarbons and radicals. These intermediates play crucial roles in the nucleation and growth of CNTs on catalytic surfaces. The interaction between heptane-derived species and metal catalysts, such as iron, nickel, or cobalt, is fundamental to the CNT growth mechanism.
The choice of heptane as a precursor also impacts the type and quality of CNTs produced. By manipulating process parameters such as temperature, pressure, and catalyst composition, researchers can influence the diameter, length, and chirality of the synthesized nanotubes. This level of control is essential for tailoring CNT properties to specific applications.
Furthermore, the use of heptane in CVD has contributed to the development of novel synthesis techniques, including floating catalyst methods and aerosol-assisted CVD. These approaches have expanded the possibilities for large-scale production and in-situ functionalization of CNTs, opening new avenues for their integration into advanced materials and devices.
The synthesis of CNTs has been a subject of intense research over the past three decades. Among the various methods developed, Chemical Vapor Deposition (CVD) has gained prominence due to its scalability, controllability, and ability to produce high-quality nanotubes. CVD involves the decomposition of carbon-containing precursors over catalytic surfaces at elevated temperatures, resulting in the growth of CNTs.
Heptane, a hydrocarbon with the molecular formula C7H16, has emerged as a significant precursor in CNT synthesis via CVD. Its role in the process is multifaceted, contributing to both the carbon source and the reaction environment. As a carbon source, heptane undergoes thermal decomposition, releasing carbon atoms that serve as building blocks for CNT growth.
The use of heptane in CNT synthesis offers several advantages. Its relatively low boiling point (98.4°C) allows for easy vaporization and control of precursor delivery. Additionally, heptane's chemical structure provides a balanced carbon-to-hydrogen ratio, which can influence the growth kinetics and morphology of the resulting nanotubes.
Research has shown that the decomposition of heptane during CVD can lead to the formation of various intermediate species, including smaller hydrocarbons and radicals. These intermediates play crucial roles in the nucleation and growth of CNTs on catalytic surfaces. The interaction between heptane-derived species and metal catalysts, such as iron, nickel, or cobalt, is fundamental to the CNT growth mechanism.
The choice of heptane as a precursor also impacts the type and quality of CNTs produced. By manipulating process parameters such as temperature, pressure, and catalyst composition, researchers can influence the diameter, length, and chirality of the synthesized nanotubes. This level of control is essential for tailoring CNT properties to specific applications.
Furthermore, the use of heptane in CVD has contributed to the development of novel synthesis techniques, including floating catalyst methods and aerosol-assisted CVD. These approaches have expanded the possibilities for large-scale production and in-situ functionalization of CNTs, opening new avenues for their integration into advanced materials and devices.
Market Analysis for CNTs
The market for carbon nanotubes (CNTs) has experienced significant growth in recent years, driven by their exceptional properties and diverse applications across various industries. The global CNT market size was valued at approximately $5.67 billion in 2020 and is projected to reach $10.68 billion by 2028, growing at a CAGR of 8.3% during the forecast period.
The electronics and semiconductor industry remains the largest consumer of CNTs, accounting for over 30% of the market share. CNTs are extensively used in the production of transistors, sensors, and conductive films, contributing to the development of smaller, faster, and more efficient electronic devices. The automotive sector is another key market for CNTs, with applications in lightweight materials, batteries, and fuel cells. The aerospace industry also shows increasing demand for CNTs in composite materials for aircraft structures.
In terms of regional distribution, Asia-Pacific dominates the CNT market, with China and Japan leading in production and consumption. North America and Europe follow closely, driven by research and development activities and advanced manufacturing sectors. Emerging economies in South America and Africa are expected to present new growth opportunities as their industrial bases expand.
The market is characterized by intense competition among key players such as Nanocyl SA, Arkema SA, and Showa Denko K.K. These companies are investing heavily in research and development to improve CNT production methods, including chemical vapor deposition (CVD) techniques using precursors like heptane. The focus on enhancing production efficiency and reducing costs is crucial for market expansion.
Despite the positive outlook, challenges remain in the CNT market. The high production costs and complexity of large-scale manufacturing processes, including those involving heptane in CVD synthesis, continue to limit widespread adoption. Additionally, concerns over potential environmental and health impacts of CNTs pose regulatory challenges that may affect market growth.
Looking ahead, the CNT market is poised for continued expansion, driven by technological advancements and increasing applications in emerging fields such as energy storage, water purification, and biomedical devices. The role of heptane in CNT synthesis by CVD is likely to gain more attention as researchers and manufacturers seek to optimize production processes and enhance the quality of CNTs for specific applications.
The electronics and semiconductor industry remains the largest consumer of CNTs, accounting for over 30% of the market share. CNTs are extensively used in the production of transistors, sensors, and conductive films, contributing to the development of smaller, faster, and more efficient electronic devices. The automotive sector is another key market for CNTs, with applications in lightweight materials, batteries, and fuel cells. The aerospace industry also shows increasing demand for CNTs in composite materials for aircraft structures.
In terms of regional distribution, Asia-Pacific dominates the CNT market, with China and Japan leading in production and consumption. North America and Europe follow closely, driven by research and development activities and advanced manufacturing sectors. Emerging economies in South America and Africa are expected to present new growth opportunities as their industrial bases expand.
The market is characterized by intense competition among key players such as Nanocyl SA, Arkema SA, and Showa Denko K.K. These companies are investing heavily in research and development to improve CNT production methods, including chemical vapor deposition (CVD) techniques using precursors like heptane. The focus on enhancing production efficiency and reducing costs is crucial for market expansion.
Despite the positive outlook, challenges remain in the CNT market. The high production costs and complexity of large-scale manufacturing processes, including those involving heptane in CVD synthesis, continue to limit widespread adoption. Additionally, concerns over potential environmental and health impacts of CNTs pose regulatory challenges that may affect market growth.
Looking ahead, the CNT market is poised for continued expansion, driven by technological advancements and increasing applications in emerging fields such as energy storage, water purification, and biomedical devices. The role of heptane in CNT synthesis by CVD is likely to gain more attention as researchers and manufacturers seek to optimize production processes and enhance the quality of CNTs for specific applications.
Heptane CVD Challenges
Chemical vapor deposition (CVD) using heptane as a carbon source for nanotube synthesis presents several significant challenges that researchers and engineers must address. One of the primary difficulties lies in controlling the decomposition rate of heptane during the CVD process. Heptane's relatively low boiling point and high vapor pressure can lead to rapid and uncontrolled decomposition, resulting in irregular carbon deposition and inconsistent nanotube growth.
Another challenge is maintaining the purity of the synthesized nanotubes. Heptane's complex molecular structure can introduce various carbon-based byproducts during the decomposition process, potentially contaminating the final nanotube product. This impurity issue can significantly affect the structural and electrical properties of the nanotubes, limiting their applicability in high-performance electronic devices and other advanced applications.
Temperature control presents a further obstacle in heptane-based CVD synthesis. The process requires precise temperature management to achieve optimal nanotube growth conditions. However, heptane's sensitivity to temperature fluctuations can lead to inconsistent decomposition rates and varying carbon availability during the synthesis process. This variability can result in nanotubes with non-uniform properties, such as varying diameters, lengths, and chiralities.
The catalytic activity of metal nanoparticles used in the CVD process can also be affected by heptane. Interactions between heptane and the catalyst surface may lead to catalyst poisoning or deactivation, reducing the efficiency of nanotube nucleation and growth. Optimizing catalyst-heptane interactions to maintain high catalytic activity throughout the synthesis process remains a significant challenge.
Scaling up heptane-based CVD processes for industrial production poses additional difficulties. Ensuring uniform gas-phase distribution of heptane vapor across large substrate areas while maintaining precise control over reaction parameters becomes increasingly complex at larger scales. This challenge is critical for achieving consistent nanotube quality and yield in industrial applications.
Environmental and safety concerns associated with heptane usage in CVD processes also need to be addressed. Heptane is a volatile organic compound (VOC) with potential health and environmental impacts. Developing effective containment, recovery, and disposal systems for heptane and its byproducts is essential for the widespread adoption of this synthesis method in industrial settings.
Lastly, optimizing the energy efficiency of heptane-based CVD processes remains a challenge. The energy required for heptane vaporization and maintaining reaction temperatures can be substantial, impacting the overall cost-effectiveness and sustainability of the synthesis method. Developing more energy-efficient reactor designs and process optimizations is crucial for improving the economic viability of heptane-based nanotube production.
Another challenge is maintaining the purity of the synthesized nanotubes. Heptane's complex molecular structure can introduce various carbon-based byproducts during the decomposition process, potentially contaminating the final nanotube product. This impurity issue can significantly affect the structural and electrical properties of the nanotubes, limiting their applicability in high-performance electronic devices and other advanced applications.
Temperature control presents a further obstacle in heptane-based CVD synthesis. The process requires precise temperature management to achieve optimal nanotube growth conditions. However, heptane's sensitivity to temperature fluctuations can lead to inconsistent decomposition rates and varying carbon availability during the synthesis process. This variability can result in nanotubes with non-uniform properties, such as varying diameters, lengths, and chiralities.
The catalytic activity of metal nanoparticles used in the CVD process can also be affected by heptane. Interactions between heptane and the catalyst surface may lead to catalyst poisoning or deactivation, reducing the efficiency of nanotube nucleation and growth. Optimizing catalyst-heptane interactions to maintain high catalytic activity throughout the synthesis process remains a significant challenge.
Scaling up heptane-based CVD processes for industrial production poses additional difficulties. Ensuring uniform gas-phase distribution of heptane vapor across large substrate areas while maintaining precise control over reaction parameters becomes increasingly complex at larger scales. This challenge is critical for achieving consistent nanotube quality and yield in industrial applications.
Environmental and safety concerns associated with heptane usage in CVD processes also need to be addressed. Heptane is a volatile organic compound (VOC) with potential health and environmental impacts. Developing effective containment, recovery, and disposal systems for heptane and its byproducts is essential for the widespread adoption of this synthesis method in industrial settings.
Lastly, optimizing the energy efficiency of heptane-based CVD processes remains a challenge. The energy required for heptane vaporization and maintaining reaction temperatures can be substantial, impacting the overall cost-effectiveness and sustainability of the synthesis method. Developing more energy-efficient reactor designs and process optimizations is crucial for improving the economic viability of heptane-based nanotube production.
Current Heptane CVD Methods
01 Use of heptane in separation processes
Heptane is utilized in various separation processes, particularly in the petrochemical industry. It serves as a solvent or extraction medium for separating different components in mixtures. This application is valuable in processes such as oil refining, where heptane can help isolate specific hydrocarbons or remove impurities.- Use of heptane in separation and extraction processes: Heptane is widely used as a solvent in various separation and extraction processes due to its non-polar nature and low boiling point. It is particularly effective in extracting oils, fats, and other non-polar compounds from mixtures. This property makes it valuable in industries such as petrochemicals, pharmaceuticals, and food processing.
- Heptane as a component in fuel formulations: Heptane is an important component in various fuel formulations, particularly in gasoline blends. It is used to improve the octane rating and combustion characteristics of fuels. The inclusion of heptane in fuel mixtures can enhance engine performance and efficiency in internal combustion engines.
- Application of heptane in polymer production: Heptane plays a role in polymer production processes, particularly in the synthesis and modification of certain types of polymers. It can be used as a reaction medium, a diluent, or a precipitating agent in polymerization reactions. This application is significant in the production of various plastic materials and synthetic rubbers.
- Heptane in analytical and laboratory applications: In analytical chemistry and laboratory settings, heptane is utilized as a solvent for various procedures such as chromatography, spectroscopy, and sample preparation. Its properties make it suitable for dissolving and separating organic compounds, making it valuable in research and quality control applications across multiple scientific disciplines.
- Environmental and safety considerations of heptane use: The use of heptane in industrial and laboratory settings requires careful consideration of environmental and safety factors. As a volatile organic compound, proper handling, storage, and disposal procedures are necessary to minimize environmental impact and ensure worker safety. Regulations and guidelines have been developed to address these concerns in various applications of heptane.
02 Heptane as a component in polymer production
Heptane plays a role in polymer production processes. It can be used as a solvent or diluent in polymerization reactions, helping to control reaction conditions and product properties. Additionally, heptane may be employed in the purification or modification of polymers after synthesis.Expand Specific Solutions03 Application of heptane in fuel formulations
Heptane is an important component in fuel formulations, particularly for internal combustion engines. It is used as a reference fuel in octane rating determinations and can be blended with other hydrocarbons to achieve desired fuel properties. Heptane's inclusion in fuel mixtures affects combustion characteristics and engine performance.Expand Specific Solutions04 Heptane in analytical and laboratory applications
Heptane finds use in various analytical and laboratory applications. It serves as a solvent for chromatography, spectroscopy, and other analytical techniques. Heptane is also employed in the preparation of samples and standards for chemical analysis, contributing to accurate and reliable results in research and quality control processes.Expand Specific Solutions05 Environmental and safety considerations for heptane use
The use of heptane in industrial processes necessitates careful consideration of environmental and safety factors. This includes developing methods for proper handling, storage, and disposal of heptane to minimize environmental impact and ensure worker safety. Additionally, research focuses on finding more environmentally friendly alternatives or optimizing processes to reduce heptane consumption.Expand Specific Solutions
Key Players in CNT Field
The field of nanotube synthesis by chemical vapor deposition using heptane is in a growth phase, with increasing market potential and technological advancements. The global market for carbon nanotubes is expanding, driven by applications in electronics, energy storage, and advanced materials. While the technology is maturing, there's still room for innovation and optimization. Key players like Samsung Electronics, LG Chem, and Tsinghua University are actively involved in research and development, pushing the boundaries of nanotube synthesis techniques. Companies such as Arkema France SA and ZEON Corp. are also contributing to the field, indicating a competitive landscape with both established corporations and specialized materials firms. The involvement of academic institutions like Nanyang Technological University and Harbin Institute of Technology suggests ongoing fundamental research to improve synthesis processes and explore new applications.
China Petroleum & Chemical Corp.
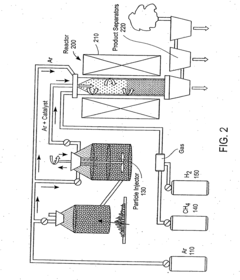
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced chemical vapor deposition (CVD) process for carbon nanotube synthesis using heptane as a carbon source. Their method involves precise control of heptane vapor concentration and temperature in the CVD reactor, resulting in high-quality single-walled carbon nanotubes (SWCNTs) with controlled diameter and chirality[1]. The process utilizes a specially designed catalyst system that enhances the decomposition of heptane and promotes nanotube growth. Sinopec's technique also incorporates in-situ purification steps to minimize amorphous carbon formation, leading to nanotubes with improved purity and structural integrity[3].
Strengths: High-quality SWCNT production, precise control over nanotube properties, and scalable process. Weaknesses: Potentially higher production costs due to the need for specialized equipment and catalysts.
ZEON Corp.
Technical Solution: ZEON Corporation has pioneered a novel approach to carbon nanotube synthesis using heptane in a modified CVD process. Their method employs a proprietary catalyst formulation that enhances the efficiency of heptane decomposition, resulting in rapid nanotube growth rates. ZEON's technique incorporates a unique reactor design that allows for continuous feed of heptane vapor, enabling sustained growth of ultra-long carbon nanotubes[2]. The company has also developed a post-synthesis treatment process that utilizes heptane as a solvent for purification and functionalization of the produced nanotubes, enhancing their compatibility with various polymer matrices[4].
Strengths: Production of ultra-long nanotubes, continuous synthesis capability, and integrated purification process. Weaknesses: Potential limitations in controlling nanotube diameter distribution.
Heptane CVD Innovations
Synthesis of small diameter single-walled carbon nanotubes
PatentActiveUS20070116632A1
Innovation
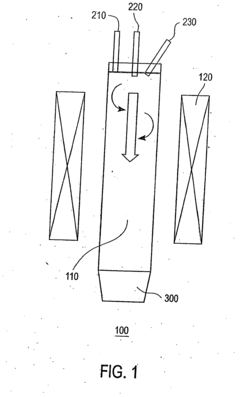
- The method involves supporting metal nanoparticles of controlled particle size on non-carbon containing powdered oxide supports, delivering them as an aerosol into a reaction chamber with a carbon precursor gas, and using a controlled gas flow to minimize wall deposition, followed by separation using cyclone separators to collect high-purity SWNTs with narrow diameter distributions.
Environmental Impact of CVD
The environmental impact of Chemical Vapor Deposition (CVD) in nanotube synthesis, particularly when using heptane as a precursor, is a critical consideration in the development and application of this technology. CVD processes, while efficient for producing high-quality nanotubes, can have significant environmental implications that must be carefully managed.
One of the primary environmental concerns associated with CVD nanotube synthesis is the emission of volatile organic compounds (VOCs). Heptane, being a hydrocarbon, contributes to these emissions when used as a carbon source. These VOCs can lead to the formation of ground-level ozone and smog, potentially impacting air quality in the surrounding areas. Additionally, incomplete combustion of heptane may result in the release of carbon monoxide and other harmful byproducts.
The energy-intensive nature of CVD processes also raises environmental concerns. High temperatures are required to facilitate the decomposition of heptane and the growth of nanotubes, leading to substantial energy consumption. This increased energy demand often translates to higher greenhouse gas emissions, particularly if the energy source is not renewable.
Waste management is another crucial aspect of the environmental impact of CVD nanotube synthesis. The process generates various waste products, including spent catalysts and unreacted precursors. Proper disposal or recycling of these materials is essential to prevent soil and water contamination. Furthermore, the potential release of nanoparticles during the synthesis or handling of nanotubes poses risks to both human health and the environment, necessitating stringent control measures.
Water usage in CVD processes, although not as significant as in some other industrial processes, still contributes to the overall environmental footprint. Cooling systems and post-synthesis cleaning procedures can consume substantial amounts of water, potentially straining local water resources in water-scarce regions.
To mitigate these environmental impacts, researchers and industries are exploring various strategies. These include the development of more efficient CVD processes that reduce energy consumption and improve precursor utilization. Closed-loop systems that recycle unreacted precursors and minimize waste generation are also being implemented. Additionally, the use of alternative, more environmentally friendly precursors to replace or complement heptane is an active area of research.
Regulatory frameworks play a crucial role in managing the environmental impact of CVD nanotube synthesis. Stringent emission controls, waste management protocols, and occupational safety standards are being implemented in many jurisdictions to ensure responsible development and application of this technology. As the field advances, continuous assessment and improvement of environmental performance will be essential to ensure the sustainable growth of nanotube production using CVD methods.
One of the primary environmental concerns associated with CVD nanotube synthesis is the emission of volatile organic compounds (VOCs). Heptane, being a hydrocarbon, contributes to these emissions when used as a carbon source. These VOCs can lead to the formation of ground-level ozone and smog, potentially impacting air quality in the surrounding areas. Additionally, incomplete combustion of heptane may result in the release of carbon monoxide and other harmful byproducts.
The energy-intensive nature of CVD processes also raises environmental concerns. High temperatures are required to facilitate the decomposition of heptane and the growth of nanotubes, leading to substantial energy consumption. This increased energy demand often translates to higher greenhouse gas emissions, particularly if the energy source is not renewable.
Waste management is another crucial aspect of the environmental impact of CVD nanotube synthesis. The process generates various waste products, including spent catalysts and unreacted precursors. Proper disposal or recycling of these materials is essential to prevent soil and water contamination. Furthermore, the potential release of nanoparticles during the synthesis or handling of nanotubes poses risks to both human health and the environment, necessitating stringent control measures.
Water usage in CVD processes, although not as significant as in some other industrial processes, still contributes to the overall environmental footprint. Cooling systems and post-synthesis cleaning procedures can consume substantial amounts of water, potentially straining local water resources in water-scarce regions.
To mitigate these environmental impacts, researchers and industries are exploring various strategies. These include the development of more efficient CVD processes that reduce energy consumption and improve precursor utilization. Closed-loop systems that recycle unreacted precursors and minimize waste generation are also being implemented. Additionally, the use of alternative, more environmentally friendly precursors to replace or complement heptane is an active area of research.
Regulatory frameworks play a crucial role in managing the environmental impact of CVD nanotube synthesis. Stringent emission controls, waste management protocols, and occupational safety standards are being implemented in many jurisdictions to ensure responsible development and application of this technology. As the field advances, continuous assessment and improvement of environmental performance will be essential to ensure the sustainable growth of nanotube production using CVD methods.
Scalability of Heptane CVD
The scalability of heptane-based chemical vapor deposition (CVD) for nanotube synthesis presents both opportunities and challenges in industrial applications. As the demand for high-quality carbon nanotubes continues to grow, the ability to scale up production processes becomes increasingly critical. Heptane, a hydrocarbon precursor, offers several advantages in terms of scalability due to its relatively low cost, availability, and ease of handling.
One of the key factors contributing to the scalability of heptane CVD is its compatibility with existing industrial infrastructure. Many chemical processing facilities already have the necessary equipment and safety protocols in place to handle hydrocarbon-based processes, making the integration of heptane CVD more straightforward compared to other precursor materials. This compatibility reduces the initial capital investment required for large-scale implementation.
Furthermore, the vapor pressure characteristics of heptane allow for efficient and controlled delivery of the precursor into the CVD reactor. This property enables precise control over the growth conditions, which is essential for maintaining consistent nanotube quality across larger production volumes. The ability to fine-tune growth parameters is crucial when scaling up, as it helps mitigate variations that can arise from increased reactor sizes or longer production runs.
However, scaling up heptane CVD also presents several challenges that need to be addressed. One of the primary concerns is the potential for increased carbon deposition on reactor walls and other surfaces as production volumes increase. This unwanted deposition can lead to reduced efficiency and increased maintenance requirements, potentially offsetting some of the scalability benefits.
Another consideration is the need for improved heat management systems in larger reactors. As the reactor size increases, maintaining uniform temperature distribution becomes more challenging, which can affect the consistency of nanotube growth. Advanced thermal management techniques and reactor designs may be necessary to ensure homogeneous growth conditions across larger production volumes.
The scalability of heptane CVD also depends on the development of more efficient catalyst systems. While current catalysts work well at laboratory scales, their performance and longevity may need to be enhanced to support continuous, high-volume production. Research into catalyst formulations that remain active for longer periods and can withstand the rigors of industrial-scale processes is ongoing.
In conclusion, while heptane CVD shows promise for scalable nanotube synthesis, realizing its full potential requires addressing several technical and engineering challenges. Ongoing research and development efforts focus on optimizing reactor designs, improving catalyst performance, and enhancing process control to overcome these hurdles and enable truly large-scale production of high-quality carbon nanotubes using heptane as a precursor.
One of the key factors contributing to the scalability of heptane CVD is its compatibility with existing industrial infrastructure. Many chemical processing facilities already have the necessary equipment and safety protocols in place to handle hydrocarbon-based processes, making the integration of heptane CVD more straightforward compared to other precursor materials. This compatibility reduces the initial capital investment required for large-scale implementation.
Furthermore, the vapor pressure characteristics of heptane allow for efficient and controlled delivery of the precursor into the CVD reactor. This property enables precise control over the growth conditions, which is essential for maintaining consistent nanotube quality across larger production volumes. The ability to fine-tune growth parameters is crucial when scaling up, as it helps mitigate variations that can arise from increased reactor sizes or longer production runs.
However, scaling up heptane CVD also presents several challenges that need to be addressed. One of the primary concerns is the potential for increased carbon deposition on reactor walls and other surfaces as production volumes increase. This unwanted deposition can lead to reduced efficiency and increased maintenance requirements, potentially offsetting some of the scalability benefits.
Another consideration is the need for improved heat management systems in larger reactors. As the reactor size increases, maintaining uniform temperature distribution becomes more challenging, which can affect the consistency of nanotube growth. Advanced thermal management techniques and reactor designs may be necessary to ensure homogeneous growth conditions across larger production volumes.
The scalability of heptane CVD also depends on the development of more efficient catalyst systems. While current catalysts work well at laboratory scales, their performance and longevity may need to be enhanced to support continuous, high-volume production. Research into catalyst formulations that remain active for longer periods and can withstand the rigors of industrial-scale processes is ongoing.
In conclusion, while heptane CVD shows promise for scalable nanotube synthesis, realizing its full potential requires addressing several technical and engineering challenges. Ongoing research and development efforts focus on optimizing reactor designs, improving catalyst performance, and enhancing process control to overcome these hurdles and enable truly large-scale production of high-quality carbon nanotubes using heptane as a precursor.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!