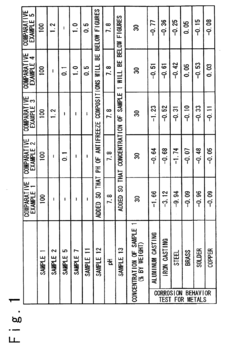
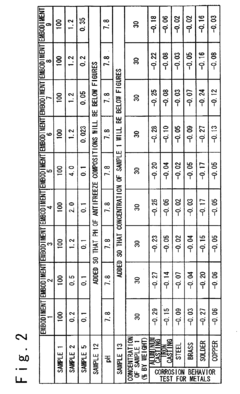
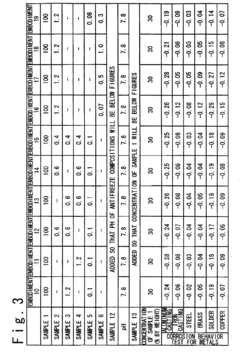
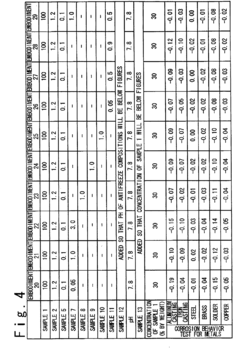
How Antifreeze Reduces Corrosion in Engine Components?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antifreeze Corrosion Protection: Background and Objectives
Antifreeze, also known as engine coolant, has been an integral component in automotive and industrial engines for nearly a century. Its primary function is to regulate engine temperature, preventing overheating in hot conditions and freezing in cold environments. However, the role of antifreeze extends beyond temperature control to include corrosion protection, which is crucial for maintaining the longevity and efficiency of engine components.
The evolution of antifreeze technology can be traced back to the early 20th century when ethylene glycol was first introduced as a coolant. Since then, the formulation of antifreeze has undergone significant improvements to enhance its corrosion protection capabilities. Modern antifreeze solutions are complex mixtures of glycols, corrosion inhibitors, and other additives designed to protect various metals and alloys found in engine systems.
The objective of antifreeze corrosion protection is to create a barrier between metal surfaces and potentially corrosive elements in the cooling system. This protection is essential because engine components are exposed to a harsh environment that includes high temperatures, pressure fluctuations, and a mixture of different metals that can lead to galvanic corrosion.
Corrosion in engine components can result in numerous problems, including reduced heat transfer efficiency, coolant leaks, and ultimately, engine failure. The financial implications of corrosion-related damage in automotive and industrial sectors are substantial, making effective corrosion protection a critical focus for manufacturers and engineers.
The development of antifreeze corrosion protection technology aims to address several key challenges. These include providing long-term protection against various types of corrosion, maintaining compatibility with different metals and alloys, and ensuring environmental safety. Additionally, as engine designs evolve and new materials are introduced, antifreeze formulations must adapt to meet these changing requirements.
Recent technological advancements have led to the creation of organic acid technology (OAT) and hybrid organic acid technology (HOAT) antifreeze formulations. These newer technologies offer improved corrosion protection and longer service life compared to traditional inorganic additive technology (IAT) formulations. The ongoing research in this field focuses on developing more efficient, environmentally friendly, and cost-effective solutions for corrosion protection in engine cooling systems.
The evolution of antifreeze technology can be traced back to the early 20th century when ethylene glycol was first introduced as a coolant. Since then, the formulation of antifreeze has undergone significant improvements to enhance its corrosion protection capabilities. Modern antifreeze solutions are complex mixtures of glycols, corrosion inhibitors, and other additives designed to protect various metals and alloys found in engine systems.
The objective of antifreeze corrosion protection is to create a barrier between metal surfaces and potentially corrosive elements in the cooling system. This protection is essential because engine components are exposed to a harsh environment that includes high temperatures, pressure fluctuations, and a mixture of different metals that can lead to galvanic corrosion.
Corrosion in engine components can result in numerous problems, including reduced heat transfer efficiency, coolant leaks, and ultimately, engine failure. The financial implications of corrosion-related damage in automotive and industrial sectors are substantial, making effective corrosion protection a critical focus for manufacturers and engineers.
The development of antifreeze corrosion protection technology aims to address several key challenges. These include providing long-term protection against various types of corrosion, maintaining compatibility with different metals and alloys, and ensuring environmental safety. Additionally, as engine designs evolve and new materials are introduced, antifreeze formulations must adapt to meet these changing requirements.
Recent technological advancements have led to the creation of organic acid technology (OAT) and hybrid organic acid technology (HOAT) antifreeze formulations. These newer technologies offer improved corrosion protection and longer service life compared to traditional inorganic additive technology (IAT) formulations. The ongoing research in this field focuses on developing more efficient, environmentally friendly, and cost-effective solutions for corrosion protection in engine cooling systems.
Market Analysis: Demand for Corrosion-Resistant Coolants
The demand for corrosion-resistant coolants in the automotive industry has been steadily increasing due to the growing awareness of engine longevity and performance optimization. As vehicles become more sophisticated and expensive, consumers and manufacturers alike are placing greater emphasis on protecting engine components from corrosion-related damage. This trend is particularly evident in regions with harsh climates, where extreme temperatures and road salt can accelerate corrosion processes.
Market research indicates that the global automotive antifreeze market is experiencing significant growth, driven by the rising production of vehicles and the increasing average lifespan of cars on the road. The market is also benefiting from the expansion of the automotive aftermarket sector, where consumers are more likely to invest in high-quality, corrosion-resistant coolants to maintain their vehicles.
In developed markets such as North America and Europe, there is a noticeable shift towards long-life coolants that offer extended protection against corrosion. These products command premium prices and are gaining market share due to their ability to reduce maintenance frequency and costs over the vehicle's lifetime. Emerging markets, particularly in Asia-Pacific and Latin America, are also showing increased demand for corrosion-resistant coolants as vehicle ownership rates rise and consumers become more educated about proper engine maintenance.
The commercial vehicle segment presents a substantial opportunity for corrosion-resistant coolant manufacturers. Fleet operators are increasingly recognizing the long-term cost benefits of using high-quality antifreeze products to protect their assets and minimize downtime. This has led to a growing demand for specialized coolants designed for heavy-duty applications.
Environmental regulations are also shaping the market landscape. There is a rising demand for eco-friendly, biodegradable coolants that offer corrosion protection without compromising environmental safety. This trend is particularly strong in regions with strict environmental policies, driving innovation in coolant formulations.
The aftermarket segment for corrosion-resistant coolants is experiencing robust growth. As vehicle owners become more proactive about maintenance, they are more likely to choose premium coolants that offer superior corrosion protection. This trend is supported by educational initiatives from both manufacturers and automotive service providers, highlighting the importance of using high-quality coolants to extend engine life.
Overall, the market analysis suggests a positive outlook for corrosion-resistant coolants, with sustained growth expected in the coming years. Manufacturers who can offer innovative, environmentally friendly, and highly effective corrosion-resistant formulations are likely to capture significant market share in this expanding sector.
Market research indicates that the global automotive antifreeze market is experiencing significant growth, driven by the rising production of vehicles and the increasing average lifespan of cars on the road. The market is also benefiting from the expansion of the automotive aftermarket sector, where consumers are more likely to invest in high-quality, corrosion-resistant coolants to maintain their vehicles.
In developed markets such as North America and Europe, there is a noticeable shift towards long-life coolants that offer extended protection against corrosion. These products command premium prices and are gaining market share due to their ability to reduce maintenance frequency and costs over the vehicle's lifetime. Emerging markets, particularly in Asia-Pacific and Latin America, are also showing increased demand for corrosion-resistant coolants as vehicle ownership rates rise and consumers become more educated about proper engine maintenance.
The commercial vehicle segment presents a substantial opportunity for corrosion-resistant coolant manufacturers. Fleet operators are increasingly recognizing the long-term cost benefits of using high-quality antifreeze products to protect their assets and minimize downtime. This has led to a growing demand for specialized coolants designed for heavy-duty applications.
Environmental regulations are also shaping the market landscape. There is a rising demand for eco-friendly, biodegradable coolants that offer corrosion protection without compromising environmental safety. This trend is particularly strong in regions with strict environmental policies, driving innovation in coolant formulations.
The aftermarket segment for corrosion-resistant coolants is experiencing robust growth. As vehicle owners become more proactive about maintenance, they are more likely to choose premium coolants that offer superior corrosion protection. This trend is supported by educational initiatives from both manufacturers and automotive service providers, highlighting the importance of using high-quality coolants to extend engine life.
Overall, the market analysis suggests a positive outlook for corrosion-resistant coolants, with sustained growth expected in the coming years. Manufacturers who can offer innovative, environmentally friendly, and highly effective corrosion-resistant formulations are likely to capture significant market share in this expanding sector.
Current Challenges in Engine Corrosion Prevention
Despite significant advancements in engine technology, corrosion prevention remains a persistent challenge in the automotive industry. The harsh operating conditions within an engine, including high temperatures, pressure fluctuations, and exposure to various chemicals, create an environment conducive to corrosion. One of the primary challenges is the constant battle against electrochemical reactions that occur between engine components and coolant fluids.
The presence of dissimilar metals in engine construction exacerbates the corrosion problem. When these metals come into contact with electrolytes in the coolant, galvanic corrosion can occur, leading to the degradation of critical engine parts. This issue is particularly prevalent in modern engines that utilize lightweight materials like aluminum alongside traditional steel components.
Another significant challenge is the formation of scale and deposits within the cooling system. As coolant circulates through the engine, it can pick up dissolved minerals and contaminants. Over time, these substances can accumulate on engine surfaces, reducing heat transfer efficiency and potentially leading to localized corrosion hot spots.
The dynamic temperature changes experienced by engine components also pose a considerable challenge. Thermal cycling can cause expansion and contraction of materials, potentially creating micro-cracks that serve as initiation points for corrosion. This thermal stress is particularly problematic in areas where different materials with varying thermal expansion coefficients are joined together.
Maintaining the proper chemical balance of the coolant is another ongoing challenge. The pH level of the coolant must be carefully controlled to prevent acidic or alkaline conditions that could accelerate corrosion. Additionally, the depletion of corrosion inhibitors in the coolant over time necessitates regular maintenance and replacement, which is often overlooked by vehicle owners.
Environmental factors further complicate corrosion prevention efforts. Exposure to road salt, atmospheric pollutants, and humidity can introduce additional corrosive elements into the engine system. These external factors can compromise the integrity of protective coatings and accelerate the corrosion process, particularly in vulnerable areas such as radiator fins and external coolant passages.
The increasing use of stop-start technology in modern vehicles introduces new challenges for corrosion prevention. Frequent engine shutdowns and restarts can lead to more frequent temperature fluctuations and potential condensation formation, creating conditions favorable for corrosion initiation.
The presence of dissimilar metals in engine construction exacerbates the corrosion problem. When these metals come into contact with electrolytes in the coolant, galvanic corrosion can occur, leading to the degradation of critical engine parts. This issue is particularly prevalent in modern engines that utilize lightweight materials like aluminum alongside traditional steel components.
Another significant challenge is the formation of scale and deposits within the cooling system. As coolant circulates through the engine, it can pick up dissolved minerals and contaminants. Over time, these substances can accumulate on engine surfaces, reducing heat transfer efficiency and potentially leading to localized corrosion hot spots.
The dynamic temperature changes experienced by engine components also pose a considerable challenge. Thermal cycling can cause expansion and contraction of materials, potentially creating micro-cracks that serve as initiation points for corrosion. This thermal stress is particularly problematic in areas where different materials with varying thermal expansion coefficients are joined together.
Maintaining the proper chemical balance of the coolant is another ongoing challenge. The pH level of the coolant must be carefully controlled to prevent acidic or alkaline conditions that could accelerate corrosion. Additionally, the depletion of corrosion inhibitors in the coolant over time necessitates regular maintenance and replacement, which is often overlooked by vehicle owners.
Environmental factors further complicate corrosion prevention efforts. Exposure to road salt, atmospheric pollutants, and humidity can introduce additional corrosive elements into the engine system. These external factors can compromise the integrity of protective coatings and accelerate the corrosion process, particularly in vulnerable areas such as radiator fins and external coolant passages.
The increasing use of stop-start technology in modern vehicles introduces new challenges for corrosion prevention. Frequent engine shutdowns and restarts can lead to more frequent temperature fluctuations and potential condensation formation, creating conditions favorable for corrosion initiation.
Existing Antifreeze Corrosion Inhibition Mechanisms
01 Use of corrosion inhibitors in antifreeze formulations
Corrosion inhibitors are added to antifreeze formulations to protect metal components in cooling systems from corrosion. These inhibitors form protective layers on metal surfaces, preventing direct contact with corrosive elements in the antifreeze solution. Common corrosion inhibitors include silicates, phosphates, and organic compounds.- Use of corrosion inhibitors in antifreeze formulations: Corrosion inhibitors are added to antifreeze formulations to protect metal components in cooling systems from corrosion. These inhibitors form protective layers on metal surfaces, preventing direct contact with corrosive elements in the antifreeze solution. Common corrosion inhibitors include silicates, phosphates, and organic compounds.
- Incorporation of pH buffers: pH buffers are used in antifreeze formulations to maintain a stable pH level, which is crucial for preventing corrosion. These buffers help neutralize acids that may form during the degradation of the antifreeze solution or from combustion byproducts. Maintaining an optimal pH range enhances the effectiveness of corrosion inhibitors and prolongs the life of the cooling system.
- Use of synergistic combinations of inhibitors: Antifreeze formulations often employ synergistic combinations of different types of corrosion inhibitors to provide comprehensive protection against various forms of corrosion. These combinations may include inorganic and organic inhibitors, each targeting specific types of corrosion or metal surfaces. The synergistic effect enhances overall corrosion protection compared to using individual inhibitors alone.
- Development of environmentally friendly formulations: There is a growing trend towards developing environmentally friendly antifreeze formulations that provide effective corrosion protection while minimizing environmental impact. These formulations may use biodegradable components, reduce or eliminate harmful substances like nitrites and borates, and focus on sustainable raw materials. The goal is to maintain or improve corrosion protection while meeting increasingly stringent environmental regulations.
- Long-life antifreeze formulations: Long-life antifreeze formulations are designed to provide extended corrosion protection and coolant life. These formulations typically use more stable corrosion inhibitors and may incorporate organic acid technology (OAT) or hybrid organic acid technology (HOAT). The extended life reduces the frequency of coolant changes, leading to cost savings and reduced environmental impact from waste coolant disposal.
02 Incorporation of pH buffers
pH buffers are used in antifreeze formulations to maintain a stable pH level, which helps prevent corrosion. By keeping the pH within an optimal range, typically between 7 and 11, the effectiveness of corrosion inhibitors is enhanced, and the overall corrosion protection of the antifreeze is improved.Expand Specific Solutions03 Use of synergistic combinations of inhibitors
Antifreeze formulations often employ synergistic combinations of different types of corrosion inhibitors to provide comprehensive protection against various forms of corrosion. These combinations may include inorganic and organic inhibitors, each targeting specific corrosion mechanisms or metal types, resulting in enhanced overall corrosion protection.Expand Specific Solutions04 Addition of anti-scaling agents
Anti-scaling agents are incorporated into antifreeze formulations to prevent the formation of scale deposits on metal surfaces. These deposits can reduce heat transfer efficiency and promote localized corrosion. Common anti-scaling agents include phosphonates and polyacrylates, which work by interfering with the crystal growth of scale-forming minerals.Expand Specific Solutions05 Use of environmentally friendly corrosion inhibitors
There is a growing trend towards using environmentally friendly corrosion inhibitors in antifreeze formulations. These inhibitors are typically derived from natural sources or designed to have minimal environmental impact. They provide effective corrosion protection while reducing the potential for environmental contamination and meeting increasingly stringent regulations.Expand Specific Solutions
Key Players in Antifreeze Industry
The antifreeze corrosion reduction market is in a mature stage, with established players and technologies. The global market size for automotive antifreeze is projected to reach $5.8 billion by 2025, driven by increasing vehicle production and aftermarket demand. Major companies like BASF, Henkel, and Prestone dominate with advanced formulations, while regional players like HAERTOL Chemie and Arteco NV focus on specialized products. The technology is well-developed, with ongoing research into more environmentally friendly and efficient solutions. Companies are investing in R&D to improve corrosion protection, heat transfer properties, and compatibility with new materials in modern engines.
BASF Corp.
Technical Solution: BASF Corp. has developed advanced antifreeze formulations that effectively reduce corrosion in engine components. Their technology utilizes a blend of organic acid technology (OAT) corrosion inhibitors, which form a thin protective layer on metal surfaces[1]. This layer prevents direct contact between the coolant and metal, significantly reducing corrosion rates. BASF's antifreeze solutions also incorporate silicate-free formulations, which minimize the risk of silicate gel formation and subsequent cooling system blockages[2]. The company has further enhanced their products with proprietary additives that provide long-lasting protection against cavitation erosion in water pumps and cylinder liners[3].
Strengths: Highly effective corrosion protection, long-lasting performance, and reduced risk of cooling system blockages. Weaknesses: May be more expensive than conventional antifreeze formulations and require specific handling procedures.
Honda Motor Co., Ltd.
Technical Solution: Honda Motor Co., Ltd. has developed a proprietary antifreeze technology that focuses on reducing corrosion in engine components, particularly in aluminum-based parts. Their approach involves using a hybrid organic acid technology (HOAT) coolant, which combines the benefits of OAT and traditional inorganic additives[4]. This formulation creates a robust protective film on metal surfaces, effectively preventing corrosion even under high-temperature conditions. Honda's antifreeze also incorporates special inhibitors that target specific areas prone to corrosion, such as water pump seals and radiator cores[5]. The company has further optimized their coolant for use in their hybrid and electric vehicle models, ensuring compatibility with advanced cooling systems and heat management technologies[6].
Strengths: Tailored protection for aluminum components, optimized for modern vehicle technologies, and extended coolant life. Weaknesses: May be less compatible with non-Honda vehicles and potentially more expensive than generic alternatives.
Innovative Corrosion Protection Technologies
Antifreezing fluids
PatentInactiveEP1707609A1
Innovation
- A propylene glycol-based antifreeze formulation that includes specific combinations of normal aliphatic dicarboxylic acids, benzimidazole skeleton compounds, triazine skeleton compounds, aromatic carboxylic acids, and nitric acid, which work together to enhance corrosion protection and reduce environmental impact.
Antifreeze/liquid coolant composition and method of use
PatentInactiveUS20070090324A1
Innovation
- A non-hazardous antifreeze coolant composition comprising glycerine, anti-oxidants, and boron-based film formers, with optional water addition, that enhances temperature stability, prevents corrosion and scale formation, and ensures effective heat transfer across a wide temperature range.
Environmental Impact of Antifreeze Formulations
The environmental impact of antifreeze formulations is a critical consideration in the automotive industry, given the widespread use of these products and their potential effects on ecosystems. Traditional antifreeze solutions, primarily composed of ethylene glycol or propylene glycol, have been known to pose significant environmental risks when improperly disposed of or leaked into the environment.
Ethylene glycol, the most common antifreeze component, is particularly concerning due to its toxicity to wildlife and aquatic organisms. When released into water bodies, it can deplete oxygen levels and create anaerobic conditions, leading to fish kills and disruption of aquatic ecosystems. Furthermore, its sweet taste attracts animals, potentially causing poisoning in terrestrial wildlife.
Propylene glycol, while less toxic than ethylene glycol, still presents environmental challenges. Although it biodegrades more rapidly, large quantities can still overwhelm natural decomposition processes, leading to oxygen depletion in water bodies and soil contamination.
Recent advancements in antifreeze formulations have focused on developing more environmentally friendly alternatives. Bio-based antifreeze solutions, derived from renewable resources such as corn or soy, offer reduced toxicity and improved biodegradability. These formulations aim to maintain the same corrosion protection and heat transfer properties while minimizing environmental impact.
Another approach involves the use of corrosion inhibitors that are less harmful to the environment. Organic acid technology (OAT) and hybrid organic acid technology (HOAT) antifreeze formulations utilize carboxylic acids as corrosion inhibitors, which are generally less toxic and more biodegradable than traditional inhibitors like silicates and phosphates.
The disposal and recycling of antifreeze also play a crucial role in mitigating environmental impact. Improved recycling technologies have made it possible to reclaim and reprocess used antifreeze, reducing the amount of waste entering the environment. Additionally, some manufacturers have implemented closed-loop systems in their production processes to minimize waste and environmental contamination.
Regulatory bodies worldwide have recognized the environmental concerns associated with antifreeze formulations and have implemented stricter guidelines for their production, use, and disposal. These regulations aim to encourage the development of more environmentally friendly products and promote responsible handling practices among consumers and industries.
As research continues, the focus remains on developing antifreeze formulations that offer optimal engine protection while minimizing environmental impact. This includes exploring novel biodegradable compounds, enhancing recycling processes, and improving product lifecycle management to ensure sustainable use of antifreeze in automotive applications.
Ethylene glycol, the most common antifreeze component, is particularly concerning due to its toxicity to wildlife and aquatic organisms. When released into water bodies, it can deplete oxygen levels and create anaerobic conditions, leading to fish kills and disruption of aquatic ecosystems. Furthermore, its sweet taste attracts animals, potentially causing poisoning in terrestrial wildlife.
Propylene glycol, while less toxic than ethylene glycol, still presents environmental challenges. Although it biodegrades more rapidly, large quantities can still overwhelm natural decomposition processes, leading to oxygen depletion in water bodies and soil contamination.
Recent advancements in antifreeze formulations have focused on developing more environmentally friendly alternatives. Bio-based antifreeze solutions, derived from renewable resources such as corn or soy, offer reduced toxicity and improved biodegradability. These formulations aim to maintain the same corrosion protection and heat transfer properties while minimizing environmental impact.
Another approach involves the use of corrosion inhibitors that are less harmful to the environment. Organic acid technology (OAT) and hybrid organic acid technology (HOAT) antifreeze formulations utilize carboxylic acids as corrosion inhibitors, which are generally less toxic and more biodegradable than traditional inhibitors like silicates and phosphates.
The disposal and recycling of antifreeze also play a crucial role in mitigating environmental impact. Improved recycling technologies have made it possible to reclaim and reprocess used antifreeze, reducing the amount of waste entering the environment. Additionally, some manufacturers have implemented closed-loop systems in their production processes to minimize waste and environmental contamination.
Regulatory bodies worldwide have recognized the environmental concerns associated with antifreeze formulations and have implemented stricter guidelines for their production, use, and disposal. These regulations aim to encourage the development of more environmentally friendly products and promote responsible handling practices among consumers and industries.
As research continues, the focus remains on developing antifreeze formulations that offer optimal engine protection while minimizing environmental impact. This includes exploring novel biodegradable compounds, enhancing recycling processes, and improving product lifecycle management to ensure sustainable use of antifreeze in automotive applications.
Regulatory Framework for Automotive Coolants
The regulatory framework for automotive coolants plays a crucial role in ensuring the safety, performance, and environmental impact of antifreeze products used in engine cooling systems. In the United States, the Environmental Protection Agency (EPA) and the Department of Transportation (DOT) are the primary regulatory bodies overseeing the production, distribution, and use of automotive coolants.
The EPA regulates antifreeze under the Toxic Substances Control Act (TSCA), which requires manufacturers to report chemical substances used in their products. This regulation aims to assess and manage the potential risks associated with these chemicals. Additionally, the EPA's Significant New Use Rule (SNUR) mandates that manufacturers notify the agency before introducing new chemical substances or significantly altering the use of existing ones in antifreeze formulations.
The DOT, through its National Highway Traffic Safety Administration (NHTSA), sets standards for vehicle safety, including requirements for cooling system performance. These standards indirectly influence the composition and properties of antifreeze products to ensure they meet the necessary cooling and corrosion protection requirements for modern engines.
At the state level, many jurisdictions have implemented regulations regarding the disposal and recycling of used antifreeze. For example, California's Department of Toxic Substances Control classifies used antifreeze as hazardous waste, requiring proper handling and disposal procedures to prevent environmental contamination.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation impacts the global automotive coolant industry. REACH requires manufacturers to register chemical substances and provide safety data, affecting the formulation and marketing of antifreeze products in the EU market and beyond.
Industry standards also play a significant role in shaping the regulatory landscape for automotive coolants. The American Society for Testing and Materials (ASTM) and the Society of Automotive Engineers (SAE) have developed specifications and test methods for antifreeze products. These standards, such as ASTM D3306 for automotive engine coolants, define performance criteria and testing procedures that manufacturers must adhere to for their products to be considered compliant.
The regulatory framework continues to evolve, with increasing focus on environmental sustainability and human health protection. Recent trends include the development of more eco-friendly antifreeze formulations, stricter limits on toxic additives, and enhanced recycling programs. These regulatory changes drive innovation in the automotive coolant industry, pushing manufacturers to develop more effective and environmentally responsible products.
The EPA regulates antifreeze under the Toxic Substances Control Act (TSCA), which requires manufacturers to report chemical substances used in their products. This regulation aims to assess and manage the potential risks associated with these chemicals. Additionally, the EPA's Significant New Use Rule (SNUR) mandates that manufacturers notify the agency before introducing new chemical substances or significantly altering the use of existing ones in antifreeze formulations.
The DOT, through its National Highway Traffic Safety Administration (NHTSA), sets standards for vehicle safety, including requirements for cooling system performance. These standards indirectly influence the composition and properties of antifreeze products to ensure they meet the necessary cooling and corrosion protection requirements for modern engines.
At the state level, many jurisdictions have implemented regulations regarding the disposal and recycling of used antifreeze. For example, California's Department of Toxic Substances Control classifies used antifreeze as hazardous waste, requiring proper handling and disposal procedures to prevent environmental contamination.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation impacts the global automotive coolant industry. REACH requires manufacturers to register chemical substances and provide safety data, affecting the formulation and marketing of antifreeze products in the EU market and beyond.
Industry standards also play a significant role in shaping the regulatory landscape for automotive coolants. The American Society for Testing and Materials (ASTM) and the Society of Automotive Engineers (SAE) have developed specifications and test methods for antifreeze products. These standards, such as ASTM D3306 for automotive engine coolants, define performance criteria and testing procedures that manufacturers must adhere to for their products to be considered compliant.
The regulatory framework continues to evolve, with increasing focus on environmental sustainability and human health protection. Recent trends include the development of more eco-friendly antifreeze formulations, stricter limits on toxic additives, and enhanced recycling programs. These regulatory changes drive innovation in the automotive coolant industry, pushing manufacturers to develop more effective and environmentally responsible products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!