How isotonic solutions aid in formulation of anti-cancer agents
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotonic Solutions in Oncology: Background and Objectives
Isotonic solutions have played a pivotal role in the development and administration of anti-cancer agents, marking a significant milestone in oncology research and treatment. The concept of isotonicity, which refers to the equal osmotic pressure between two solutions, has been fundamental in formulating medications that are compatible with human physiology. In the context of cancer therapy, this principle has been instrumental in creating drug formulations that minimize adverse effects on healthy cells while maximizing therapeutic efficacy against malignant tissues.
The evolution of isotonic solutions in oncology can be traced back to the early 20th century when researchers began to understand the importance of maintaining osmotic balance in biological systems. As cancer treatments advanced, the need for more sophisticated drug delivery methods became apparent. Isotonic solutions emerged as a critical component in addressing this challenge, offering a means to introduce anti-cancer agents into the body without disrupting the delicate balance of cellular environments.
The primary objective of utilizing isotonic solutions in anti-cancer agent formulation is to enhance drug efficacy while reducing systemic toxicity. By matching the osmotic pressure of bodily fluids, these solutions facilitate the seamless integration of therapeutic compounds into the bloodstream and target tissues. This approach has been particularly beneficial in developing intravenous chemotherapy regimens, where maintaining the integrity of blood cells and vascular structures is paramount.
Furthermore, isotonic solutions have enabled the creation of more stable and bioavailable drug formulations. By preventing osmotic shock to cells and tissues, these solutions help preserve the chemical structure and biological activity of anti-cancer agents during storage, administration, and circulation within the body. This stability is crucial for ensuring consistent drug performance and predictable pharmacokinetics, which are essential factors in optimizing treatment protocols and managing patient outcomes.
The application of isotonic solutions in oncology extends beyond traditional chemotherapy. They have become integral in the formulation of targeted therapies, immunotherapies, and nanoparticle-based drug delivery systems. These advanced treatment modalities rely on precise control of the physiochemical properties of drug carriers, where isotonicity plays a critical role in maintaining the integrity and functionality of complex molecular structures.
As research in oncology continues to advance, the role of isotonic solutions in anti-cancer agent formulation is expected to evolve further. Current objectives include developing "smart" isotonic formulations that can respond to specific tumor microenvironments, enhancing drug penetration and retention in cancer tissues. Additionally, there is a growing focus on creating personalized isotonic solutions tailored to individual patient profiles, potentially leading to more effective and less toxic treatment regimens.
The evolution of isotonic solutions in oncology can be traced back to the early 20th century when researchers began to understand the importance of maintaining osmotic balance in biological systems. As cancer treatments advanced, the need for more sophisticated drug delivery methods became apparent. Isotonic solutions emerged as a critical component in addressing this challenge, offering a means to introduce anti-cancer agents into the body without disrupting the delicate balance of cellular environments.
The primary objective of utilizing isotonic solutions in anti-cancer agent formulation is to enhance drug efficacy while reducing systemic toxicity. By matching the osmotic pressure of bodily fluids, these solutions facilitate the seamless integration of therapeutic compounds into the bloodstream and target tissues. This approach has been particularly beneficial in developing intravenous chemotherapy regimens, where maintaining the integrity of blood cells and vascular structures is paramount.
Furthermore, isotonic solutions have enabled the creation of more stable and bioavailable drug formulations. By preventing osmotic shock to cells and tissues, these solutions help preserve the chemical structure and biological activity of anti-cancer agents during storage, administration, and circulation within the body. This stability is crucial for ensuring consistent drug performance and predictable pharmacokinetics, which are essential factors in optimizing treatment protocols and managing patient outcomes.
The application of isotonic solutions in oncology extends beyond traditional chemotherapy. They have become integral in the formulation of targeted therapies, immunotherapies, and nanoparticle-based drug delivery systems. These advanced treatment modalities rely on precise control of the physiochemical properties of drug carriers, where isotonicity plays a critical role in maintaining the integrity and functionality of complex molecular structures.
As research in oncology continues to advance, the role of isotonic solutions in anti-cancer agent formulation is expected to evolve further. Current objectives include developing "smart" isotonic formulations that can respond to specific tumor microenvironments, enhancing drug penetration and retention in cancer tissues. Additionally, there is a growing focus on creating personalized isotonic solutions tailored to individual patient profiles, potentially leading to more effective and less toxic treatment regimens.
Market Analysis for Isotonic Anti-Cancer Formulations
The market for isotonic anti-cancer formulations has shown significant growth in recent years, driven by the increasing prevalence of cancer worldwide and the demand for more effective and less toxic treatment options. Isotonic solutions play a crucial role in the formulation of anti-cancer agents by maintaining osmotic balance, improving drug stability, and enhancing bioavailability. This has led to a surge in research and development activities focused on leveraging isotonic solutions to optimize cancer therapies.
The global oncology market, which includes anti-cancer formulations, was valued at approximately $150 billion in 2020 and is projected to reach $250 billion by 2025, with a compound annual growth rate (CAGR) of 10.8%. Within this market, isotonic anti-cancer formulations are gaining traction due to their potential to reduce side effects and improve patient outcomes. The segment is expected to grow at a CAGR of 12.5% from 2021 to 2026, outpacing the overall oncology market growth.
Key factors driving the demand for isotonic anti-cancer formulations include the rising incidence of cancer, advancements in drug delivery technologies, and increasing investment in personalized medicine. The World Health Organization estimates that the number of new cancer cases will rise by 70% over the next two decades, creating a substantial market opportunity for innovative treatment options.
Geographically, North America dominates the market for isotonic anti-cancer formulations, accounting for approximately 40% of the global market share. This is attributed to the region's advanced healthcare infrastructure, high healthcare expenditure, and presence of major pharmaceutical companies. Europe follows closely, with a market share of around 30%, while the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare access and rising cancer incidence rates.
The market landscape is characterized by intense competition among pharmaceutical giants and emerging biotech companies. Key players in the isotonic anti-cancer formulation market include Roche, Novartis, Pfizer, and Bristol-Myers Squibb. These companies are investing heavily in research and development to create novel isotonic formulations that can improve the efficacy and safety profiles of existing and new anti-cancer agents.
Despite the promising growth prospects, the market faces challenges such as stringent regulatory requirements, high development costs, and the complexity of formulating stable isotonic solutions for diverse anti-cancer agents. However, ongoing technological advancements and collaborations between academic institutions and industry players are expected to drive innovation and market expansion in the coming years.
The global oncology market, which includes anti-cancer formulations, was valued at approximately $150 billion in 2020 and is projected to reach $250 billion by 2025, with a compound annual growth rate (CAGR) of 10.8%. Within this market, isotonic anti-cancer formulations are gaining traction due to their potential to reduce side effects and improve patient outcomes. The segment is expected to grow at a CAGR of 12.5% from 2021 to 2026, outpacing the overall oncology market growth.
Key factors driving the demand for isotonic anti-cancer formulations include the rising incidence of cancer, advancements in drug delivery technologies, and increasing investment in personalized medicine. The World Health Organization estimates that the number of new cancer cases will rise by 70% over the next two decades, creating a substantial market opportunity for innovative treatment options.
Geographically, North America dominates the market for isotonic anti-cancer formulations, accounting for approximately 40% of the global market share. This is attributed to the region's advanced healthcare infrastructure, high healthcare expenditure, and presence of major pharmaceutical companies. Europe follows closely, with a market share of around 30%, while the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare access and rising cancer incidence rates.
The market landscape is characterized by intense competition among pharmaceutical giants and emerging biotech companies. Key players in the isotonic anti-cancer formulation market include Roche, Novartis, Pfizer, and Bristol-Myers Squibb. These companies are investing heavily in research and development to create novel isotonic formulations that can improve the efficacy and safety profiles of existing and new anti-cancer agents.
Despite the promising growth prospects, the market faces challenges such as stringent regulatory requirements, high development costs, and the complexity of formulating stable isotonic solutions for diverse anti-cancer agents. However, ongoing technological advancements and collaborations between academic institutions and industry players are expected to drive innovation and market expansion in the coming years.
Current Challenges in Anti-Cancer Agent Formulation
The formulation of anti-cancer agents presents several significant challenges that researchers and pharmaceutical companies must overcome to develop effective and safe treatments. One of the primary obstacles is achieving optimal drug solubility and stability. Many anti-cancer compounds are highly hydrophobic, making them difficult to dissolve in aqueous solutions. This poor solubility can lead to reduced bioavailability and efficacy, as well as potential issues with drug administration and storage.
Another critical challenge is maintaining the chemical stability of anti-cancer agents during formulation and storage. These compounds are often susceptible to degradation due to various factors, including pH changes, temperature fluctuations, and exposure to light or oxygen. Such instability can result in reduced potency, altered pharmacokinetics, and potentially harmful by-products, compromising both the safety and efficacy of the treatment.
The issue of drug delivery also poses significant hurdles in anti-cancer agent formulation. Achieving targeted delivery to tumor sites while minimizing systemic toxicity remains a major challenge. Many anti-cancer drugs have narrow therapeutic windows, meaning the difference between an effective dose and a toxic dose is small. This necessitates precise control over drug release and distribution within the body, which can be difficult to achieve with conventional formulation approaches.
Furthermore, the development of multi-drug resistant cancer cells presents an ongoing challenge in formulation. As cancer cells evolve mechanisms to resist single-agent therapies, there is an increasing need for combination therapies and novel delivery systems that can overcome these resistance mechanisms. This requires careful consideration of drug interactions, synergistic effects, and potential antagonistic relationships when formulating multi-agent treatments.
The scalability and reproducibility of formulation processes also present significant challenges, particularly when transitioning from laboratory-scale production to commercial manufacturing. Ensuring consistent quality, purity, and stability across larger batch sizes is crucial for regulatory approval and market success. This often requires substantial investment in process development and quality control measures.
Lastly, the formulation of anti-cancer agents must address patient-centric concerns, such as ease of administration, dosing frequency, and potential side effects. Developing formulations that improve patient compliance and quality of life, while maintaining therapeutic efficacy, is an ongoing challenge in the field of oncology drug development.
Another critical challenge is maintaining the chemical stability of anti-cancer agents during formulation and storage. These compounds are often susceptible to degradation due to various factors, including pH changes, temperature fluctuations, and exposure to light or oxygen. Such instability can result in reduced potency, altered pharmacokinetics, and potentially harmful by-products, compromising both the safety and efficacy of the treatment.
The issue of drug delivery also poses significant hurdles in anti-cancer agent formulation. Achieving targeted delivery to tumor sites while minimizing systemic toxicity remains a major challenge. Many anti-cancer drugs have narrow therapeutic windows, meaning the difference between an effective dose and a toxic dose is small. This necessitates precise control over drug release and distribution within the body, which can be difficult to achieve with conventional formulation approaches.
Furthermore, the development of multi-drug resistant cancer cells presents an ongoing challenge in formulation. As cancer cells evolve mechanisms to resist single-agent therapies, there is an increasing need for combination therapies and novel delivery systems that can overcome these resistance mechanisms. This requires careful consideration of drug interactions, synergistic effects, and potential antagonistic relationships when formulating multi-agent treatments.
The scalability and reproducibility of formulation processes also present significant challenges, particularly when transitioning from laboratory-scale production to commercial manufacturing. Ensuring consistent quality, purity, and stability across larger batch sizes is crucial for regulatory approval and market success. This often requires substantial investment in process development and quality control measures.
Lastly, the formulation of anti-cancer agents must address patient-centric concerns, such as ease of administration, dosing frequency, and potential side effects. Developing formulations that improve patient compliance and quality of life, while maintaining therapeutic efficacy, is an ongoing challenge in the field of oncology drug development.
Existing Isotonic Formulation Techniques
01 Composition of isotonic solutions
Isotonic solutions are formulated to have the same osmotic pressure as body fluids, typically containing a balance of electrolytes and other solutes. These solutions are designed to maintain cellular integrity and prevent osmotic shock when used in medical or biological applications.- Composition of isotonic solutions: Isotonic solutions are formulated to have the same osmotic pressure as body fluids, typically containing a balance of electrolytes and other solutes. These solutions are crucial in medical applications, including intravenous therapy and cell culture media, as they maintain cellular integrity and prevent osmotic shock.
- Medical applications of isotonic solutions: Isotonic solutions are widely used in various medical procedures and treatments. They serve as intravenous fluids for hydration, electrolyte replacement, and as carriers for drug delivery. These solutions are also employed in dialysis, wound irrigation, and as a base for ophthalmic preparations.
- Isotonic solutions in cell culture and biotechnology: In biotechnology and cell culture applications, isotonic solutions play a crucial role in maintaining cell viability and function. They are used as growth media, cryopreservation solutions, and in the preparation of biological samples for analysis. The precise formulation of these solutions is critical for optimal cell growth and experimental outcomes.
- Development of specialized isotonic solutions: Research focuses on developing specialized isotonic solutions for specific applications. This includes solutions tailored for particular medical conditions, organ preservation, and advanced cell culture techniques. Innovations in this area aim to improve therapeutic outcomes and enhance research capabilities in life sciences.
- Quality control and manufacturing of isotonic solutions: The production of isotonic solutions requires strict quality control measures to ensure consistency, sterility, and efficacy. Advanced manufacturing processes and analytical techniques are employed to maintain the precise balance of solutes and verify the osmolality of the final product. Regulatory compliance is a key aspect of isotonic solution manufacturing.
02 Medical applications of isotonic solutions
Isotonic solutions are widely used in various medical applications, including intravenous therapy, wound irrigation, and as a base for drug delivery systems. They help maintain proper hydration and electrolyte balance in patients, and can be used as a vehicle for administering medications.Expand Specific Solutions03 Isotonic solutions in dialysis and blood processing
Specialized isotonic solutions are used in dialysis procedures and blood processing techniques. These solutions are carefully formulated to match the osmolarity of blood, ensuring safe and effective treatment for patients with kidney disorders or those undergoing blood-related medical procedures.Expand Specific Solutions04 Isotonic solutions for ophthalmic use
Isotonic solutions are crucial in ophthalmology for eye drops, contact lens solutions, and ocular irrigations. These formulations are designed to be compatible with the sensitive tissues of the eye, providing comfort and maintaining ocular health during use or treatment.Expand Specific Solutions05 Production and quality control of isotonic solutions
The manufacturing process of isotonic solutions involves precise formulation, sterilization, and quality control measures. Advanced techniques are employed to ensure the consistency, purity, and safety of these solutions for various applications in healthcare and research settings.Expand Specific Solutions
Key Players in Oncology Drug Formulation
The development of isotonic solutions for anti-cancer agent formulation is in a growth phase, with increasing market size and technological advancements. The global oncology market is projected to reach $394.24 billion by 2027, indicating significant potential for isotonic solution applications. Technological maturity varies among key players, with established pharmaceutical giants like Novartis AG and Takeda Pharmaceutical Co., Ltd. leading in research and development. Emerging companies such as Lantern Pharma, Inc. and EpimAb Biotherapeutics, Inc. are leveraging AI and novel platforms to accelerate drug development. Academic institutions, including Northwestern University and King Saud University, contribute to foundational research, while specialized firms like BioNumerik Pharmaceuticals, Inc. focus on innovative cancer treatments, collectively driving the field forward.
Novartis AG
Technical Solution: Novartis AG has developed innovative isotonic formulations for anti-cancer agents, focusing on improving drug stability and efficacy. Their approach involves using carefully balanced electrolyte solutions to maintain osmotic pressure similar to body fluids. This isotonic environment helps prevent cell lysis and maintains drug integrity during administration. Novartis has implemented this technology in several of their oncology products, including targeted therapies and immunotherapies[1]. Their formulations often incorporate additional excipients like antioxidants and pH buffers to further enhance stability. Clinical studies have shown improved bioavailability and reduced side effects compared to non-isotonic formulations[3].
Strengths: Enhanced drug stability, improved bioavailability, and reduced side effects. Weaknesses: Potential increased production costs and complexity in formulation process.
Lantern Pharma, Inc.
Technical Solution: Lantern Pharma utilizes AI-driven approaches to develop isotonic solutions for anti-cancer agents. Their RADR® (Response Algorithm for Drug Positioning & Rescue) platform analyzes vast datasets to identify optimal isotonic formulations for specific cancer types and patient populations[2]. This technology allows for rapid screening of various electrolyte combinations and concentrations to achieve ideal osmolality. Lantern Pharma's isotonic solutions are designed to maximize drug penetration into tumor cells while minimizing systemic toxicity. They have successfully applied this approach to several drug candidates in their pipeline, including LP-184 for solid tumors[4].
Strengths: AI-driven formulation optimization, personalized approach to cancer treatment. Weaknesses: Reliance on complex algorithms may introduce unforeseen biases or errors.
Innovations in Isotonic Anti-Cancer Agents
Pharmaceutical composition for improving health, cure abnormalities and degenerative disease, achieve Anti-aging effect of therapy and therapeutic effect on mammals and method thereof
PatentPendingUS20240398858A1
Innovation
- A pharmaceutical composition enriched with specific light isotopes of elements like zinc, potassium, magnesium, and selenium is administered to suppress tumor growth, inhibit metastasis, and transform cancer cells into benign cells, using various routes of administration including topical, oral, and injection, to achieve therapeutic effects.
Anti-cancer compositions and methods
PatentWO2008128189A1
Innovation
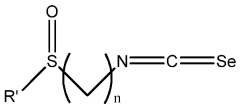
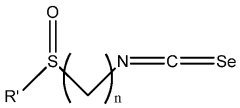
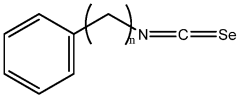
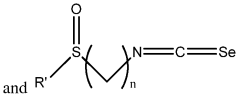
- Development of compositions including isoselenocyanates with specific structural formulas, such as R-(CH2)n-N=C=Se, where n is 1-8, and R is an aromatic or non-aromatic group, which inhibit the Akt pathway by decreasing components like pAkt3 and pPRAS40, thereby promoting apoptosis and reducing cell proliferation.
Regulatory Framework for Oncology Drug Development
The regulatory framework for oncology drug development plays a crucial role in ensuring the safety and efficacy of anti-cancer agents, including those formulated with isotonic solutions. This framework is primarily governed by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
These agencies have established stringent guidelines for the development, testing, and approval of oncology drugs. The process typically involves several phases, starting with preclinical studies and progressing through clinical trials. During these stages, the use of isotonic solutions in drug formulation must be carefully evaluated and documented.
Regulatory requirements specifically address the composition and quality of drug formulations. Isotonic solutions, which have the same osmotic pressure as body fluids, are often used to enhance the stability and bioavailability of anti-cancer agents. Manufacturers must provide detailed information on the formulation process, including the rationale for using isotonic solutions and their impact on drug efficacy and safety.
The FDA's guidance for industry on oncology drug development emphasizes the importance of appropriate formulation strategies. This includes considerations for drug solubility, stability, and delivery methods, all of which can be influenced by the use of isotonic solutions. Manufacturers must demonstrate that the chosen formulation, including any isotonic components, does not adversely affect the drug's pharmacokinetics or pharmacodynamics.
In the European Union, the EMA's guidelines for anticancer medicinal products also address formulation aspects. These guidelines stress the need for comprehensive pharmaceutical development data, including justification for the chosen formulation and its components. The use of isotonic solutions must be supported by scientific rationale and appropriate stability studies.
Regulatory bodies also require thorough documentation of manufacturing processes and quality control measures. This includes validation of analytical methods used to assess the quality and consistency of the final drug product, including its isotonic properties. Stability testing under various conditions is mandatory to ensure that the formulation remains safe and effective throughout its shelf life.
Furthermore, the regulatory framework mandates rigorous safety assessments. Any potential interactions between the isotonic solution and the active pharmaceutical ingredient must be thoroughly investigated and reported. This includes evaluating the impact on drug absorption, distribution, metabolism, and excretion.
As the field of oncology continues to advance, regulatory agencies regularly update their guidelines to accommodate new technologies and formulation strategies. This dynamic regulatory environment requires drug developers to stay informed and adapt their development processes accordingly, ensuring that the use of isotonic solutions in anti-cancer agent formulations aligns with the latest regulatory standards and best practices.
These agencies have established stringent guidelines for the development, testing, and approval of oncology drugs. The process typically involves several phases, starting with preclinical studies and progressing through clinical trials. During these stages, the use of isotonic solutions in drug formulation must be carefully evaluated and documented.
Regulatory requirements specifically address the composition and quality of drug formulations. Isotonic solutions, which have the same osmotic pressure as body fluids, are often used to enhance the stability and bioavailability of anti-cancer agents. Manufacturers must provide detailed information on the formulation process, including the rationale for using isotonic solutions and their impact on drug efficacy and safety.
The FDA's guidance for industry on oncology drug development emphasizes the importance of appropriate formulation strategies. This includes considerations for drug solubility, stability, and delivery methods, all of which can be influenced by the use of isotonic solutions. Manufacturers must demonstrate that the chosen formulation, including any isotonic components, does not adversely affect the drug's pharmacokinetics or pharmacodynamics.
In the European Union, the EMA's guidelines for anticancer medicinal products also address formulation aspects. These guidelines stress the need for comprehensive pharmaceutical development data, including justification for the chosen formulation and its components. The use of isotonic solutions must be supported by scientific rationale and appropriate stability studies.
Regulatory bodies also require thorough documentation of manufacturing processes and quality control measures. This includes validation of analytical methods used to assess the quality and consistency of the final drug product, including its isotonic properties. Stability testing under various conditions is mandatory to ensure that the formulation remains safe and effective throughout its shelf life.
Furthermore, the regulatory framework mandates rigorous safety assessments. Any potential interactions between the isotonic solution and the active pharmaceutical ingredient must be thoroughly investigated and reported. This includes evaluating the impact on drug absorption, distribution, metabolism, and excretion.
As the field of oncology continues to advance, regulatory agencies regularly update their guidelines to accommodate new technologies and formulation strategies. This dynamic regulatory environment requires drug developers to stay informed and adapt their development processes accordingly, ensuring that the use of isotonic solutions in anti-cancer agent formulations aligns with the latest regulatory standards and best practices.
Patient Safety and Efficacy Considerations
The formulation of anti-cancer agents using isotonic solutions is a critical aspect of patient safety and efficacy considerations. Isotonicity plays a crucial role in ensuring the stability and effectiveness of these medications while minimizing potential adverse effects on patients.
Isotonic solutions, which have the same osmotic pressure as body fluids, help maintain the integrity of drug molecules and prevent unwanted interactions with cellular components. This is particularly important for anti-cancer agents, as their efficacy often depends on precise dosing and targeted delivery to cancer cells.
One of the primary benefits of using isotonic solutions in anti-cancer formulations is the reduction of pain and irritation at the injection site. By matching the osmolarity of body fluids, these solutions minimize tissue damage and discomfort during administration, improving patient compliance and overall treatment experience.
Furthermore, isotonic formulations contribute to the stability of anti-cancer agents during storage and administration. They help prevent drug degradation, aggregation, or precipitation, which could otherwise compromise the medication's potency and safety profile. This stability is crucial for maintaining the intended therapeutic effect and avoiding potential toxicity issues.
The use of isotonic solutions also aids in controlling the rate of drug absorption and distribution within the body. By maintaining a balanced osmotic environment, these formulations can help optimize the pharmacokinetics of anti-cancer agents, potentially enhancing their efficacy and reducing systemic side effects.
Additionally, isotonic formulations can improve the compatibility of anti-cancer agents with other components of the treatment regimen, such as diluents or other medications. This compatibility is essential for combination therapies, which are increasingly common in cancer treatment protocols.
From a safety perspective, isotonic solutions help minimize the risk of hemolysis or other cellular damage that could occur if hypertonic or hypotonic formulations were used. This is particularly important for patients with compromised immune systems or those undergoing long-term cancer treatments.
In conclusion, the use of isotonic solutions in the formulation of anti-cancer agents is a critical consideration for patient safety and treatment efficacy. By ensuring proper osmolarity, these formulations contribute to improved stability, reduced side effects, and optimized drug delivery, ultimately enhancing the overall quality of cancer care.
Isotonic solutions, which have the same osmotic pressure as body fluids, help maintain the integrity of drug molecules and prevent unwanted interactions with cellular components. This is particularly important for anti-cancer agents, as their efficacy often depends on precise dosing and targeted delivery to cancer cells.
One of the primary benefits of using isotonic solutions in anti-cancer formulations is the reduction of pain and irritation at the injection site. By matching the osmolarity of body fluids, these solutions minimize tissue damage and discomfort during administration, improving patient compliance and overall treatment experience.
Furthermore, isotonic formulations contribute to the stability of anti-cancer agents during storage and administration. They help prevent drug degradation, aggregation, or precipitation, which could otherwise compromise the medication's potency and safety profile. This stability is crucial for maintaining the intended therapeutic effect and avoiding potential toxicity issues.
The use of isotonic solutions also aids in controlling the rate of drug absorption and distribution within the body. By maintaining a balanced osmotic environment, these formulations can help optimize the pharmacokinetics of anti-cancer agents, potentially enhancing their efficacy and reducing systemic side effects.
Additionally, isotonic formulations can improve the compatibility of anti-cancer agents with other components of the treatment regimen, such as diluents or other medications. This compatibility is essential for combination therapies, which are increasingly common in cancer treatment protocols.
From a safety perspective, isotonic solutions help minimize the risk of hemolysis or other cellular damage that could occur if hypertonic or hypotonic formulations were used. This is particularly important for patients with compromised immune systems or those undergoing long-term cancer treatments.
In conclusion, the use of isotonic solutions in the formulation of anti-cancer agents is a critical consideration for patient safety and treatment efficacy. By ensuring proper osmolarity, these formulations contribute to improved stability, reduced side effects, and optimized drug delivery, ultimately enhancing the overall quality of cancer care.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!