How Battery Acid Influences Polymer Electrolyte Membranes
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Acid-PEM Interaction Background and Objectives
The interaction between battery acid and polymer electrolyte membranes (PEMs) has been a critical area of research in the field of energy storage and conversion technologies. This complex relationship has significant implications for the performance, durability, and efficiency of various electrochemical devices, particularly in fuel cells and advanced battery systems.
The development of PEMs dates back to the 1960s, with the introduction of Nafion by DuPont. Since then, the technology has evolved considerably, driven by the growing demand for clean energy solutions and the need for more efficient energy storage systems. The primary goal of studying the influence of battery acid on PEMs is to enhance the overall performance and longevity of electrochemical devices while mitigating potential degradation mechanisms.
As we delve into this topic, it is essential to understand the fundamental principles governing the interaction between battery acid and PEMs. Battery acid, typically sulfuric acid in lead-acid batteries, can have profound effects on the chemical and physical properties of PEMs. These effects include changes in proton conductivity, mechanical stability, and chemical resistance of the membrane.
The objectives of this technical research report are multifaceted. Firstly, we aim to provide a comprehensive overview of the current state of knowledge regarding the influence of battery acid on PEMs. This includes an examination of the chemical and physical mechanisms underlying this interaction, as well as the resulting changes in membrane properties and performance.
Secondly, we seek to identify the key challenges and limitations in current PEM technologies when exposed to battery acid environments. This involves analyzing the degradation mechanisms, performance losses, and potential failure modes that can occur due to prolonged exposure to acidic conditions.
Thirdly, our goal is to explore innovative approaches and materials that can enhance the resistance of PEMs to battery acid, thereby improving their durability and performance in harsh electrochemical environments. This includes investigating novel polymer compositions, surface modifications, and composite materials that show promise in mitigating acid-induced degradation.
Lastly, we aim to provide insights into the future directions of research and development in this field. By identifying emerging trends and potential breakthroughs, we hope to guide future efforts in developing more robust and efficient PEMs for advanced energy storage and conversion applications.
The development of PEMs dates back to the 1960s, with the introduction of Nafion by DuPont. Since then, the technology has evolved considerably, driven by the growing demand for clean energy solutions and the need for more efficient energy storage systems. The primary goal of studying the influence of battery acid on PEMs is to enhance the overall performance and longevity of electrochemical devices while mitigating potential degradation mechanisms.
As we delve into this topic, it is essential to understand the fundamental principles governing the interaction between battery acid and PEMs. Battery acid, typically sulfuric acid in lead-acid batteries, can have profound effects on the chemical and physical properties of PEMs. These effects include changes in proton conductivity, mechanical stability, and chemical resistance of the membrane.
The objectives of this technical research report are multifaceted. Firstly, we aim to provide a comprehensive overview of the current state of knowledge regarding the influence of battery acid on PEMs. This includes an examination of the chemical and physical mechanisms underlying this interaction, as well as the resulting changes in membrane properties and performance.
Secondly, we seek to identify the key challenges and limitations in current PEM technologies when exposed to battery acid environments. This involves analyzing the degradation mechanisms, performance losses, and potential failure modes that can occur due to prolonged exposure to acidic conditions.
Thirdly, our goal is to explore innovative approaches and materials that can enhance the resistance of PEMs to battery acid, thereby improving their durability and performance in harsh electrochemical environments. This includes investigating novel polymer compositions, surface modifications, and composite materials that show promise in mitigating acid-induced degradation.
Lastly, we aim to provide insights into the future directions of research and development in this field. By identifying emerging trends and potential breakthroughs, we hope to guide future efforts in developing more robust and efficient PEMs for advanced energy storage and conversion applications.
Market Analysis for Acid-Resistant PEMs
The market for acid-resistant Polymer Electrolyte Membranes (PEMs) is experiencing significant growth, driven by the increasing demand for high-performance batteries in various applications. The global PEM market is projected to expand substantially over the next decade, with acid-resistant membranes playing a crucial role in this growth.
The automotive sector represents a major market for acid-resistant PEMs, particularly in the rapidly expanding electric vehicle (EV) industry. As EV adoption accelerates worldwide, the demand for advanced battery technologies that can withstand harsh acidic environments is surging. This trend is further supported by government initiatives promoting clean energy and sustainable transportation solutions.
In addition to the automotive sector, the stationary energy storage market is emerging as a key driver for acid-resistant PEMs. The growing integration of renewable energy sources into power grids necessitates efficient and durable energy storage systems, where acid-resistant membranes can significantly enhance battery performance and longevity.
The telecommunications industry is another significant consumer of acid-resistant PEMs, particularly for backup power systems in remote locations. The need for reliable, long-lasting power solutions in challenging environments is propelling the demand for advanced membrane technologies that can withstand acidic conditions.
Geographically, Asia-Pacific is expected to dominate the acid-resistant PEM market, with China and South Korea leading in both production and consumption. This regional dominance is attributed to the presence of major battery manufacturers and the rapid growth of the EV market in these countries. North America and Europe are also witnessing substantial growth in demand for acid-resistant PEMs, driven by increasing investments in renewable energy and energy storage projects.
The market landscape for acid-resistant PEMs is characterized by intense competition and rapid technological advancements. Key players are focusing on developing innovative membrane materials that offer improved acid resistance, higher conductivity, and enhanced durability. This competitive environment is fostering significant research and development activities, leading to the introduction of novel membrane technologies.
Despite the positive growth outlook, the acid-resistant PEM market faces challenges such as high production costs and complex manufacturing processes. However, ongoing research efforts and economies of scale are expected to gradually address these issues, making acid-resistant PEMs more cost-effective and accessible to a broader range of applications.
In conclusion, the market for acid-resistant PEMs is poised for substantial growth, driven by the expanding EV industry, increasing adoption of renewable energy storage systems, and the need for reliable power solutions in various sectors. As technology continues to advance and production costs decrease, acid-resistant PEMs are likely to play an increasingly critical role in shaping the future of energy storage and conversion technologies.
The automotive sector represents a major market for acid-resistant PEMs, particularly in the rapidly expanding electric vehicle (EV) industry. As EV adoption accelerates worldwide, the demand for advanced battery technologies that can withstand harsh acidic environments is surging. This trend is further supported by government initiatives promoting clean energy and sustainable transportation solutions.
In addition to the automotive sector, the stationary energy storage market is emerging as a key driver for acid-resistant PEMs. The growing integration of renewable energy sources into power grids necessitates efficient and durable energy storage systems, where acid-resistant membranes can significantly enhance battery performance and longevity.
The telecommunications industry is another significant consumer of acid-resistant PEMs, particularly for backup power systems in remote locations. The need for reliable, long-lasting power solutions in challenging environments is propelling the demand for advanced membrane technologies that can withstand acidic conditions.
Geographically, Asia-Pacific is expected to dominate the acid-resistant PEM market, with China and South Korea leading in both production and consumption. This regional dominance is attributed to the presence of major battery manufacturers and the rapid growth of the EV market in these countries. North America and Europe are also witnessing substantial growth in demand for acid-resistant PEMs, driven by increasing investments in renewable energy and energy storage projects.
The market landscape for acid-resistant PEMs is characterized by intense competition and rapid technological advancements. Key players are focusing on developing innovative membrane materials that offer improved acid resistance, higher conductivity, and enhanced durability. This competitive environment is fostering significant research and development activities, leading to the introduction of novel membrane technologies.
Despite the positive growth outlook, the acid-resistant PEM market faces challenges such as high production costs and complex manufacturing processes. However, ongoing research efforts and economies of scale are expected to gradually address these issues, making acid-resistant PEMs more cost-effective and accessible to a broader range of applications.
In conclusion, the market for acid-resistant PEMs is poised for substantial growth, driven by the expanding EV industry, increasing adoption of renewable energy storage systems, and the need for reliable power solutions in various sectors. As technology continues to advance and production costs decrease, acid-resistant PEMs are likely to play an increasingly critical role in shaping the future of energy storage and conversion technologies.
Current Challenges in PEM Acid Resistance
Polymer electrolyte membranes (PEMs) face significant challenges in maintaining their integrity and performance when exposed to battery acid environments. The primary issue lies in the chemical degradation of the membrane material, which can lead to reduced proton conductivity, increased gas crossover, and ultimately, decreased battery efficiency and lifespan.
One of the most pressing challenges is the acid-induced hydrolysis of the polymer backbone. This process can cause chain scission and the formation of low molecular weight fragments, leading to a loss of mechanical strength and dimensional stability. The rate of hydrolysis is often accelerated at elevated temperatures, which are common in battery operating conditions.
Another critical issue is the leaching of functional groups responsible for proton conduction. Sulfonic acid groups, commonly used in PEMs for their excellent proton conductivity, are particularly susceptible to acid attack. Their gradual loss over time results in a decrease in the membrane's ion exchange capacity and overall proton conductivity.
The formation of pinholes and microcracks in the membrane structure is also a significant concern. These defects can arise from localized chemical attack or mechanical stress induced by swelling and deswelling cycles in the acidic environment. Such imperfections compromise the membrane's barrier properties, allowing unwanted crossover of reactants and products between electrode compartments.
Furthermore, the presence of metal ions, often introduced as contaminants or catalysts, can exacerbate membrane degradation. These ions can catalyze the decomposition of hydrogen peroxide, a byproduct of oxygen reduction, leading to the formation of highly reactive hydroxyl and peroxyl radicals. These radicals can cause severe oxidative damage to the polymer structure.
The development of acid-resistant PEMs is further complicated by the need to balance multiple, often conflicting, properties. While increasing the degree of crosslinking or the use of fluorinated polymers can enhance acid resistance, these modifications may negatively impact proton conductivity or increase production costs.
Addressing these challenges requires a multifaceted approach, combining advanced material science, electrochemistry, and polymer engineering. Researchers are exploring various strategies, including the development of composite membranes, surface modifications, and novel polymer architectures. However, finding a solution that simultaneously addresses all aspects of acid resistance while maintaining other critical membrane properties remains an ongoing challenge in the field of PEM technology for battery applications.
One of the most pressing challenges is the acid-induced hydrolysis of the polymer backbone. This process can cause chain scission and the formation of low molecular weight fragments, leading to a loss of mechanical strength and dimensional stability. The rate of hydrolysis is often accelerated at elevated temperatures, which are common in battery operating conditions.
Another critical issue is the leaching of functional groups responsible for proton conduction. Sulfonic acid groups, commonly used in PEMs for their excellent proton conductivity, are particularly susceptible to acid attack. Their gradual loss over time results in a decrease in the membrane's ion exchange capacity and overall proton conductivity.
The formation of pinholes and microcracks in the membrane structure is also a significant concern. These defects can arise from localized chemical attack or mechanical stress induced by swelling and deswelling cycles in the acidic environment. Such imperfections compromise the membrane's barrier properties, allowing unwanted crossover of reactants and products between electrode compartments.
Furthermore, the presence of metal ions, often introduced as contaminants or catalysts, can exacerbate membrane degradation. These ions can catalyze the decomposition of hydrogen peroxide, a byproduct of oxygen reduction, leading to the formation of highly reactive hydroxyl and peroxyl radicals. These radicals can cause severe oxidative damage to the polymer structure.
The development of acid-resistant PEMs is further complicated by the need to balance multiple, often conflicting, properties. While increasing the degree of crosslinking or the use of fluorinated polymers can enhance acid resistance, these modifications may negatively impact proton conductivity or increase production costs.
Addressing these challenges requires a multifaceted approach, combining advanced material science, electrochemistry, and polymer engineering. Researchers are exploring various strategies, including the development of composite membranes, surface modifications, and novel polymer architectures. However, finding a solution that simultaneously addresses all aspects of acid resistance while maintaining other critical membrane properties remains an ongoing challenge in the field of PEM technology for battery applications.
Existing Acid-Resistant PEM Solutions
01 Composition and structure of polymer electrolyte membranes
The composition and structure of polymer electrolyte membranes significantly influence their performance in fuel cells. Various polymers and additives are used to enhance properties such as proton conductivity, mechanical strength, and chemical stability. Membrane thickness and morphology also play crucial roles in determining overall efficiency.- Composition and structure of polymer electrolyte membranes: The composition and structure of polymer electrolyte membranes significantly influence their performance in fuel cells. Various polymers and additives are used to enhance properties such as proton conductivity, mechanical strength, and chemical stability. Membrane thickness and morphology also play crucial roles in determining overall efficiency.
- Proton conductivity enhancement techniques: Improving proton conductivity is a key focus in polymer electrolyte membrane development. Techniques include incorporating acidic functional groups, using composite materials, and optimizing water retention properties. These enhancements aim to increase fuel cell performance and efficiency under various operating conditions.
- Durability and chemical stability improvements: Enhancing the durability and chemical stability of polymer electrolyte membranes is crucial for long-term fuel cell operation. Strategies include developing cross-linked structures, incorporating reinforcing materials, and using chemically resistant polymers to withstand harsh operating environments and extend membrane lifespan.
- Water management and hydration effects: Proper water management in polymer electrolyte membranes is essential for maintaining optimal performance. The influence of hydration on proton conductivity, mechanical properties, and gas permeability is studied. Techniques to balance water content and prevent flooding or drying are developed to ensure consistent fuel cell operation.
- Temperature and pressure effects on membrane performance: The influence of temperature and pressure on polymer electrolyte membrane performance is significant. Research focuses on developing membranes that maintain high proton conductivity and mechanical integrity across a wide range of operating conditions. This includes studying thermal stability, pressure-induced changes, and the impact on overall fuel cell efficiency.
02 Proton conductivity enhancement techniques
Improving proton conductivity is a key focus in polymer electrolyte membrane development. Techniques include incorporating acidic functional groups, using composite materials, and optimizing water retention properties. These enhancements aim to increase fuel cell performance and efficiency across various operating conditions.Expand Specific Solutions03 Durability and chemical stability improvements
Enhancing the durability and chemical stability of polymer electrolyte membranes is crucial for long-term fuel cell operation. Researchers focus on developing membranes resistant to degradation from factors such as radical attack, mechanical stress, and temperature fluctuations. Cross-linking and reinforcement strategies are often employed to achieve these improvements.Expand Specific Solutions04 Water management and hydration effects
Proper water management in polymer electrolyte membranes is essential for optimal fuel cell performance. The influence of membrane hydration on proton conductivity, gas permeability, and mechanical properties is a critical area of study. Researchers investigate various approaches to balance water content and distribution within the membrane.Expand Specific Solutions05 Temperature and pressure effects on membrane performance
The influence of operating temperature and pressure on polymer electrolyte membrane performance is a significant consideration in fuel cell design. Researchers study how these factors affect membrane properties such as conductivity, gas crossover, and mechanical integrity. Developing membranes that maintain high performance across a wide range of conditions is a key objective.Expand Specific Solutions
Key Players in PEM and Battery Industries
The battery acid influence on polymer electrolyte membranes is a critical area of research in the advanced energy storage sector. This field is currently in a growth phase, with increasing market size driven by the rising demand for electric vehicles and renewable energy storage solutions. The global market for polymer electrolyte membranes is expected to expand significantly in the coming years. Technologically, the field is advancing rapidly, with companies like LG Chem, Samsung SDI, and Contemporary Amperex Technology leading the way in innovation. These firms are investing heavily in R&D to improve membrane performance, durability, and cost-effectiveness. Emerging players such as Anthro Energy and Brightvolt are also contributing to technological advancements, particularly in the area of solid-state batteries, which could potentially revolutionize the industry.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced polymer electrolyte membranes (PEMs) that are highly resistant to battery acid influence. Their proprietary technology involves the use of perfluorosulfonic acid (PFSA) ionomers with enhanced chemical stability[1]. These membranes incorporate cross-linked structures and reinforced backbone polymers to withstand the harsh acidic environment in batteries. LG Chem's PEMs also feature optimized ion channels for improved proton conductivity, which helps maintain performance even under acidic conditions[3]. The company has implemented a multi-layer membrane design, with an acid-resistant outer layer protecting the inner functional layers[5].
Strengths: High chemical stability in acidic environments, improved proton conductivity, and multi-layer protection. Weaknesses: Potentially higher production costs due to complex membrane structure and materials used.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has focused on developing polymer electrolyte membranes with enhanced acid resistance through the incorporation of inorganic nanoparticles. Their approach involves dispersing acid-scavenging nanoparticles, such as metal oxides, throughout the polymer matrix[2]. This technique helps neutralize acid molecules that come into contact with the membrane, prolonging its lifespan. Samsung SDI has also implemented a gradient distribution of these nanoparticles, with higher concentrations near the membrane surface for improved acid resistance[4]. Additionally, they have developed a surface modification technique that creates a hydrophobic layer on the membrane, further protecting it from acid attack[6].
Strengths: Effective acid neutralization, extended membrane lifespan, and innovative surface protection. Weaknesses: Potential for nanoparticle agglomeration, which could affect membrane uniformity and performance over time.
Core Innovations in Acid-Resistant PEM Materials
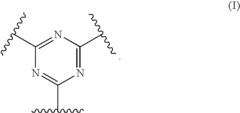
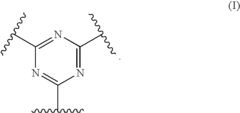
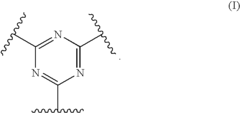
Polymer electrolyte with aromatic sulfone crosslinking
PatentInactiveUS8802793B2
Innovation
- Crosslinking highly fluorinated polymers with aromatic crosslinkers to form aromatic sulfones, creating crosslinked polymers with pendent sulfonic acid groups, which are then converted to sulfonic acid form, resulting in membranes with specific hydration products and equivalent weights suitable for fuel cell applications.
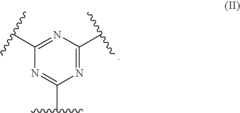
Polymer electrolyte membranes crosslinked by nitrile trimerization
PatentInactiveUS7435498B2
Innovation
- A method involving a highly fluorinated polymer with sulfonyl halide and nitrile groups, where nitrile groups are trimerized to form crosslinks, and sulfonyl halide groups are converted to sulfonic acid groups, creating a crosslinked polymer electrolyte membrane with trivalent groups, suitable for use in fuel cells.
Environmental Impact of PEM-Acid Interactions
The interaction between polymer electrolyte membranes (PEMs) and battery acid has significant environmental implications that extend beyond the immediate performance of the battery system. As PEMs are exposed to acidic environments, they undergo chemical and physical changes that can lead to the release of potentially harmful substances into the environment.
One of the primary environmental concerns is the degradation of PEM materials when in contact with battery acid. This degradation process can result in the leaching of polymer components, including fluorinated compounds in the case of perfluorosulfonic acid (PFSA) membranes, which are commonly used in fuel cells and some battery applications. These compounds, if released into soil or water systems, can persist in the environment for extended periods due to their chemical stability.
The acidic environment can also accelerate the breakdown of PEM materials, potentially releasing metal ions used as catalysts or structural components. These metal ions, such as platinum or ruthenium, can have toxic effects on aquatic ecosystems if they accumulate in sufficient concentrations. The environmental impact of these metal contaminants depends on factors such as their bioavailability and the specific ecosystem characteristics.
Furthermore, the acid-PEM interaction can lead to the formation of secondary pollutants. As the membrane degrades, it may produce organic byproducts that can react with the acid or other environmental components, forming new compounds with unknown ecological effects. This chemical transformation process adds complexity to environmental risk assessments and remediation efforts.
The disposal of spent PEMs and associated battery components presents another environmental challenge. Improper disposal can lead to soil and water contamination, as residual acid and degraded membrane materials leach into the surrounding environment. This underscores the importance of developing effective recycling and disposal protocols for PEM-based energy storage systems.
On a broader scale, the environmental impact of PEM-acid interactions extends to the carbon footprint of battery production and recycling processes. The need for acid-resistant membranes may necessitate more energy-intensive manufacturing techniques or the use of more environmentally persistent materials, potentially offsetting some of the environmental benefits of battery-powered technologies.
To mitigate these environmental risks, ongoing research focuses on developing more environmentally benign PEM materials and improving acid management within battery systems. This includes exploring bio-based polymers, enhancing membrane stability to reduce degradation, and designing closed-loop systems that minimize acid exposure and contain potential contaminants.
One of the primary environmental concerns is the degradation of PEM materials when in contact with battery acid. This degradation process can result in the leaching of polymer components, including fluorinated compounds in the case of perfluorosulfonic acid (PFSA) membranes, which are commonly used in fuel cells and some battery applications. These compounds, if released into soil or water systems, can persist in the environment for extended periods due to their chemical stability.
The acidic environment can also accelerate the breakdown of PEM materials, potentially releasing metal ions used as catalysts or structural components. These metal ions, such as platinum or ruthenium, can have toxic effects on aquatic ecosystems if they accumulate in sufficient concentrations. The environmental impact of these metal contaminants depends on factors such as their bioavailability and the specific ecosystem characteristics.
Furthermore, the acid-PEM interaction can lead to the formation of secondary pollutants. As the membrane degrades, it may produce organic byproducts that can react with the acid or other environmental components, forming new compounds with unknown ecological effects. This chemical transformation process adds complexity to environmental risk assessments and remediation efforts.
The disposal of spent PEMs and associated battery components presents another environmental challenge. Improper disposal can lead to soil and water contamination, as residual acid and degraded membrane materials leach into the surrounding environment. This underscores the importance of developing effective recycling and disposal protocols for PEM-based energy storage systems.
On a broader scale, the environmental impact of PEM-acid interactions extends to the carbon footprint of battery production and recycling processes. The need for acid-resistant membranes may necessitate more energy-intensive manufacturing techniques or the use of more environmentally persistent materials, potentially offsetting some of the environmental benefits of battery-powered technologies.
To mitigate these environmental risks, ongoing research focuses on developing more environmentally benign PEM materials and improving acid management within battery systems. This includes exploring bio-based polymers, enhancing membrane stability to reduce degradation, and designing closed-loop systems that minimize acid exposure and contain potential contaminants.
Safety Regulations for Battery-PEM Systems
Safety regulations for battery-PEM systems are crucial for ensuring the safe operation and handling of these complex electrochemical devices. These regulations typically cover various aspects of battery and polymer electrolyte membrane (PEM) design, manufacturing, transportation, and disposal. One of the primary concerns addressed by these regulations is the potential for acid leakage and its impact on the PEM.
Regulatory bodies, such as the International Electrotechnical Commission (IEC) and the United Nations Economic Commission for Europe (UNECE), have established standards for battery safety. These standards often include specific requirements for the containment of battery acid and the protection of PEM components. For instance, IEC 62133 outlines safety requirements for portable sealed secondary cells and batteries, including those using PEM technology.
The transportation of batteries and PEM systems is subject to strict regulations due to the potential hazards associated with battery acid. The International Air Transport Association (IATA) and the U.S. Department of Transportation (DOT) have implemented guidelines for the packaging and labeling of batteries to prevent acid spills during transit. These regulations often require the use of acid-resistant containers and absorbent materials to mitigate the risk of leakage.
Workplace safety regulations also play a significant role in protecting workers who handle battery-PEM systems. Organizations such as the Occupational Safety and Health Administration (OSHA) in the United States mandate the use of personal protective equipment (PPE) when working with battery acid. This includes acid-resistant gloves, aprons, and eye protection. Additionally, facilities that manufacture or service battery-PEM systems are required to have proper ventilation systems and emergency eyewash stations.
Environmental regulations address the disposal and recycling of battery-PEM systems to prevent acid contamination of soil and water resources. The European Union's Battery Directive (2006/66/EC) and similar regulations in other regions set standards for the collection, treatment, and recycling of batteries, including those with PEM technology. These regulations often require manufacturers to implement take-back programs and ensure proper disposal of battery acid and other hazardous components.
Research and development in battery-PEM systems are also subject to safety regulations. Laboratories and testing facilities must adhere to strict protocols when handling battery acid and conducting experiments on PEM materials. This includes the use of fume hoods, proper storage of acids, and regular safety inspections. Regulatory compliance in this area is essential for obtaining funding and publishing research results.
As battery technology continues to evolve, safety regulations are regularly updated to address new challenges and risks. For example, the growing use of lithium-ion batteries in electric vehicles has led to the development of specific safety standards for high-voltage battery systems, which often incorporate advanced PEM technology. These regulations focus on preventing thermal runaway and managing the unique risks associated with large-scale battery installations.
Regulatory bodies, such as the International Electrotechnical Commission (IEC) and the United Nations Economic Commission for Europe (UNECE), have established standards for battery safety. These standards often include specific requirements for the containment of battery acid and the protection of PEM components. For instance, IEC 62133 outlines safety requirements for portable sealed secondary cells and batteries, including those using PEM technology.
The transportation of batteries and PEM systems is subject to strict regulations due to the potential hazards associated with battery acid. The International Air Transport Association (IATA) and the U.S. Department of Transportation (DOT) have implemented guidelines for the packaging and labeling of batteries to prevent acid spills during transit. These regulations often require the use of acid-resistant containers and absorbent materials to mitigate the risk of leakage.
Workplace safety regulations also play a significant role in protecting workers who handle battery-PEM systems. Organizations such as the Occupational Safety and Health Administration (OSHA) in the United States mandate the use of personal protective equipment (PPE) when working with battery acid. This includes acid-resistant gloves, aprons, and eye protection. Additionally, facilities that manufacture or service battery-PEM systems are required to have proper ventilation systems and emergency eyewash stations.
Environmental regulations address the disposal and recycling of battery-PEM systems to prevent acid contamination of soil and water resources. The European Union's Battery Directive (2006/66/EC) and similar regulations in other regions set standards for the collection, treatment, and recycling of batteries, including those with PEM technology. These regulations often require manufacturers to implement take-back programs and ensure proper disposal of battery acid and other hazardous components.
Research and development in battery-PEM systems are also subject to safety regulations. Laboratories and testing facilities must adhere to strict protocols when handling battery acid and conducting experiments on PEM materials. This includes the use of fume hoods, proper storage of acids, and regular safety inspections. Regulatory compliance in this area is essential for obtaining funding and publishing research results.
As battery technology continues to evolve, safety regulations are regularly updated to address new challenges and risks. For example, the growing use of lithium-ion batteries in electric vehicles has led to the development of specific safety standards for high-voltage battery systems, which often incorporate advanced PEM technology. These regulations focus on preventing thermal runaway and managing the unique risks associated with large-scale battery installations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!