Occlusive Dressing Integration With Sensors For Wound Monitoring
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wound Monitoring Tech Evolution and Objectives
Wound monitoring technology has undergone significant evolution over the past few decades, driven by the need for more effective and efficient wound care management. The journey began with simple visual inspection and manual measurements, which were subjective and time-consuming. As healthcare demands increased, the focus shifted towards developing more sophisticated and automated monitoring systems.
The 1980s saw the introduction of basic electronic devices for measuring wound dimensions and capturing images. These early innovations laid the groundwork for more advanced technologies. In the 1990s and early 2000s, the integration of digital imaging and computer-aided analysis marked a significant leap forward, enabling more precise wound assessment and documentation.
The advent of smart sensors and Internet of Things (IoT) technologies in the 2010s revolutionized wound monitoring. These innovations allowed for continuous, real-time data collection on various wound parameters such as moisture levels, pH, temperature, and exudate composition. This shift towards sensor-based monitoring systems provided healthcare professionals with more comprehensive and timely information about wound healing progress.
Recent years have witnessed a growing interest in integrating sensors directly into wound dressings. This approach aims to create "smart dressings" capable of monitoring wound conditions without the need for dressing removal, thereby reducing the risk of infection and improving patient comfort. The integration of occlusive dressings with sensors represents a cutting-edge development in this field, combining the benefits of moisture-retentive wound healing with advanced monitoring capabilities.
The primary objectives of current research in occlusive dressing integration with sensors for wound monitoring are multifaceted. Firstly, there is a focus on developing sensors that are thin, flexible, and biocompatible to ensure seamless integration with occlusive dressings without compromising their protective functions. Secondly, researchers aim to enhance the accuracy and reliability of sensor measurements in the challenging environment of a wound bed.
Another key objective is to improve data transmission and analysis capabilities. This involves developing robust wireless communication systems and advanced algorithms for interpreting sensor data to provide meaningful insights into wound healing progress. Additionally, there is a strong emphasis on creating user-friendly interfaces that allow healthcare professionals to easily access and interpret the collected data.
Looking ahead, the field is moving towards the development of "closed-loop" systems that not only monitor wound conditions but also automatically adjust treatment parameters based on the collected data. This could include controlled release of medications or adjustment of environmental factors to optimize healing conditions. The ultimate goal is to create a comprehensive wound care system that combines continuous monitoring with intelligent, adaptive treatment strategies, thereby improving patient outcomes and reducing the burden on healthcare resources.
The 1980s saw the introduction of basic electronic devices for measuring wound dimensions and capturing images. These early innovations laid the groundwork for more advanced technologies. In the 1990s and early 2000s, the integration of digital imaging and computer-aided analysis marked a significant leap forward, enabling more precise wound assessment and documentation.
The advent of smart sensors and Internet of Things (IoT) technologies in the 2010s revolutionized wound monitoring. These innovations allowed for continuous, real-time data collection on various wound parameters such as moisture levels, pH, temperature, and exudate composition. This shift towards sensor-based monitoring systems provided healthcare professionals with more comprehensive and timely information about wound healing progress.
Recent years have witnessed a growing interest in integrating sensors directly into wound dressings. This approach aims to create "smart dressings" capable of monitoring wound conditions without the need for dressing removal, thereby reducing the risk of infection and improving patient comfort. The integration of occlusive dressings with sensors represents a cutting-edge development in this field, combining the benefits of moisture-retentive wound healing with advanced monitoring capabilities.
The primary objectives of current research in occlusive dressing integration with sensors for wound monitoring are multifaceted. Firstly, there is a focus on developing sensors that are thin, flexible, and biocompatible to ensure seamless integration with occlusive dressings without compromising their protective functions. Secondly, researchers aim to enhance the accuracy and reliability of sensor measurements in the challenging environment of a wound bed.
Another key objective is to improve data transmission and analysis capabilities. This involves developing robust wireless communication systems and advanced algorithms for interpreting sensor data to provide meaningful insights into wound healing progress. Additionally, there is a strong emphasis on creating user-friendly interfaces that allow healthcare professionals to easily access and interpret the collected data.
Looking ahead, the field is moving towards the development of "closed-loop" systems that not only monitor wound conditions but also automatically adjust treatment parameters based on the collected data. This could include controlled release of medications or adjustment of environmental factors to optimize healing conditions. The ultimate goal is to create a comprehensive wound care system that combines continuous monitoring with intelligent, adaptive treatment strategies, thereby improving patient outcomes and reducing the burden on healthcare resources.
Market Analysis for Smart Wound Care Solutions
The smart wound care solutions market is experiencing significant growth, driven by the increasing prevalence of chronic wounds, rising geriatric population, and advancements in sensor technologies. This market segment encompasses a range of innovative products that integrate occlusive dressings with sensors for real-time wound monitoring, offering improved patient outcomes and reduced healthcare costs.
The global smart wound care market is projected to expand rapidly in the coming years, with a compound annual growth rate (CAGR) exceeding 5% from 2021 to 2026. North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its high healthcare expenditure and early adoption of advanced medical technologies.
Key factors driving market demand include the rising incidence of chronic wounds such as diabetic foot ulcers, pressure ulcers, and venous leg ulcers. The growing diabetic population, which is more susceptible to chronic wounds, is a significant contributor to market growth. Additionally, the aging population in developed countries is fueling demand for advanced wound care solutions that can provide better monitoring and management of complex wounds.
The COVID-19 pandemic has further accelerated the adoption of smart wound care solutions, as healthcare providers seek ways to reduce in-person visits and minimize the risk of infection transmission. Remote monitoring capabilities offered by these technologies have become increasingly valuable in this context.
Hospital-acquired pressure ulcers represent another substantial market opportunity, as healthcare facilities strive to improve patient care and reduce associated costs. Smart wound care solutions can help in early detection and prevention of these complications, aligning with the broader trend towards value-based healthcare.
The market is also benefiting from increasing awareness among healthcare professionals and patients about the benefits of advanced wound care technologies. Government initiatives and favorable reimbursement policies in some regions are further supporting market growth by improving access to these innovative solutions.
However, challenges such as high product costs and limited awareness in developing regions may hinder market expansion. Additionally, concerns about data privacy and security in connected wound care devices need to be addressed to foster wider adoption.
In conclusion, the market for smart wound care solutions, particularly those integrating occlusive dressings with sensors for wound monitoring, shows strong growth potential. As technology continues to advance and healthcare systems increasingly focus on preventive and personalized care, this market segment is poised for continued expansion and innovation.
The global smart wound care market is projected to expand rapidly in the coming years, with a compound annual growth rate (CAGR) exceeding 5% from 2021 to 2026. North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its high healthcare expenditure and early adoption of advanced medical technologies.
Key factors driving market demand include the rising incidence of chronic wounds such as diabetic foot ulcers, pressure ulcers, and venous leg ulcers. The growing diabetic population, which is more susceptible to chronic wounds, is a significant contributor to market growth. Additionally, the aging population in developed countries is fueling demand for advanced wound care solutions that can provide better monitoring and management of complex wounds.
The COVID-19 pandemic has further accelerated the adoption of smart wound care solutions, as healthcare providers seek ways to reduce in-person visits and minimize the risk of infection transmission. Remote monitoring capabilities offered by these technologies have become increasingly valuable in this context.
Hospital-acquired pressure ulcers represent another substantial market opportunity, as healthcare facilities strive to improve patient care and reduce associated costs. Smart wound care solutions can help in early detection and prevention of these complications, aligning with the broader trend towards value-based healthcare.
The market is also benefiting from increasing awareness among healthcare professionals and patients about the benefits of advanced wound care technologies. Government initiatives and favorable reimbursement policies in some regions are further supporting market growth by improving access to these innovative solutions.
However, challenges such as high product costs and limited awareness in developing regions may hinder market expansion. Additionally, concerns about data privacy and security in connected wound care devices need to be addressed to foster wider adoption.
In conclusion, the market for smart wound care solutions, particularly those integrating occlusive dressings with sensors for wound monitoring, shows strong growth potential. As technology continues to advance and healthcare systems increasingly focus on preventive and personalized care, this market segment is poised for continued expansion and innovation.
Current Challenges in Sensor-Integrated Dressings
The integration of sensors into occlusive dressings for wound monitoring presents several significant challenges that researchers and developers must address. One of the primary obstacles is the miniaturization of sensors without compromising their functionality. As wound dressings need to be thin and flexible, the sensors must be compact enough to be incorporated seamlessly without causing discomfort or interfering with the healing process.
Another major challenge lies in ensuring the biocompatibility and safety of sensor-integrated dressings. The materials used for sensors and their integration methods must not cause adverse reactions or introduce potential infection risks to the wound site. This requires extensive testing and careful selection of materials that are both functional for sensing and safe for prolonged contact with wounds.
Power management presents a significant hurdle in the development of sensor-integrated dressings. Traditional batteries are often too bulky and rigid for integration into flexible dressings. Researchers are exploring alternative power sources such as thin-film batteries, energy harvesting technologies, or wireless power transfer methods. However, each of these solutions comes with its own set of challenges related to efficiency, reliability, and safety.
Data transmission and connectivity pose another set of challenges. Wireless communication technologies must be implemented in a way that ensures reliable data transfer while minimizing power consumption. The integration of antennas and communication modules into the dressing without compromising its primary wound-healing function requires innovative design approaches.
Durability and reliability of the sensor systems in the moist wound environment are critical concerns. Sensors must maintain accuracy and functionality despite exposure to wound exudates, cleaning solutions, and potential physical stresses. Developing robust encapsulation methods that protect electronic components without impeding their sensing capabilities is a complex engineering challenge.
Calibration and standardization of sensor readings across different wound types and patient conditions represent another significant challenge. Variations in wound characteristics, such as depth, exudate levels, and tissue composition, can affect sensor readings. Developing algorithms and calibration methods that account for these variables to provide accurate and clinically relevant data is an ongoing area of research.
Cost-effectiveness and scalability of production are also important considerations. For sensor-integrated dressings to be widely adopted, they must be manufacturable at a scale and cost that makes them accessible for routine clinical use. This requires optimization of materials and production processes to balance performance with economic viability.
Another major challenge lies in ensuring the biocompatibility and safety of sensor-integrated dressings. The materials used for sensors and their integration methods must not cause adverse reactions or introduce potential infection risks to the wound site. This requires extensive testing and careful selection of materials that are both functional for sensing and safe for prolonged contact with wounds.
Power management presents a significant hurdle in the development of sensor-integrated dressings. Traditional batteries are often too bulky and rigid for integration into flexible dressings. Researchers are exploring alternative power sources such as thin-film batteries, energy harvesting technologies, or wireless power transfer methods. However, each of these solutions comes with its own set of challenges related to efficiency, reliability, and safety.
Data transmission and connectivity pose another set of challenges. Wireless communication technologies must be implemented in a way that ensures reliable data transfer while minimizing power consumption. The integration of antennas and communication modules into the dressing without compromising its primary wound-healing function requires innovative design approaches.
Durability and reliability of the sensor systems in the moist wound environment are critical concerns. Sensors must maintain accuracy and functionality despite exposure to wound exudates, cleaning solutions, and potential physical stresses. Developing robust encapsulation methods that protect electronic components without impeding their sensing capabilities is a complex engineering challenge.
Calibration and standardization of sensor readings across different wound types and patient conditions represent another significant challenge. Variations in wound characteristics, such as depth, exudate levels, and tissue composition, can affect sensor readings. Developing algorithms and calibration methods that account for these variables to provide accurate and clinically relevant data is an ongoing area of research.
Cost-effectiveness and scalability of production are also important considerations. For sensor-integrated dressings to be widely adopted, they must be manufacturable at a scale and cost that makes them accessible for routine clinical use. This requires optimization of materials and production processes to balance performance with economic viability.
Existing Sensor-Integrated Occlusive Dressing Solutions
01 Integrated sensors for wound monitoring
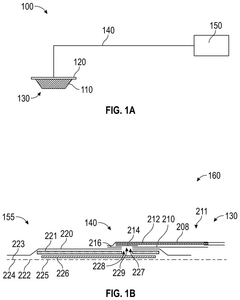
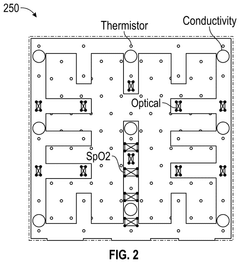
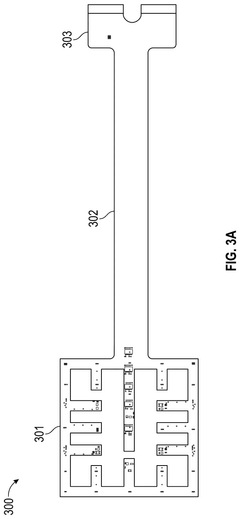
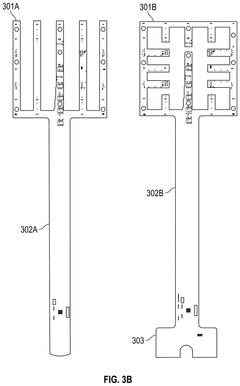
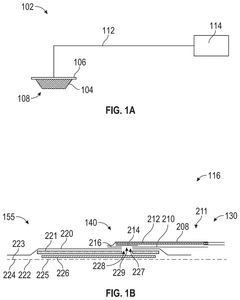
Occlusive dressings with integrated sensors allow for continuous monitoring of wound conditions. These sensors can measure various parameters such as temperature, pH, moisture levels, and bacterial growth, providing real-time data on wound healing progress. This technology enables healthcare professionals to make informed decisions about wound management without disturbing the healing environment.- Occlusive dressings with integrated sensors for wound monitoring: These dressings incorporate sensors directly into the occlusive wound covering to continuously monitor various parameters of the wound environment. The integrated sensors can measure factors such as moisture levels, pH, temperature, and bacterial presence, providing real-time data on wound healing progress without the need to remove the dressing.
- Smart wound dressings with wireless communication capabilities: Advanced wound dressings equipped with wireless communication modules allow for remote monitoring of wound conditions. These smart dressings can transmit data collected by integrated sensors to healthcare providers or mobile devices, enabling timely interventions and personalized treatment adjustments without frequent dressing changes.
- Multi-layered occlusive dressings with embedded sensor arrays: These dressings feature multiple layers designed to optimize wound healing while incorporating an array of sensors. The layered structure may include moisture-wicking, antimicrobial, and occlusive components, with sensors strategically placed to monitor different aspects of wound healing across various depths of the wound bed.
- Biodegradable occlusive dressings with integrated biosensors: Innovative dressings made from biodegradable materials incorporate biosensors that can detect specific biomarkers indicative of wound healing or infection. These eco-friendly dressings provide valuable diagnostic information while naturally breaking down over time, reducing the need for dressing removal and minimizing disruption to the wound site.
- Occlusive dressings with drug delivery systems and integrated monitoring: These advanced dressings combine occlusive properties with controlled drug delivery mechanisms and integrated sensors. The dressings can release therapeutic agents based on wound conditions detected by the sensors, providing a closed-loop system for optimized wound treatment and real-time monitoring of drug efficacy.
02 Smart dressings with data transmission capabilities
Advanced occlusive dressings incorporate wireless communication technologies to transmit wound data to external devices or healthcare systems. These smart dressings can alert medical professionals to changes in wound status, enabling timely interventions. The integration of data transmission capabilities enhances remote patient monitoring and improves overall wound care management.Expand Specific Solutions03 Customizable and adaptive wound dressings
Innovative occlusive dressings feature adaptable designs that can be customized to fit various wound types and sizes. These dressings may incorporate shape-memory materials or modular components that allow for adjustments as the wound heals. The ability to customize the dressing enhances patient comfort and optimizes the healing environment for different wound stages.Expand Specific Solutions04 Integration of therapeutic agents in smart dressings
Advanced occlusive dressings combine monitoring capabilities with the ability to deliver therapeutic agents directly to the wound site. These dressings may incorporate drug-releasing mechanisms triggered by sensor data or external controls. The integration of treatment delivery with wound monitoring allows for targeted and timely interventions, potentially accelerating the healing process.Expand Specific Solutions05 Enhanced visualization and imaging of wound progress
Some occlusive dressings incorporate transparent materials or imaging technologies that allow for visual inspection of the wound without removing the dressing. These may include integrated cameras or specialized materials that change color in response to wound conditions. Such features enable healthcare providers to assess wound healing progress while maintaining the integrity of the occlusive environment.Expand Specific Solutions
Key Players in Smart Wound Care Industry
The research on occlusive dressing integration with sensors for wound monitoring is in an emerging stage, with the market showing significant growth potential. The global advanced wound care market, which includes smart dressings, is expected to expand rapidly due to increasing chronic wounds and technological advancements. While the technology is still evolving, several key players are making strides in this field. Companies like Smith & Nephew plc and Coloplast A/S are leveraging their expertise in wound care to develop innovative sensor-integrated dressings. Academic institutions such as University College Cork and RWTH Aachen University are contributing to research and development efforts. The collaboration between industry and academia is accelerating the maturation of this technology, although widespread clinical adoption is still in progress.
Smith & Nephew plc
Technical Solution: Smith & Nephew has developed advanced wound monitoring systems integrating sensors into occlusive dressings. Their PICO™ system combines negative pressure wound therapy with a smart dressing that incorporates sensors to monitor wound exudate levels and pressure[1]. The dressing uses a proprietary Airlock™ Technology layer to distribute negative pressure evenly and efficiently remove exudate. The integrated sensors provide real-time data on wound healing progress, allowing for remote monitoring and timely interventions. Smith & Nephew has also been working on incorporating pH and temperature sensors into their dressings to detect early signs of infection[2]. These smart dressings aim to optimize wound healing by providing continuous monitoring and personalized care.
Strengths: Established brand in wound care, extensive R&D capabilities, and existing product lines that can be enhanced with sensor technology. Weaknesses: High cost of advanced dressings may limit adoption in some markets.
Coloplast A/S
Technical Solution: Coloplast has been investing in smart wound care solutions, focusing on integrating sensors into their existing wound dressing products. Their research includes developing dressings with embedded moisture sensors to monitor wound exudate levels and prevent maceration[3]. The company is also exploring the use of temperature and pH sensors to detect early signs of infection. Coloplast's approach involves creating a thin, flexible sensor array that can be incorporated into their Biatain® line of foam dressings without compromising the dressing's primary functions. The sensor data is transmitted wirelessly to a smartphone app, allowing healthcare providers to remotely monitor wound healing progress and make informed decisions about treatment adjustments[4]. Coloplast is also investigating the use of machine learning algorithms to analyze sensor data and predict wound healing outcomes.
Strengths: Strong presence in ostomy and continence care markets, which can be leveraged for wound care innovations. Extensive distribution network. Weaknesses: Relatively new entrant in advanced wound monitoring technology, may face challenges in competing with established players.
Core Innovations in Wound Monitoring Sensors
Systems and method for applying biocompatible encapsulation to sensor enabled wound monitoring and therapy dressings
PatentPendingUS20250090380A1
Innovation
- The development of sensor-enabled wound dressings with embedded electronic components that collect and transmit data on tissue conditions, integrated into existing treatment regimes, including wound dressings and orthopedic devices, to provide comprehensive monitoring and feedback.
Positioning of sensors for sensor enabled wound monitoring or therapy
PatentPendingUS20240423543A1
Innovation
- A wound monitoring and therapy system with a wound dressing incorporating sensors to measure various wound characteristics, a controller to determine sensor positioning, and a method to provide real-time feedback on sensor placement and wound status.
Regulatory Framework for Medical Wearables
The regulatory framework for medical wearables, including occlusive dressings integrated with sensors for wound monitoring, is a complex and evolving landscape. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing these devices. The FDA categorizes such products under the broader umbrella of medical devices, with specific classifications based on their intended use and risk level.
For wound monitoring dressings with integrated sensors, the FDA typically considers them as Class II medical devices. This classification requires manufacturers to submit a 510(k) premarket notification, demonstrating that the device is substantially equivalent to a legally marketed predicate device. The process involves rigorous testing and documentation to ensure safety and efficacy.
In the European Union, medical wearables fall under the purview of the Medical Device Regulation (MDR). The MDR, which came into full effect in May 2021, has introduced more stringent requirements for medical device manufacturers. Occlusive dressings with integrated sensors would likely be classified as Class IIa or IIb devices under the MDR, depending on their specific features and intended use.
The regulatory framework also addresses data privacy and security concerns, particularly relevant for sensor-integrated dressings that collect and transmit patient data. In the EU, the General Data Protection Regulation (GDPR) sets strict guidelines for the handling of personal health data. Similarly, in the US, the Health Insurance Portability and Accountability Act (HIPAA) governs the protection of sensitive patient health information.
Manufacturers must also comply with quality management standards, such as ISO 13485, which specifies requirements for a quality management system for medical devices. This standard ensures that devices consistently meet customer and regulatory requirements throughout their lifecycle.
As the field of medical wearables continues to advance, regulatory bodies are adapting their frameworks to keep pace with technological innovations. For instance, the FDA has introduced the Digital Health Software Precertification (Pre-Cert) Program, aimed at streamlining the regulatory process for software-based medical technologies, which may include certain types of sensor-integrated dressings.
Globally, there is a trend towards harmonization of regulatory standards, as evidenced by the Medical Device Single Audit Program (MDSAP). This program allows for a single regulatory audit of a medical device manufacturer's quality management system to satisfy the requirements of multiple regulatory jurisdictions.
For wound monitoring dressings with integrated sensors, the FDA typically considers them as Class II medical devices. This classification requires manufacturers to submit a 510(k) premarket notification, demonstrating that the device is substantially equivalent to a legally marketed predicate device. The process involves rigorous testing and documentation to ensure safety and efficacy.
In the European Union, medical wearables fall under the purview of the Medical Device Regulation (MDR). The MDR, which came into full effect in May 2021, has introduced more stringent requirements for medical device manufacturers. Occlusive dressings with integrated sensors would likely be classified as Class IIa or IIb devices under the MDR, depending on their specific features and intended use.
The regulatory framework also addresses data privacy and security concerns, particularly relevant for sensor-integrated dressings that collect and transmit patient data. In the EU, the General Data Protection Regulation (GDPR) sets strict guidelines for the handling of personal health data. Similarly, in the US, the Health Insurance Portability and Accountability Act (HIPAA) governs the protection of sensitive patient health information.
Manufacturers must also comply with quality management standards, such as ISO 13485, which specifies requirements for a quality management system for medical devices. This standard ensures that devices consistently meet customer and regulatory requirements throughout their lifecycle.
As the field of medical wearables continues to advance, regulatory bodies are adapting their frameworks to keep pace with technological innovations. For instance, the FDA has introduced the Digital Health Software Precertification (Pre-Cert) Program, aimed at streamlining the regulatory process for software-based medical technologies, which may include certain types of sensor-integrated dressings.
Globally, there is a trend towards harmonization of regulatory standards, as evidenced by the Medical Device Single Audit Program (MDSAP). This program allows for a single regulatory audit of a medical device manufacturer's quality management system to satisfy the requirements of multiple regulatory jurisdictions.
Biocompatibility and Safety Considerations
The integration of sensors with occlusive dressings for wound monitoring presents significant biocompatibility and safety considerations that must be carefully addressed. The primary concern is ensuring that the materials used in both the dressing and the sensors are non-toxic and do not cause adverse reactions when in contact with the wound and surrounding skin.
Biocompatibility is crucial for wound healing, as any foreign material introduced to the wound site must not impede the natural healing process or cause inflammation. The materials used in the sensors and dressing should be thoroughly tested for cytotoxicity, sensitization, and irritation potential. This typically involves in vitro and in vivo studies to assess the interaction between the materials and living tissues.
The adhesives used to secure the dressing and sensors must also be carefully selected to minimize skin irritation while maintaining a secure seal. Hypoallergenic adhesives are often preferred to reduce the risk of allergic reactions in patients with sensitive skin. Additionally, the adhesive should allow for easy removal of the dressing without causing trauma to the healing wound or surrounding skin.
Safety considerations extend beyond biocompatibility to include the potential risks associated with the electronic components of the sensors. These components must be properly insulated to prevent electrical leakage that could harm the patient or interfere with the wound healing process. The sensors should also be designed to withstand the moist environment of a healing wound without compromising their functionality or safety.
Another important aspect is the prevention of bacterial colonization on the surface of the dressing and sensors. Antimicrobial coatings or materials may be incorporated to reduce the risk of infection, but these must be carefully evaluated to ensure they do not negatively impact wound healing or cause resistance in microbial populations.
The integration of sensors into occlusive dressings also raises concerns about the potential for increased moisture retention at the wound site. While maintaining a moist wound environment is generally beneficial for healing, excessive moisture can lead to maceration of the surrounding skin and potentially increase the risk of infection. The design of the integrated system must therefore carefully balance moisture management with the need for continuous monitoring.
Lastly, the disposal of used dressings with integrated sensors presents both environmental and biohazard concerns. The materials used should be selected with consideration for their environmental impact, and clear protocols must be established for the safe disposal of used dressings containing electronic components.
Biocompatibility is crucial for wound healing, as any foreign material introduced to the wound site must not impede the natural healing process or cause inflammation. The materials used in the sensors and dressing should be thoroughly tested for cytotoxicity, sensitization, and irritation potential. This typically involves in vitro and in vivo studies to assess the interaction between the materials and living tissues.
The adhesives used to secure the dressing and sensors must also be carefully selected to minimize skin irritation while maintaining a secure seal. Hypoallergenic adhesives are often preferred to reduce the risk of allergic reactions in patients with sensitive skin. Additionally, the adhesive should allow for easy removal of the dressing without causing trauma to the healing wound or surrounding skin.
Safety considerations extend beyond biocompatibility to include the potential risks associated with the electronic components of the sensors. These components must be properly insulated to prevent electrical leakage that could harm the patient or interfere with the wound healing process. The sensors should also be designed to withstand the moist environment of a healing wound without compromising their functionality or safety.
Another important aspect is the prevention of bacterial colonization on the surface of the dressing and sensors. Antimicrobial coatings or materials may be incorporated to reduce the risk of infection, but these must be carefully evaluated to ensure they do not negatively impact wound healing or cause resistance in microbial populations.
The integration of sensors into occlusive dressings also raises concerns about the potential for increased moisture retention at the wound site. While maintaining a moist wound environment is generally beneficial for healing, excessive moisture can lead to maceration of the surrounding skin and potentially increase the risk of infection. The design of the integrated system must therefore carefully balance moisture management with the need for continuous monitoring.
Lastly, the disposal of used dressings with integrated sensors presents both environmental and biohazard concerns. The materials used should be selected with consideration for their environmental impact, and clear protocols must be established for the safe disposal of used dressings containing electronic components.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!