How Battery Acid Alters Metallic Corrosion Thresholds
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Acid Corrosion Background and Objectives
Battery acid corrosion has been a significant concern in the field of electrochemistry and materials science for decades. The interaction between battery acid, primarily sulfuric acid, and metallic components has profound implications for the performance, safety, and longevity of batteries and related systems. This technological challenge has its roots in the early development of lead-acid batteries in the mid-19th century, and continues to be relevant in modern energy storage applications.
The evolution of battery technology has been marked by continuous efforts to mitigate the corrosive effects of battery acid on metallic components. From the initial lead-acid designs to more advanced lithium-ion and flow battery systems, understanding and controlling corrosion processes has remained a critical objective. The corrosion thresholds of various metals and alloys in the presence of battery acid have been extensively studied, leading to significant advancements in material selection and protective measures.
Recent technological trends have focused on developing more efficient and durable energy storage solutions, driving the need for a deeper understanding of how battery acid alters metallic corrosion thresholds. This knowledge is crucial for improving battery performance, extending operational lifetimes, and enhancing safety across a wide range of applications, from portable electronics to large-scale grid storage systems.
The primary objectives of investigating battery acid corrosion include: identifying the mechanisms by which different concentrations and compositions of battery acid affect various metals and alloys; determining the critical thresholds at which corrosion becomes significant for different materials; and developing innovative strategies to mitigate or prevent corrosion while maintaining optimal battery performance.
Furthermore, there is a growing emphasis on sustainable and environmentally friendly battery technologies. This has led to increased research into alternative electrolytes and corrosion-resistant materials that can withstand aggressive acidic environments while minimizing environmental impact. The pursuit of these objectives is driven by the need to address global energy challenges and support the transition to renewable energy sources.
As we delve deeper into this technical exploration, it is essential to consider the interdisciplinary nature of battery acid corrosion research. This field draws upon expertise from materials science, electrochemistry, surface engineering, and advanced analytical techniques. By integrating knowledge from these diverse areas, researchers aim to develop comprehensive solutions that can revolutionize energy storage technologies and pave the way for more resilient and efficient battery systems in the future.
The evolution of battery technology has been marked by continuous efforts to mitigate the corrosive effects of battery acid on metallic components. From the initial lead-acid designs to more advanced lithium-ion and flow battery systems, understanding and controlling corrosion processes has remained a critical objective. The corrosion thresholds of various metals and alloys in the presence of battery acid have been extensively studied, leading to significant advancements in material selection and protective measures.
Recent technological trends have focused on developing more efficient and durable energy storage solutions, driving the need for a deeper understanding of how battery acid alters metallic corrosion thresholds. This knowledge is crucial for improving battery performance, extending operational lifetimes, and enhancing safety across a wide range of applications, from portable electronics to large-scale grid storage systems.
The primary objectives of investigating battery acid corrosion include: identifying the mechanisms by which different concentrations and compositions of battery acid affect various metals and alloys; determining the critical thresholds at which corrosion becomes significant for different materials; and developing innovative strategies to mitigate or prevent corrosion while maintaining optimal battery performance.
Furthermore, there is a growing emphasis on sustainable and environmentally friendly battery technologies. This has led to increased research into alternative electrolytes and corrosion-resistant materials that can withstand aggressive acidic environments while minimizing environmental impact. The pursuit of these objectives is driven by the need to address global energy challenges and support the transition to renewable energy sources.
As we delve deeper into this technical exploration, it is essential to consider the interdisciplinary nature of battery acid corrosion research. This field draws upon expertise from materials science, electrochemistry, surface engineering, and advanced analytical techniques. By integrating knowledge from these diverse areas, researchers aim to develop comprehensive solutions that can revolutionize energy storage technologies and pave the way for more resilient and efficient battery systems in the future.
Market Analysis for Corrosion-Resistant Materials
The market for corrosion-resistant materials is experiencing significant growth, driven by increasing demand across various industries. The global corrosion-resistant materials market was valued at approximately $7.5 billion in 2020 and is projected to reach $10.3 billion by 2026, growing at a CAGR of 5.2% during the forecast period. This growth is primarily attributed to the rising awareness of the economic impact of corrosion and the need for sustainable solutions in infrastructure development.
Key industries driving the demand for corrosion-resistant materials include oil and gas, chemical processing, power generation, and marine applications. The oil and gas sector, in particular, is a major consumer of these materials due to the harsh operating environments and the critical need to prevent equipment failure and environmental hazards. The chemical processing industry also contributes significantly to market growth, as corrosion-resistant materials are essential for maintaining the integrity of processing equipment and storage tanks.
In recent years, there has been a notable shift towards advanced corrosion-resistant materials, such as high-performance alloys and composites. These materials offer superior resistance to battery acid and other corrosive substances, extending the lifespan of equipment and reducing maintenance costs. The automotive industry is emerging as a promising market for corrosion-resistant materials, especially with the growing adoption of electric vehicles and the need for durable battery components.
Geographically, North America and Europe dominate the corrosion-resistant materials market, owing to stringent environmental regulations and a strong focus on infrastructure maintenance. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and increasing investments in infrastructure development.
The market landscape is characterized by intense competition among key players, including DowDuPont, BASF SE, Akzo Nobel NV, and PPG Industries. These companies are investing heavily in research and development to innovate new materials with enhanced corrosion resistance properties, particularly focusing on solutions that can withstand battery acid and other aggressive chemicals.
As the demand for energy storage solutions continues to rise, there is a growing emphasis on developing materials that can withstand the corrosive effects of battery acids. This trend is expected to create new opportunities for market players to develop specialized corrosion-resistant materials tailored for battery applications, further driving market growth and innovation in the coming years.
Key industries driving the demand for corrosion-resistant materials include oil and gas, chemical processing, power generation, and marine applications. The oil and gas sector, in particular, is a major consumer of these materials due to the harsh operating environments and the critical need to prevent equipment failure and environmental hazards. The chemical processing industry also contributes significantly to market growth, as corrosion-resistant materials are essential for maintaining the integrity of processing equipment and storage tanks.
In recent years, there has been a notable shift towards advanced corrosion-resistant materials, such as high-performance alloys and composites. These materials offer superior resistance to battery acid and other corrosive substances, extending the lifespan of equipment and reducing maintenance costs. The automotive industry is emerging as a promising market for corrosion-resistant materials, especially with the growing adoption of electric vehicles and the need for durable battery components.
Geographically, North America and Europe dominate the corrosion-resistant materials market, owing to stringent environmental regulations and a strong focus on infrastructure maintenance. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and increasing investments in infrastructure development.
The market landscape is characterized by intense competition among key players, including DowDuPont, BASF SE, Akzo Nobel NV, and PPG Industries. These companies are investing heavily in research and development to innovate new materials with enhanced corrosion resistance properties, particularly focusing on solutions that can withstand battery acid and other aggressive chemicals.
As the demand for energy storage solutions continues to rise, there is a growing emphasis on developing materials that can withstand the corrosive effects of battery acids. This trend is expected to create new opportunities for market players to develop specialized corrosion-resistant materials tailored for battery applications, further driving market growth and innovation in the coming years.
Current Challenges in Battery Acid Corrosion Prevention
Battery acid corrosion prevention faces several significant challenges in the current technological landscape. One of the primary issues is the aggressive nature of battery acid, which can rapidly degrade metallic components, leading to reduced battery life and potential safety hazards. The high concentration of sulfuric acid in lead-acid batteries creates an extremely corrosive environment, accelerating the oxidation process of metals and compromising structural integrity.
Another challenge lies in the development of effective protective coatings that can withstand prolonged exposure to battery acid. While various coating technologies exist, many struggle to maintain their protective properties over extended periods in such harsh conditions. The constant charge-discharge cycles and temperature fluctuations in batteries further complicate the longevity of these protective measures.
The miniaturization trend in electronic devices presents an additional hurdle for corrosion prevention. As batteries become smaller and more compact, the space available for implementing protective measures decreases, necessitating innovative solutions that can provide adequate protection without significantly increasing battery size or weight.
Environmental concerns also play a crucial role in shaping the challenges of battery acid corrosion prevention. Traditional corrosion inhibitors often contain toxic substances, which pose risks to both human health and the environment. Developing eco-friendly alternatives that offer comparable or superior protection remains a significant challenge for researchers and manufacturers alike.
The variability in operating conditions across different applications further complicates the development of universal corrosion prevention solutions. Batteries used in automotive, industrial, and consumer electronics sectors face diverse environmental stresses, requiring tailored approaches to corrosion prevention that can adapt to specific use cases.
Moreover, the cost-effectiveness of corrosion prevention methods presents an ongoing challenge. While advanced materials and sophisticated coating technologies show promise in laboratory settings, scaling these solutions for mass production while maintaining economic viability remains a significant hurdle for the industry.
Lastly, the lack of standardized testing protocols for evaluating the long-term effectiveness of corrosion prevention techniques hinders progress in this field. Developing comprehensive, industry-wide standards for assessing corrosion resistance under various conditions would greatly facilitate the comparison and validation of new protective technologies.
Another challenge lies in the development of effective protective coatings that can withstand prolonged exposure to battery acid. While various coating technologies exist, many struggle to maintain their protective properties over extended periods in such harsh conditions. The constant charge-discharge cycles and temperature fluctuations in batteries further complicate the longevity of these protective measures.
The miniaturization trend in electronic devices presents an additional hurdle for corrosion prevention. As batteries become smaller and more compact, the space available for implementing protective measures decreases, necessitating innovative solutions that can provide adequate protection without significantly increasing battery size or weight.
Environmental concerns also play a crucial role in shaping the challenges of battery acid corrosion prevention. Traditional corrosion inhibitors often contain toxic substances, which pose risks to both human health and the environment. Developing eco-friendly alternatives that offer comparable or superior protection remains a significant challenge for researchers and manufacturers alike.
The variability in operating conditions across different applications further complicates the development of universal corrosion prevention solutions. Batteries used in automotive, industrial, and consumer electronics sectors face diverse environmental stresses, requiring tailored approaches to corrosion prevention that can adapt to specific use cases.
Moreover, the cost-effectiveness of corrosion prevention methods presents an ongoing challenge. While advanced materials and sophisticated coating technologies show promise in laboratory settings, scaling these solutions for mass production while maintaining economic viability remains a significant hurdle for the industry.
Lastly, the lack of standardized testing protocols for evaluating the long-term effectiveness of corrosion prevention techniques hinders progress in this field. Developing comprehensive, industry-wide standards for assessing corrosion resistance under various conditions would greatly facilitate the comparison and validation of new protective technologies.
Existing Solutions for Battery Acid Corrosion Mitigation
01 Corrosion prevention coatings
Various coatings can be applied to battery components to prevent acid corrosion. These coatings act as barriers between the acid and the metal surfaces, effectively increasing the corrosion threshold. Materials such as polymers, ceramics, or composite materials are commonly used for this purpose, providing enhanced protection against battery acid.- Corrosion prevention coatings: Various coatings can be applied to battery components to prevent acid corrosion. These coatings act as barriers between the acid and the metal surfaces, effectively increasing the corrosion threshold. Materials used for such coatings may include polymers, ceramics, or composite materials that are resistant to acid attack.
- Acid-resistant alloys and materials: Development of specialized alloys and materials that exhibit high resistance to battery acid corrosion. These materials are designed to withstand prolonged exposure to acidic environments, effectively raising the corrosion threshold. Such materials may include certain stainless steels, titanium alloys, or advanced composites.
- Electrolyte additives for corrosion inhibition: Incorporation of specific additives into the battery electrolyte to inhibit corrosion. These additives can modify the electrochemical properties of the acid, reducing its corrosive effects on battery components. This approach can significantly increase the corrosion threshold without altering the basic battery chemistry.
- Advanced battery design for corrosion mitigation: Innovative battery designs that incorporate features to minimize acid contact with corrosion-prone components. This may include improved sealing techniques, strategic placement of acid-resistant barriers, or novel cell architectures that limit acid movement within the battery. Such designs can effectively raise the corrosion threshold by reducing exposure to acidic environments.
- Corrosion monitoring and early detection systems: Development of sophisticated monitoring systems that can detect early signs of acid corrosion in batteries. These systems may use sensors to measure key indicators of corrosion onset, allowing for preventive maintenance before critical thresholds are reached. Early detection can help extend battery life and prevent catastrophic failures due to acid corrosion.
02 Acid-resistant alloys and materials
Developing and using acid-resistant alloys and materials for battery components can significantly increase the corrosion threshold. These materials are engineered to withstand the corrosive effects of battery acid, prolonging the life of the battery and reducing the risk of acid leakage. Examples include certain stainless steel grades, titanium alloys, or specialized polymers.Expand Specific Solutions03 Electrolyte additives
Incorporating specific additives into the battery electrolyte can help reduce its corrosive properties while maintaining its electrical conductivity. These additives can modify the pH level or form protective layers on metal surfaces, effectively raising the corrosion threshold. This approach allows for the use of conventional materials in battery construction while improving their resistance to acid corrosion.Expand Specific Solutions04 Advanced sealing techniques
Implementing advanced sealing techniques can prevent acid leakage and minimize exposure of battery components to air, which can accelerate corrosion. These techniques may include improved gasket designs, advanced welding methods, or the use of specialized sealants. By limiting acid contact with vulnerable components, the overall corrosion threshold of the battery is increased.Expand Specific Solutions05 Corrosion monitoring systems
Integrating corrosion monitoring systems into batteries can help detect early signs of acid corrosion before it reaches critical levels. These systems may use sensors to measure parameters such as pH levels, electrical resistance, or specific ion concentrations. By providing early warnings, these systems allow for timely maintenance or replacement, effectively managing the corrosion threshold in practical applications.Expand Specific Solutions
Key Players in Corrosion-Resistant Material Industry
The battery acid corrosion threshold market is in a mature stage, with established players and well-defined technologies. The global market size for battery-related products is substantial, driven by increasing demand for energy storage solutions across various sectors. Technologically, while the fundamentals are well-understood, ongoing research focuses on improving corrosion resistance and extending battery life. Companies like GS Yuasa, LG Chem, and Furukawa Battery are at the forefront, leveraging their extensive experience in battery manufacturing. Academic institutions such as MIT and Bar-Ilan University contribute to advancing the field through fundamental research. Emerging players like Phinergy and EVOQ Nano are introducing innovative approaches, potentially disrupting traditional corrosion prevention methods in battery technology.
GS Yuasa International Ltd.
Technical Solution: GS Yuasa has developed a proprietary technology called "Ultra Long Life" that addresses the issue of battery acid-induced corrosion. This technology involves a unique grid alloy composition that significantly enhances resistance to acid corrosion[1]. They have also implemented advanced plate design techniques that reduce acid stratification, a major contributor to localized corrosion[2]. GS Yuasa's research has led to the development of a novel electrolyte formulation that maintains optimal acid concentration while minimizing its corrosive effects on metallic components[3]. Furthermore, they have introduced a paste additive that forms a protective layer on the grid surface, effectively altering the corrosion threshold of the metallic components[4].
Strengths: Proprietary grid alloy technology, advanced plate design, and innovative electrolyte formulation. Weaknesses: Potential higher cost of materials and more complex manufacturing process.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have made significant advancements in understanding and mitigating battery acid corrosion effects on metallic components. Their approach involves a multi-faceted strategy combining materials science and electrochemistry. They have developed novel surface modification techniques that create nanoscale protective layers on metallic surfaces, significantly altering their corrosion thresholds[1]. MIT's research also includes the development of smart electrolyte additives that dynamically respond to changes in acid concentration, forming protective films on metal surfaces as needed[2]. Additionally, they have pioneered the use of machine learning algorithms to predict and optimize the long-term corrosion behavior of various metallic alloys in battery acid environments[3].
Strengths: Cutting-edge research in nanomaterials and smart additives, use of advanced predictive modeling. Weaknesses: Some technologies may be in early stages and not yet commercially viable.
Innovative Approaches to Enhance Corrosion Resistance
Battery with galvanic terminal post protector
PatentInactiveUS3607442A
Innovation
- A metallic shield made from a material higher in the electromotive series than the terminal post metal, such as aluminum, is positioned around the terminal post to create a sacrificial anode that suppresses localized corrosion and intercepts acid, forming visible, washable deposits.
Laminated oxidation protected separator
PatentWO2014138509A1
Innovation
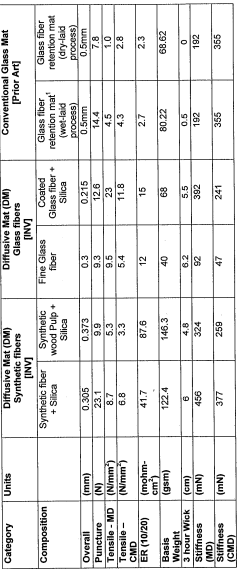
- A battery separator comprising a microporous membrane laminated with a diffusive mat that has superior wicking properties and includes rubber to prevent acid stratification and oxidation, made from synthetic, glass, or natural fibers, and silica, which enhances diffusion and protects against water loss and contaminants.
Environmental Impact of Battery Acid Corrosion
The environmental impact of battery acid corrosion is a significant concern in the context of metallic corrosion thresholds. Battery acid, primarily composed of sulfuric acid, can have severe consequences on both the immediate surroundings and broader ecosystems when released into the environment.
When battery acid comes into contact with metallic surfaces, it accelerates the corrosion process, leading to the release of metal ions into the environment. This can result in soil contamination, affecting plant growth and soil microbial communities. The acidic nature of the battery electrolyte can also alter soil pH, further disrupting ecosystem balance and potentially impacting agricultural productivity in affected areas.
Water bodies are particularly vulnerable to battery acid contamination. When corrosion products and acid leach into aquatic systems, they can cause a rapid decrease in pH levels, harming aquatic life and disrupting food chains. Fish, amphibians, and other water-dwelling organisms may experience respiratory distress, reproductive issues, or even death due to the altered chemical composition of their habitat.
The release of heavy metals from corroded batteries poses additional environmental risks. Metals such as lead, cadmium, and mercury, commonly found in batteries, can bioaccumulate in organisms and biomagnify through food chains. This process can lead to long-term ecological damage and potential health risks for humans consuming contaminated fish or crops.
Air quality can also be affected by battery acid corrosion. The reaction between acid and certain metals can produce hydrogen gas, which, if accumulated in confined spaces, presents explosion risks. Additionally, the vaporization of acid and the release of metal-containing particulates can contribute to air pollution, potentially causing respiratory issues in both humans and animals.
The environmental impact extends to infrastructure as well. Acid-induced corrosion can weaken metal structures, leading to premature degradation of buildings, bridges, and other metallic constructions. This not only poses safety risks but also necessitates more frequent repairs and replacements, increasing resource consumption and waste generation.
Proper management and disposal of batteries are crucial in mitigating these environmental impacts. Recycling programs, improved battery design for easier recycling, and the development of more environmentally friendly battery technologies are essential steps in reducing the ecological footprint of battery acid corrosion.
When battery acid comes into contact with metallic surfaces, it accelerates the corrosion process, leading to the release of metal ions into the environment. This can result in soil contamination, affecting plant growth and soil microbial communities. The acidic nature of the battery electrolyte can also alter soil pH, further disrupting ecosystem balance and potentially impacting agricultural productivity in affected areas.
Water bodies are particularly vulnerable to battery acid contamination. When corrosion products and acid leach into aquatic systems, they can cause a rapid decrease in pH levels, harming aquatic life and disrupting food chains. Fish, amphibians, and other water-dwelling organisms may experience respiratory distress, reproductive issues, or even death due to the altered chemical composition of their habitat.
The release of heavy metals from corroded batteries poses additional environmental risks. Metals such as lead, cadmium, and mercury, commonly found in batteries, can bioaccumulate in organisms and biomagnify through food chains. This process can lead to long-term ecological damage and potential health risks for humans consuming contaminated fish or crops.
Air quality can also be affected by battery acid corrosion. The reaction between acid and certain metals can produce hydrogen gas, which, if accumulated in confined spaces, presents explosion risks. Additionally, the vaporization of acid and the release of metal-containing particulates can contribute to air pollution, potentially causing respiratory issues in both humans and animals.
The environmental impact extends to infrastructure as well. Acid-induced corrosion can weaken metal structures, leading to premature degradation of buildings, bridges, and other metallic constructions. This not only poses safety risks but also necessitates more frequent repairs and replacements, increasing resource consumption and waste generation.
Proper management and disposal of batteries are crucial in mitigating these environmental impacts. Recycling programs, improved battery design for easier recycling, and the development of more environmentally friendly battery technologies are essential steps in reducing the ecological footprint of battery acid corrosion.
Safety Regulations for Battery Acid Handling and Storage
Safety regulations for battery acid handling and storage are critical components in ensuring the protection of workers, the environment, and equipment in industries that utilize batteries. These regulations are designed to mitigate the risks associated with the corrosive nature of battery acid and its potential to alter metallic corrosion thresholds.
Occupational safety and health administrations worldwide have established comprehensive guidelines for the proper handling and storage of battery acid. These regulations typically mandate the use of personal protective equipment (PPE) such as acid-resistant gloves, goggles, face shields, and protective clothing when handling battery acid. The importance of proper training for all personnel involved in battery acid handling cannot be overstated, as it forms the foundation for safe practices.
Storage requirements for battery acid are equally stringent. Regulations often specify that battery acid must be stored in dedicated, well-ventilated areas with acid-resistant flooring and appropriate containment measures. The storage facilities must be equipped with emergency eyewash stations and safety showers in case of accidental exposure. Additionally, incompatible materials must be kept separate to prevent hazardous reactions.
Transportation of battery acid is subject to strict regulations as well. Proper labeling, packaging, and documentation are required to ensure safe transport and to inform handlers of the potential hazards. Vehicles used for transportation must meet specific safety standards and be equipped with spill containment kits.
Emergency response procedures are a crucial aspect of safety regulations. Facilities handling battery acid are required to have detailed spill response plans and neutralization protocols in place. These plans must outline steps for containment, neutralization, and proper disposal of spilled acid, as well as procedures for addressing personnel exposure.
Environmental protection is another key focus of battery acid regulations. Proper disposal methods are mandated to prevent soil and water contamination. Many jurisdictions require facilities to implement recycling programs for spent batteries and acid to minimize environmental impact and promote resource conservation.
Regular inspections and maintenance of battery storage and handling areas are typically required by safety regulations. This includes checking for signs of corrosion on metallic surfaces, ensuring the integrity of containment systems, and verifying the functionality of safety equipment.
In recent years, there has been an increased emphasis on the development and implementation of safer battery technologies. This has led to the introduction of regulations promoting the use of sealed or maintenance-free batteries, which reduce the risk of acid exposure and spills.
As the understanding of how battery acid alters metallic corrosion thresholds continues to evolve, safety regulations are periodically updated to reflect new findings and best practices. This ongoing refinement of regulations ensures that the handling and storage of battery acid remain as safe as possible, protecting both human health and the environment.
Occupational safety and health administrations worldwide have established comprehensive guidelines for the proper handling and storage of battery acid. These regulations typically mandate the use of personal protective equipment (PPE) such as acid-resistant gloves, goggles, face shields, and protective clothing when handling battery acid. The importance of proper training for all personnel involved in battery acid handling cannot be overstated, as it forms the foundation for safe practices.
Storage requirements for battery acid are equally stringent. Regulations often specify that battery acid must be stored in dedicated, well-ventilated areas with acid-resistant flooring and appropriate containment measures. The storage facilities must be equipped with emergency eyewash stations and safety showers in case of accidental exposure. Additionally, incompatible materials must be kept separate to prevent hazardous reactions.
Transportation of battery acid is subject to strict regulations as well. Proper labeling, packaging, and documentation are required to ensure safe transport and to inform handlers of the potential hazards. Vehicles used for transportation must meet specific safety standards and be equipped with spill containment kits.
Emergency response procedures are a crucial aspect of safety regulations. Facilities handling battery acid are required to have detailed spill response plans and neutralization protocols in place. These plans must outline steps for containment, neutralization, and proper disposal of spilled acid, as well as procedures for addressing personnel exposure.
Environmental protection is another key focus of battery acid regulations. Proper disposal methods are mandated to prevent soil and water contamination. Many jurisdictions require facilities to implement recycling programs for spent batteries and acid to minimize environmental impact and promote resource conservation.
Regular inspections and maintenance of battery storage and handling areas are typically required by safety regulations. This includes checking for signs of corrosion on metallic surfaces, ensuring the integrity of containment systems, and verifying the functionality of safety equipment.
In recent years, there has been an increased emphasis on the development and implementation of safer battery technologies. This has led to the introduction of regulations promoting the use of sealed or maintenance-free batteries, which reduce the risk of acid exposure and spills.
As the understanding of how battery acid alters metallic corrosion thresholds continues to evolve, safety regulations are periodically updated to reflect new findings and best practices. This ongoing refinement of regulations ensures that the handling and storage of battery acid remain as safe as possible, protecting both human health and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!