How To Design Occlusive Dressings For Negative Pressure Therapy
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NPWT Dressing Evolution
The evolution of Negative Pressure Wound Therapy (NPWT) dressings has been a significant advancement in wound care management. Initially introduced in the 1990s, NPWT dressings have undergone substantial improvements to enhance their efficacy and patient comfort.
Early NPWT dressings consisted of simple foam or gauze materials placed directly on the wound bed, covered with an adhesive drape to create an airtight seal. These basic designs were effective but often caused discomfort and skin damage during dressing changes.
As the technology progressed, manufacturers developed more sophisticated dressing systems. The introduction of non-adherent layers between the wound and the foam reduced tissue ingrowth, making dressing changes less painful. This innovation marked a significant step in improving patient experience and reducing the risk of secondary trauma to healing tissues.
The next major evolution came with the development of specialized foam materials. These new foams were designed to distribute negative pressure more evenly across the wound surface, promoting better granulation tissue formation and faster healing. Some foams were also infused with antimicrobial agents to help prevent infection in high-risk wounds.
Advancements in adhesive technology led to the creation of more skin-friendly drapes. These new drapes provided a better seal while reducing the risk of skin maceration and irritation around the wound edges. Some designs incorporated silicone-based adhesives, which allowed for gentler removal and repositioning of the dressing.
The integration of instillation therapy with NPWT represented another significant leap forward. These systems allowed for the controlled delivery of wound cleansing solutions or antimicrobial agents directly to the wound bed, enhancing the therapy's effectiveness in managing complex or infected wounds.
Recent innovations have focused on creating more user-friendly and portable NPWT systems. Disposable, single-use NPWT devices have been developed, offering increased mobility for patients and reducing the need for bulky equipment. These portable systems have greatly improved patient compliance and quality of life during treatment.
The latest trend in NPWT dressing evolution is the incorporation of smart technologies. Some advanced dressings now include sensors that can monitor wound healing progress, detect infection, or alert healthcare providers to dressing integrity issues. This integration of digital health technology with NPWT promises to revolutionize wound care management and patient monitoring.
Early NPWT dressings consisted of simple foam or gauze materials placed directly on the wound bed, covered with an adhesive drape to create an airtight seal. These basic designs were effective but often caused discomfort and skin damage during dressing changes.
As the technology progressed, manufacturers developed more sophisticated dressing systems. The introduction of non-adherent layers between the wound and the foam reduced tissue ingrowth, making dressing changes less painful. This innovation marked a significant step in improving patient experience and reducing the risk of secondary trauma to healing tissues.
The next major evolution came with the development of specialized foam materials. These new foams were designed to distribute negative pressure more evenly across the wound surface, promoting better granulation tissue formation and faster healing. Some foams were also infused with antimicrobial agents to help prevent infection in high-risk wounds.
Advancements in adhesive technology led to the creation of more skin-friendly drapes. These new drapes provided a better seal while reducing the risk of skin maceration and irritation around the wound edges. Some designs incorporated silicone-based adhesives, which allowed for gentler removal and repositioning of the dressing.
The integration of instillation therapy with NPWT represented another significant leap forward. These systems allowed for the controlled delivery of wound cleansing solutions or antimicrobial agents directly to the wound bed, enhancing the therapy's effectiveness in managing complex or infected wounds.
Recent innovations have focused on creating more user-friendly and portable NPWT systems. Disposable, single-use NPWT devices have been developed, offering increased mobility for patients and reducing the need for bulky equipment. These portable systems have greatly improved patient compliance and quality of life during treatment.
The latest trend in NPWT dressing evolution is the incorporation of smart technologies. Some advanced dressings now include sensors that can monitor wound healing progress, detect infection, or alert healthcare providers to dressing integrity issues. This integration of digital health technology with NPWT promises to revolutionize wound care management and patient monitoring.
Market Demand Analysis
The market demand for occlusive dressings in negative pressure therapy has been experiencing significant growth in recent years, driven by the increasing prevalence of chronic wounds and the rising adoption of advanced wound care technologies. Negative pressure wound therapy (NPWT) has become a standard treatment for various types of complex wounds, including diabetic foot ulcers, pressure ulcers, and surgical incisions.
The global NPWT market size was valued at $2.7 billion in 2020 and is projected to reach $3.9 billion by 2026, growing at a CAGR of 6.2% during the forecast period. Occlusive dressings play a crucial role in NPWT systems, as they create an airtight seal around the wound, allowing for the application of controlled negative pressure.
The aging population and the rising incidence of chronic diseases, such as diabetes and obesity, are key factors driving the demand for NPWT and associated occlusive dressings. According to the International Diabetes Federation, the number of adults with diabetes is expected to reach 700 million by 2045, potentially leading to an increase in diabetic foot ulcers and other chronic wounds requiring NPWT.
Healthcare facilities, including hospitals and wound care centers, represent the largest end-user segment for occlusive dressings in NPWT. However, there is a growing trend towards home-based NPWT, driven by the need for cost-effective healthcare solutions and patient preference for at-home treatment. This shift is creating new opportunities for portable NPWT devices and associated dressings designed for ease of use in non-clinical settings.
The market demand for occlusive dressings in NPWT is also influenced by technological advancements in wound care materials. There is a growing interest in dressings that not only provide an effective seal but also offer additional benefits such as antimicrobial properties, improved moisture management, and enhanced patient comfort. Manufacturers are focusing on developing innovative materials that can address these requirements while maintaining compatibility with various NPWT systems.
Geographically, North America holds the largest market share for NPWT devices and dressings, followed by Europe. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing awareness of advanced wound care techniques, and rising healthcare expenditure in countries like China and India.
The competitive landscape of the occlusive dressings market for NPWT is characterized by the presence of both established medical device companies and specialized wound care product manufacturers. Key players are investing in research and development to introduce novel dressing designs that can improve treatment outcomes and patient experience. Additionally, there is a growing emphasis on developing cost-effective solutions to address the needs of price-sensitive markets and healthcare systems focused on value-based care.
The global NPWT market size was valued at $2.7 billion in 2020 and is projected to reach $3.9 billion by 2026, growing at a CAGR of 6.2% during the forecast period. Occlusive dressings play a crucial role in NPWT systems, as they create an airtight seal around the wound, allowing for the application of controlled negative pressure.
The aging population and the rising incidence of chronic diseases, such as diabetes and obesity, are key factors driving the demand for NPWT and associated occlusive dressings. According to the International Diabetes Federation, the number of adults with diabetes is expected to reach 700 million by 2045, potentially leading to an increase in diabetic foot ulcers and other chronic wounds requiring NPWT.
Healthcare facilities, including hospitals and wound care centers, represent the largest end-user segment for occlusive dressings in NPWT. However, there is a growing trend towards home-based NPWT, driven by the need for cost-effective healthcare solutions and patient preference for at-home treatment. This shift is creating new opportunities for portable NPWT devices and associated dressings designed for ease of use in non-clinical settings.
The market demand for occlusive dressings in NPWT is also influenced by technological advancements in wound care materials. There is a growing interest in dressings that not only provide an effective seal but also offer additional benefits such as antimicrobial properties, improved moisture management, and enhanced patient comfort. Manufacturers are focusing on developing innovative materials that can address these requirements while maintaining compatibility with various NPWT systems.
Geographically, North America holds the largest market share for NPWT devices and dressings, followed by Europe. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing awareness of advanced wound care techniques, and rising healthcare expenditure in countries like China and India.
The competitive landscape of the occlusive dressings market for NPWT is characterized by the presence of both established medical device companies and specialized wound care product manufacturers. Key players are investing in research and development to introduce novel dressing designs that can improve treatment outcomes and patient experience. Additionally, there is a growing emphasis on developing cost-effective solutions to address the needs of price-sensitive markets and healthcare systems focused on value-based care.
Current Challenges
Negative pressure wound therapy (NPWT) has revolutionized the treatment of complex wounds, but designing effective occlusive dressings for this therapy presents several significant challenges. One of the primary obstacles is achieving a perfect seal around the wound site. Any air leakage can compromise the negative pressure, reducing the therapy's effectiveness and potentially leading to treatment failure.
Material selection poses another critical challenge. The dressing must be flexible enough to conform to various wound shapes and body contours while maintaining structural integrity under sustained negative pressure. It should also be biocompatible, non-adherent to the wound bed, and allow for easy removal without causing trauma to the healing tissue.
Moisture management is a complex issue in NPWT dressing design. The dressing must effectively remove excess exudate to prevent maceration of the surrounding skin, yet maintain an optimal moist environment for wound healing. Striking this balance is particularly challenging given the variability in wound exudate production across different wound types and healing stages.
Durability is another key concern. NPWT dressings often need to remain in place for several days, withstanding movement, shear forces, and constant negative pressure without degradation or loss of function. This requirement necessitates advanced materials and construction techniques that can maintain performance over extended periods.
Pain management during dressing changes represents a significant challenge. Traditional NPWT dressings can adhere to the wound bed, causing pain and potential tissue damage upon removal. Developing dressings that minimize adhesion while maintaining effective negative pressure is an ongoing area of research and development.
Cost-effectiveness is an increasingly important consideration in healthcare, and NPWT dressings are no exception. Designing dressings that meet all performance requirements while remaining economically viable for widespread use is a substantial challenge, particularly given the complex materials and manufacturing processes involved.
Lastly, the integration of smart technologies into NPWT dressings presents both opportunities and challenges. Incorporating sensors for real-time monitoring of wound healing, pressure levels, and exudate volume could greatly enhance treatment efficacy. However, this integration must be achieved without compromising the dressing's primary functions or significantly increasing costs.
Addressing these challenges requires a multidisciplinary approach, combining expertise in materials science, bioengineering, wound healing biology, and clinical practice. As the field of NPWT continues to evolve, overcoming these obstacles will be crucial in developing next-generation occlusive dressings that improve patient outcomes and expand the applicability of this important therapeutic modality.
Material selection poses another critical challenge. The dressing must be flexible enough to conform to various wound shapes and body contours while maintaining structural integrity under sustained negative pressure. It should also be biocompatible, non-adherent to the wound bed, and allow for easy removal without causing trauma to the healing tissue.
Moisture management is a complex issue in NPWT dressing design. The dressing must effectively remove excess exudate to prevent maceration of the surrounding skin, yet maintain an optimal moist environment for wound healing. Striking this balance is particularly challenging given the variability in wound exudate production across different wound types and healing stages.
Durability is another key concern. NPWT dressings often need to remain in place for several days, withstanding movement, shear forces, and constant negative pressure without degradation or loss of function. This requirement necessitates advanced materials and construction techniques that can maintain performance over extended periods.
Pain management during dressing changes represents a significant challenge. Traditional NPWT dressings can adhere to the wound bed, causing pain and potential tissue damage upon removal. Developing dressings that minimize adhesion while maintaining effective negative pressure is an ongoing area of research and development.
Cost-effectiveness is an increasingly important consideration in healthcare, and NPWT dressings are no exception. Designing dressings that meet all performance requirements while remaining economically viable for widespread use is a substantial challenge, particularly given the complex materials and manufacturing processes involved.
Lastly, the integration of smart technologies into NPWT dressings presents both opportunities and challenges. Incorporating sensors for real-time monitoring of wound healing, pressure levels, and exudate volume could greatly enhance treatment efficacy. However, this integration must be achieved without compromising the dressing's primary functions or significantly increasing costs.
Addressing these challenges requires a multidisciplinary approach, combining expertise in materials science, bioengineering, wound healing biology, and clinical practice. As the field of NPWT continues to evolve, overcoming these obstacles will be crucial in developing next-generation occlusive dressings that improve patient outcomes and expand the applicability of this important therapeutic modality.
Existing Dressing Designs
01 Occlusive dressing design for wound healing
Occlusive dressings are designed to create a moist environment that promotes wound healing. These dressings form a barrier over the wound, preventing external contamination while maintaining optimal moisture levels. This environment facilitates cell migration, enhances autolytic debridement, and accelerates the healing process.- Occlusive dressing materials and composition: Occlusive dressings are made from various materials and compositions to create a barrier over wounds. These materials can include polymers, hydrocolloids, and adhesive substances that provide a moist environment for wound healing while protecting against external contaminants. The composition of these dressings often includes components that enhance their occlusive properties and promote optimal wound healing conditions.
- Application techniques for occlusive dressings: Specific techniques are employed for applying occlusive dressings to ensure proper adherence and effectiveness. These methods may involve preparation of the wound area, careful placement of the dressing, and techniques to secure the edges for complete occlusion. Some applications may require specialized tools or applicators to ensure proper placement and to minimize air pockets between the dressing and the wound surface.
- Occlusive dressings with medication delivery: Some occlusive dressings are designed to incorporate and deliver medications directly to the wound site. These dressings may contain antimicrobial agents, growth factors, or other therapeutic substances that are released into the wound environment over time. The occlusive nature of the dressing helps to maintain high local concentrations of the medication, potentially enhancing its effectiveness.
- Specialized occlusive dressings for specific wound types: Different types of wounds may require specialized occlusive dressings. For example, burn wounds, surgical incisions, or chronic ulcers may each benefit from dressings with specific occlusive properties tailored to their unique healing requirements. These specialized dressings may vary in their level of occlusion, moisture retention, and ability to conform to different body contours.
- Monitoring and management of occluded wounds: Techniques and devices have been developed for monitoring and managing wounds under occlusive dressings. These may include transparent dressings that allow visual inspection, sensors that can detect changes in the wound environment, or systems for controlled removal of excess exudate without compromising the occlusive seal. Such innovations aim to optimize the healing process while minimizing the need for frequent dressing changes.
02 Moisture management in occlusive dressings
Effective occlusive dressings incorporate moisture management features to maintain an ideal wound environment. These dressings may include absorbent layers to handle excess exudate, while still maintaining a moist wound bed. Some designs incorporate semi-permeable films that allow vapor transmission while preventing liquid penetration.Expand Specific Solutions03 Occlusive dressings with medication delivery
Some occlusive dressings are designed to deliver medications directly to the wound site. These dressings may incorporate antimicrobial agents, growth factors, or other therapeutic substances within their structure. The occlusive nature of the dressing helps to maintain the medication in contact with the wound, potentially improving its efficacy.Expand Specific Solutions04 Specialized occlusive dressings for specific wound types
Occlusive dressings are developed for specific wound types or body locations. For example, specialized dressings may be designed for burn wounds, surgical incisions, or pressure ulcers. These dressings take into account factors such as wound shape, location, and specific healing requirements to provide optimal occlusion and support.Expand Specific Solutions05 Occlusive dressing removal and wound monitoring
Innovations in occlusive dressings focus on ease of removal and wound monitoring capabilities. Some dressings incorporate features that allow for gentle removal without damaging newly formed tissue. Others may include transparent windows or sensors that enable wound assessment without removing the dressing, maintaining the occlusive environment.Expand Specific Solutions
Key NPWT Players
The market for occlusive dressings in negative pressure therapy is in a mature growth stage, with a significant global market size driven by increasing prevalence of chronic wounds and surgical procedures. The technology has reached a high level of maturity, with established players like KCI Licensing, Inc., 3M Innovative Properties Co., and Smith & Nephew plc dominating the market. These companies have developed advanced solutions and hold substantial intellectual property. Emerging players such as Aatru Medical LLC and Worldwide Innovative Healthcare, Inc. are introducing innovative designs to challenge incumbents. The competitive landscape is characterized by ongoing R&D efforts to improve efficacy, patient comfort, and cost-effectiveness, with a focus on portable and disposable solutions.
KCI Licensing, Inc.
Technical Solution: KCI Licensing, Inc. has developed advanced occlusive dressings for negative pressure wound therapy (NPWT). Their V.A.C. (Vacuum Assisted Closure) Therapy System utilizes a specialized foam dressing with an adhesive drape to create a sealed environment. The foam dressing is cut to fit the wound shape and placed directly into the wound bed. A tube is then inserted into the foam, and the entire area is covered with an adhesive drape to create an airtight seal. This design allows for even distribution of negative pressure across the wound surface, promoting granulation tissue formation and wound contraction[1][3]. The company has also introduced the V.A.C. VERAFLO™ Therapy, which combines NPWT with automated topical wound solution delivery and removal, enhancing the wound cleansing process[2].
Strengths: Proven clinical efficacy, versatile application for various wound types, and integrated solution delivery. Weaknesses: Potential for tissue ingrowth into foam, which may cause pain during dressing changes, and the need for frequent dressing changes in some cases.
3M Innovative Properties Co.
Technical Solution: 3M Innovative Properties Co. has developed the V.A.C.ULTA™ Negative Pressure Wound Therapy System, which incorporates their proprietary SensaT.R.A.C.™ technology. This system uses a specialized foam dressing with a hydrophobic layer to prevent fluid from entering the tubing. The dressing is covered with a transparent adhesive film to create an airtight seal. The SensaT.R.A.C.™ pad, connected to the suction tube, is placed over a small cut in the film. This design allows for precise pressure control and continuous monitoring of the wound environment[4]. 3M has also introduced the Snap™ Therapy System, a mechanically powered, disposable NPWT device for smaller wounds, offering a more portable solution[5].
Strengths: Advanced pressure control technology, range of solutions for different wound sizes, and disposable options for improved portability. Weaknesses: Potential for skin irritation from adhesive film in some patients, and the need for careful application to prevent air leaks.
Core Innovations
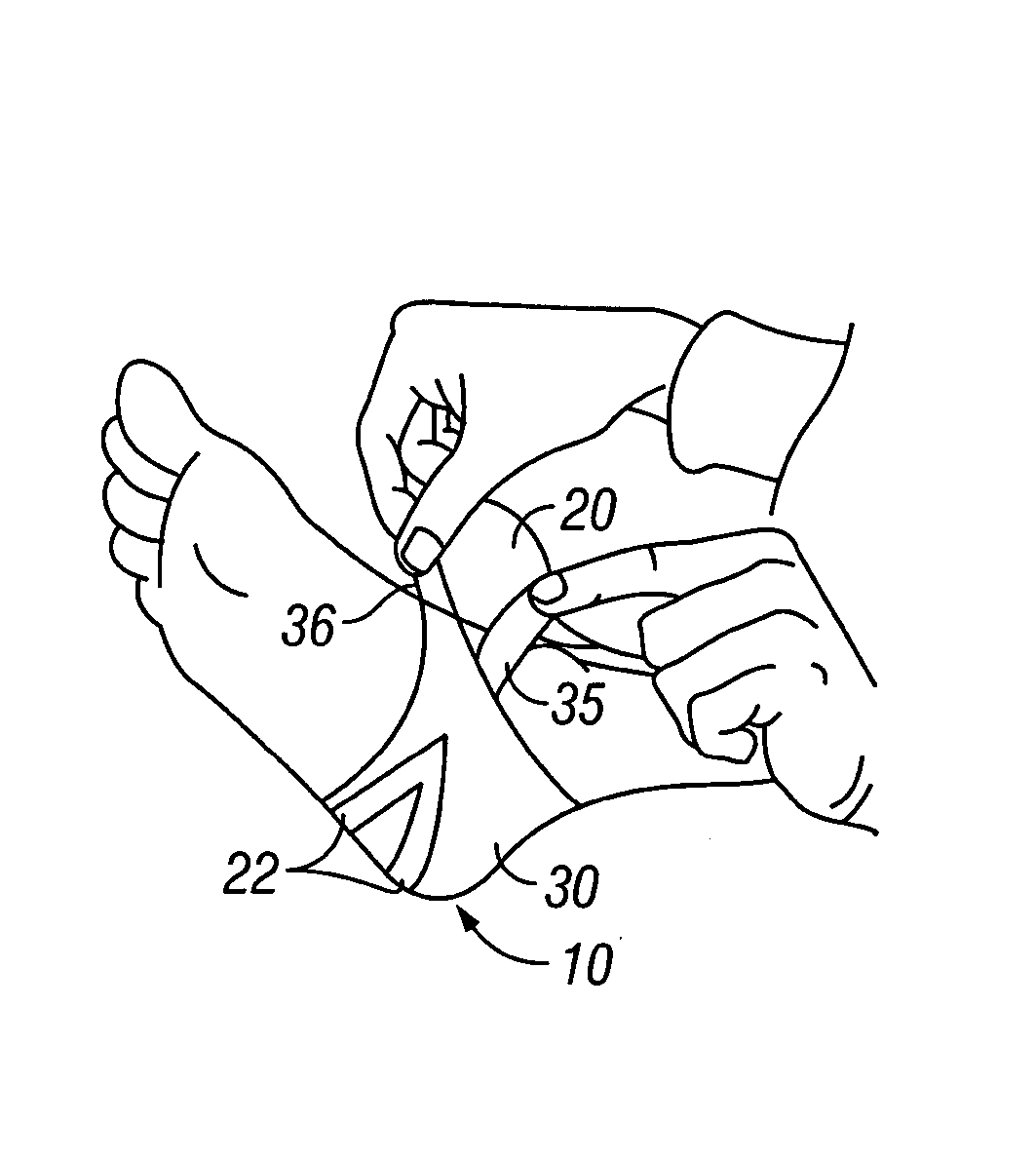
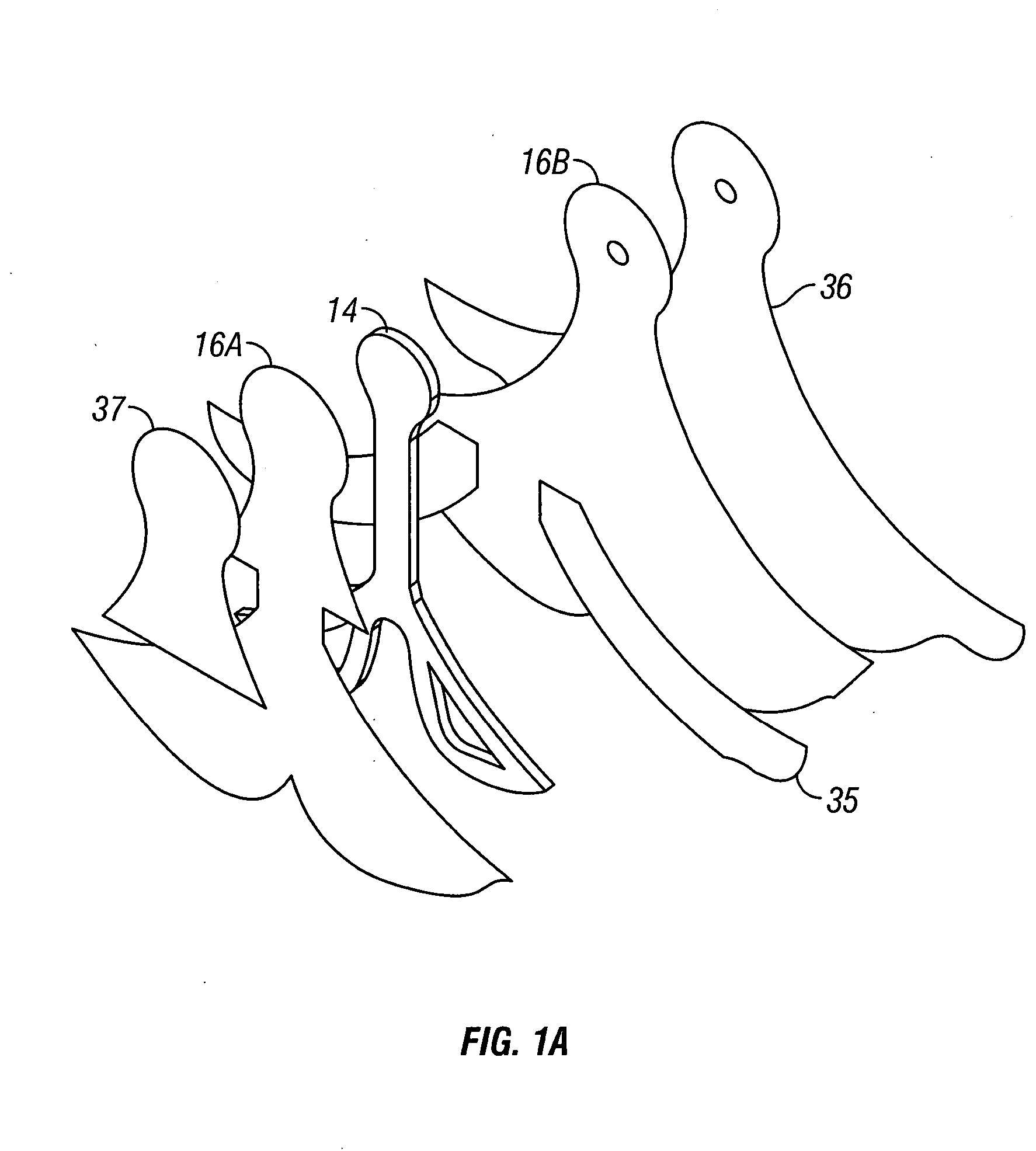
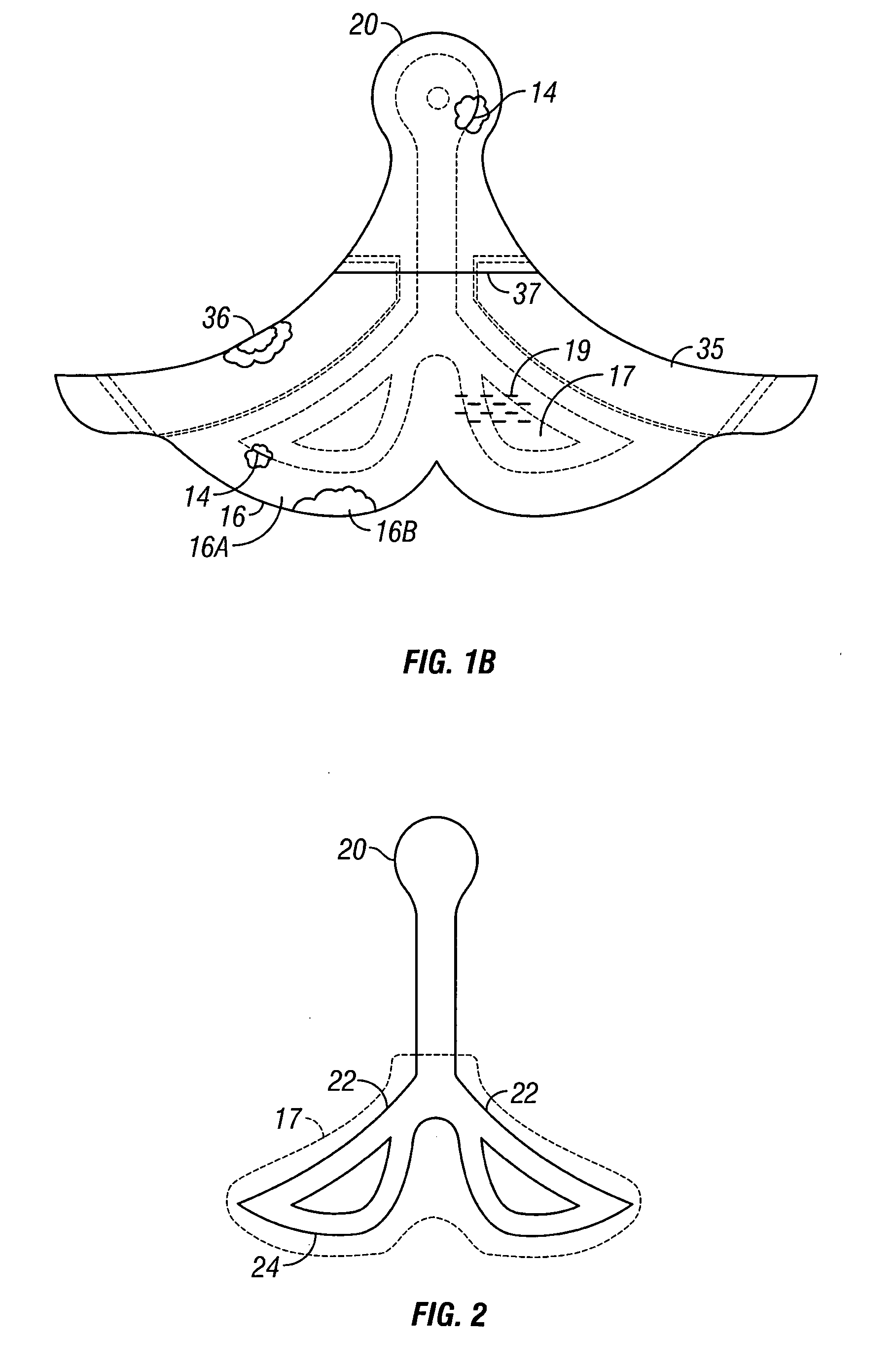
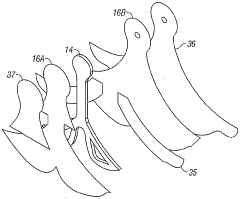
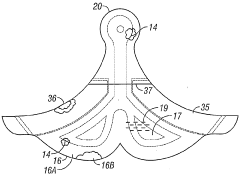
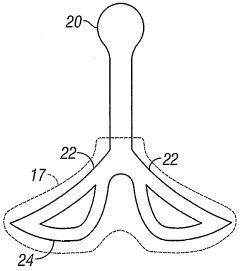
Negative pressure wound treatment dressing
PatentActiveUS20050020955A1
Innovation
- A contoured porous pad is placed within a fenestrated and occlusive wrapping, with flexible tubing for fluid communication of negative pressure, and adhesive attachments to secure the dressing, ensuring airtight sealing and maintaining pressure during ambulation.
Negative pressure wound treatment dressing
PatentWO2005009488A2
Innovation
- A contoured porous pad is positioned within a fenestrated and occlusive wrapping, with flexible tubing for fluid communication of negative pressure, and adhesive attachments to secure the dressing, ensuring airtight sealing and maintaining negative pressure over the wound despite movement, using a collection canister to manage effluents.
Regulatory Compliance
Regulatory compliance is a critical aspect of designing occlusive dressings for negative pressure therapy. The development and marketing of these medical devices are subject to stringent regulations to ensure patient safety and product efficacy. In the United States, the Food and Drug Administration (FDA) oversees the approval process for such devices under the Medical Device Regulations. Manufacturers must adhere to the FDA's Quality System Regulation (QSR) and obtain 510(k) clearance or Premarket Approval (PMA) depending on the device classification.
The European Union regulates these devices under the Medical Device Regulation (MDR), which replaced the previous Medical Device Directive (MDD) in 2021. Manufacturers seeking to market their products in the EU must obtain CE marking, demonstrating compliance with the MDR's essential requirements. This process involves conducting clinical evaluations, risk assessments, and implementing a quality management system.
In addition to regional regulations, international standards play a crucial role in ensuring global consistency and quality. ISO 13485, the international standard for quality management systems in medical devices, is widely recognized and often required for regulatory compliance. Manufacturers must also consider ISO 10993 for biocompatibility testing and ISO 14155 for clinical investigations of medical devices.
Specific to negative pressure wound therapy devices, standards such as ISO 10079-1 for medical suction equipment and IEC 60601-1 for medical electrical equipment safety are particularly relevant. These standards address performance, safety, and usability aspects of the devices.
Regulatory bodies also focus on the materials used in occlusive dressings. Manufacturers must demonstrate that the materials are biocompatible and suitable for their intended use. This involves conducting extensive testing according to ISO 10993 series, including cytotoxicity, sensitization, and irritation tests.
Post-market surveillance is another crucial aspect of regulatory compliance. Manufacturers are required to implement systems for monitoring the performance and safety of their devices once they are in use. This includes collecting and analyzing data on adverse events, conducting post-market clinical follow-up studies, and updating risk assessments based on real-world evidence.
Compliance with data protection regulations, such as the General Data Protection Regulation (GDPR) in the EU, is also essential, particularly for devices that collect or process patient data. Manufacturers must ensure that their devices and associated software systems are designed with privacy and data security in mind.
As regulations evolve, manufacturers must stay informed about changes and updates to maintain compliance. This often requires dedicated regulatory affairs teams and ongoing investment in quality management systems. Failure to comply with regulatory requirements can result in significant penalties, product recalls, and damage to a company's reputation.
The European Union regulates these devices under the Medical Device Regulation (MDR), which replaced the previous Medical Device Directive (MDD) in 2021. Manufacturers seeking to market their products in the EU must obtain CE marking, demonstrating compliance with the MDR's essential requirements. This process involves conducting clinical evaluations, risk assessments, and implementing a quality management system.
In addition to regional regulations, international standards play a crucial role in ensuring global consistency and quality. ISO 13485, the international standard for quality management systems in medical devices, is widely recognized and often required for regulatory compliance. Manufacturers must also consider ISO 10993 for biocompatibility testing and ISO 14155 for clinical investigations of medical devices.
Specific to negative pressure wound therapy devices, standards such as ISO 10079-1 for medical suction equipment and IEC 60601-1 for medical electrical equipment safety are particularly relevant. These standards address performance, safety, and usability aspects of the devices.
Regulatory bodies also focus on the materials used in occlusive dressings. Manufacturers must demonstrate that the materials are biocompatible and suitable for their intended use. This involves conducting extensive testing according to ISO 10993 series, including cytotoxicity, sensitization, and irritation tests.
Post-market surveillance is another crucial aspect of regulatory compliance. Manufacturers are required to implement systems for monitoring the performance and safety of their devices once they are in use. This includes collecting and analyzing data on adverse events, conducting post-market clinical follow-up studies, and updating risk assessments based on real-world evidence.
Compliance with data protection regulations, such as the General Data Protection Regulation (GDPR) in the EU, is also essential, particularly for devices that collect or process patient data. Manufacturers must ensure that their devices and associated software systems are designed with privacy and data security in mind.
As regulations evolve, manufacturers must stay informed about changes and updates to maintain compliance. This often requires dedicated regulatory affairs teams and ongoing investment in quality management systems. Failure to comply with regulatory requirements can result in significant penalties, product recalls, and damage to a company's reputation.
Material Advancements
Material advancements play a crucial role in the design and effectiveness of occlusive dressings for negative pressure therapy. Recent developments in this field have focused on enhancing the performance, biocompatibility, and versatility of these dressings.
One significant advancement is the development of smart polymers that can respond to changes in the wound environment. These materials can adapt their properties based on factors such as pH, temperature, or bacterial presence. For instance, some polymers can release antimicrobial agents when bacterial colonization is detected, providing targeted treatment and reducing the risk of infection.
Nanofiber technology has also emerged as a promising avenue for improving occlusive dressings. Electrospun nanofibers offer high surface area-to-volume ratios, allowing for enhanced fluid absorption and retention. These nanofibers can be engineered to incorporate various therapeutic agents, such as growth factors or antibiotics, enabling controlled release over time.
Hydrogel-based materials have gained attention for their ability to maintain a moist wound environment while allowing for gas exchange. Advanced hydrogels can now be designed with tunable mechanical properties, making them suitable for different wound types and locations. Some hydrogels also exhibit self-healing properties, which can help maintain the integrity of the dressing during therapy.
Composite materials that combine multiple functionalities are becoming increasingly popular. For example, dressings incorporating both absorbent layers and antimicrobial components can simultaneously manage wound exudate and prevent infection. These multi-functional materials can simplify wound care protocols and potentially improve patient outcomes.
Biodegradable materials are another area of focus, addressing the need for dressings that can be left in place for extended periods without requiring frequent changes. These materials gradually break down into harmless byproducts, reducing the risk of tissue damage during dressing removal and minimizing patient discomfort.
Advances in surface modification techniques have led to the development of dressings with improved adhesion properties. This is particularly important for maintaining an effective seal during negative pressure therapy. Some materials now feature micro-patterned surfaces that enhance adhesion without increasing skin irritation.
Researchers are also exploring the use of biomimetic materials that mimic the structure and properties of natural tissues. These materials can potentially promote faster wound healing by providing a more physiologically relevant environment for cell growth and tissue regeneration.
As material science continues to evolve, we can expect further innovations in occlusive dressings for negative pressure therapy. Future developments may include materials with enhanced sensing capabilities, improved integration with wound monitoring systems, and even more sophisticated drug delivery mechanisms.
One significant advancement is the development of smart polymers that can respond to changes in the wound environment. These materials can adapt their properties based on factors such as pH, temperature, or bacterial presence. For instance, some polymers can release antimicrobial agents when bacterial colonization is detected, providing targeted treatment and reducing the risk of infection.
Nanofiber technology has also emerged as a promising avenue for improving occlusive dressings. Electrospun nanofibers offer high surface area-to-volume ratios, allowing for enhanced fluid absorption and retention. These nanofibers can be engineered to incorporate various therapeutic agents, such as growth factors or antibiotics, enabling controlled release over time.
Hydrogel-based materials have gained attention for their ability to maintain a moist wound environment while allowing for gas exchange. Advanced hydrogels can now be designed with tunable mechanical properties, making them suitable for different wound types and locations. Some hydrogels also exhibit self-healing properties, which can help maintain the integrity of the dressing during therapy.
Composite materials that combine multiple functionalities are becoming increasingly popular. For example, dressings incorporating both absorbent layers and antimicrobial components can simultaneously manage wound exudate and prevent infection. These multi-functional materials can simplify wound care protocols and potentially improve patient outcomes.
Biodegradable materials are another area of focus, addressing the need for dressings that can be left in place for extended periods without requiring frequent changes. These materials gradually break down into harmless byproducts, reducing the risk of tissue damage during dressing removal and minimizing patient discomfort.
Advances in surface modification techniques have led to the development of dressings with improved adhesion properties. This is particularly important for maintaining an effective seal during negative pressure therapy. Some materials now feature micro-patterned surfaces that enhance adhesion without increasing skin irritation.
Researchers are also exploring the use of biomimetic materials that mimic the structure and properties of natural tissues. These materials can potentially promote faster wound healing by providing a more physiologically relevant environment for cell growth and tissue regeneration.
As material science continues to evolve, we can expect further innovations in occlusive dressings for negative pressure therapy. Future developments may include materials with enhanced sensing capabilities, improved integration with wound monitoring systems, and even more sophisticated drug delivery mechanisms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!