How To Validate Controlled Release From Occlusive Dressing Patches
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Occlusive Dressing Patch Release Validation Goals
The primary goal of validating controlled release from occlusive dressing patches is to ensure the consistent and predictable delivery of therapeutic agents to the wound site over an extended period. This validation process aims to optimize the patch's performance, enhance patient outcomes, and meet regulatory requirements for medical devices.
One key objective is to establish a reliable method for measuring the release kinetics of active ingredients from the patch. This involves developing analytical techniques to quantify the amount of drug released at various time points, typically using methods such as high-performance liquid chromatography (HPLC) or spectrophotometry. The validation process should demonstrate the accuracy, precision, and reproducibility of these analytical methods.
Another critical goal is to determine the factors influencing the release rate and profile. This includes evaluating the impact of patch composition, such as the type and concentration of polymers used in the matrix, as well as the physicochemical properties of the active ingredients. Environmental factors like temperature and humidity should also be assessed to ensure consistent performance under various storage and application conditions.
Validating the uniformity of drug distribution within the patch is essential for ensuring consistent release across the entire surface area. This may involve techniques such as imaging or mapping to visualize the spatial distribution of active ingredients. Additionally, the validation process should assess the patch's adhesion properties and their potential impact on drug release, as poor adhesion could lead to inconsistent delivery.
The validation goals should also include evaluating the patch's stability over time, both in storage and during use. This involves accelerated aging studies to predict long-term performance and shelf life. The integrity of the occlusive barrier must be verified to ensure that it maintains its protective function while allowing controlled drug release.
Furthermore, the validation process should aim to establish in vitro-in vivo correlation (IVIVC) models. These models help predict the patch's performance in clinical settings based on laboratory data, reducing the need for extensive human trials and facilitating future product development.
Lastly, the validation goals should encompass the development of quality control measures and acceptance criteria for batch-to-batch consistency. This includes setting specifications for drug content, release rates, and physical properties of the patches to ensure reproducible manufacturing and consistent therapeutic efficacy.
One key objective is to establish a reliable method for measuring the release kinetics of active ingredients from the patch. This involves developing analytical techniques to quantify the amount of drug released at various time points, typically using methods such as high-performance liquid chromatography (HPLC) or spectrophotometry. The validation process should demonstrate the accuracy, precision, and reproducibility of these analytical methods.
Another critical goal is to determine the factors influencing the release rate and profile. This includes evaluating the impact of patch composition, such as the type and concentration of polymers used in the matrix, as well as the physicochemical properties of the active ingredients. Environmental factors like temperature and humidity should also be assessed to ensure consistent performance under various storage and application conditions.
Validating the uniformity of drug distribution within the patch is essential for ensuring consistent release across the entire surface area. This may involve techniques such as imaging or mapping to visualize the spatial distribution of active ingredients. Additionally, the validation process should assess the patch's adhesion properties and their potential impact on drug release, as poor adhesion could lead to inconsistent delivery.
The validation goals should also include evaluating the patch's stability over time, both in storage and during use. This involves accelerated aging studies to predict long-term performance and shelf life. The integrity of the occlusive barrier must be verified to ensure that it maintains its protective function while allowing controlled drug release.
Furthermore, the validation process should aim to establish in vitro-in vivo correlation (IVIVC) models. These models help predict the patch's performance in clinical settings based on laboratory data, reducing the need for extensive human trials and facilitating future product development.
Lastly, the validation goals should encompass the development of quality control measures and acceptance criteria for batch-to-batch consistency. This includes setting specifications for drug content, release rates, and physical properties of the patches to ensure reproducible manufacturing and consistent therapeutic efficacy.
Market Demand for Controlled Release Patches
The market demand for controlled release patches, particularly occlusive dressing patches, has been steadily growing due to their numerous advantages in wound care and drug delivery. These patches offer a controlled and sustained release of active ingredients, providing prolonged therapeutic effects and reducing the frequency of dressing changes. This technology has gained significant traction in both medical and consumer healthcare sectors.
In the medical field, controlled release patches are increasingly used for chronic wound management, post-surgical care, and treatment of various skin conditions. The aging population and rising incidence of chronic diseases have been key drivers for market growth. Healthcare professionals appreciate the ability of these patches to maintain a moist wound environment, promote healing, and reduce the risk of infections.
The pharmaceutical industry has shown keen interest in controlled release patches for transdermal drug delivery. This method offers advantages such as improved patient compliance, avoidance of first-pass metabolism, and the potential for continuous drug administration. The market has seen a surge in demand for patches delivering pain medications, hormones, and various other therapeutic agents.
Consumer healthcare is another sector driving demand for controlled release patches. Over-the-counter products for pain relief, smoking cessation, and skincare have gained popularity. Consumers value the convenience and ease of use offered by these patches, contributing to market expansion in the personal care segment.
The global wound care market, which includes controlled release patches, has been experiencing robust growth. Factors such as the increasing prevalence of chronic wounds, diabetic foot ulcers, and pressure ulcers have fueled this growth. Additionally, the rise in surgical procedures worldwide has boosted the demand for advanced wound care products, including occlusive dressing patches.
Emerging economies present significant growth opportunities for the controlled release patch market. As healthcare infrastructure improves and awareness of advanced wound care technologies increases, these regions are expected to contribute substantially to market expansion. However, challenges such as high production costs and regulatory hurdles in some countries may impact market penetration.
Innovation in materials science and drug delivery technologies continues to drive the evolution of controlled release patches. There is a growing demand for smart patches that can monitor wound healing progress or adjust drug release based on physiological parameters. This trend aligns with the broader movement towards personalized medicine and patient-centric healthcare solutions.
In the medical field, controlled release patches are increasingly used for chronic wound management, post-surgical care, and treatment of various skin conditions. The aging population and rising incidence of chronic diseases have been key drivers for market growth. Healthcare professionals appreciate the ability of these patches to maintain a moist wound environment, promote healing, and reduce the risk of infections.
The pharmaceutical industry has shown keen interest in controlled release patches for transdermal drug delivery. This method offers advantages such as improved patient compliance, avoidance of first-pass metabolism, and the potential for continuous drug administration. The market has seen a surge in demand for patches delivering pain medications, hormones, and various other therapeutic agents.
Consumer healthcare is another sector driving demand for controlled release patches. Over-the-counter products for pain relief, smoking cessation, and skincare have gained popularity. Consumers value the convenience and ease of use offered by these patches, contributing to market expansion in the personal care segment.
The global wound care market, which includes controlled release patches, has been experiencing robust growth. Factors such as the increasing prevalence of chronic wounds, diabetic foot ulcers, and pressure ulcers have fueled this growth. Additionally, the rise in surgical procedures worldwide has boosted the demand for advanced wound care products, including occlusive dressing patches.
Emerging economies present significant growth opportunities for the controlled release patch market. As healthcare infrastructure improves and awareness of advanced wound care technologies increases, these regions are expected to contribute substantially to market expansion. However, challenges such as high production costs and regulatory hurdles in some countries may impact market penetration.
Innovation in materials science and drug delivery technologies continues to drive the evolution of controlled release patches. There is a growing demand for smart patches that can monitor wound healing progress or adjust drug release based on physiological parameters. This trend aligns with the broader movement towards personalized medicine and patient-centric healthcare solutions.
Current Challenges in Patch Release Validation
Validating controlled release from occlusive dressing patches presents several significant challenges in the current landscape of medical device development and quality control. One of the primary difficulties lies in the complexity of the release mechanisms involved. Occlusive patches typically contain multiple layers and components, each contributing to the controlled release of active ingredients. This intricate structure makes it challenging to develop standardized validation protocols that can accurately assess the release kinetics across different patch designs and formulations.
Another major hurdle is the variability in physiological conditions that patches encounter when applied to human skin. Factors such as skin temperature, moisture levels, pH, and individual patient characteristics can significantly influence the release profile of active ingredients. Developing validation methods that can account for this biological variability while maintaining reproducibility and reliability is a considerable challenge for researchers and manufacturers.
The long-term nature of many occlusive patch applications further complicates the validation process. Some patches are designed for extended wear, lasting days or even weeks. Validating the controlled release over such prolonged periods requires sophisticated in vitro models or extensive clinical trials, both of which can be time-consuming and costly. Moreover, ensuring that laboratory tests accurately predict real-world performance over these extended timeframes remains a significant technical challenge.
Regulatory requirements add another layer of complexity to patch release validation. Different regulatory bodies may have varying standards and expectations for demonstrating controlled release, necessitating the development of validation protocols that can satisfy multiple regulatory frameworks. This often requires a delicate balance between scientific rigor and practical feasibility in validation methodologies.
The selection of appropriate analytical techniques for quantifying drug release also poses challenges. Traditional dissolution testing methods may not always be suitable for occlusive patches, given their unique delivery mechanisms and the potential for interference from patch components. Developing and validating alternative analytical methods that can accurately measure drug release from patches in a manner that correlates with in vivo performance is an ongoing area of research and development.
Furthermore, the increasing trend towards personalized medicine and the development of smart patches with programmable release profiles introduces new validation challenges. These advanced patches may incorporate sensors or responsive materials that alter release rates based on patient needs or environmental cues. Validating such dynamic release systems requires innovative approaches that can assess both the baseline release profile and the patch's ability to modulate release under various conditions.
Another major hurdle is the variability in physiological conditions that patches encounter when applied to human skin. Factors such as skin temperature, moisture levels, pH, and individual patient characteristics can significantly influence the release profile of active ingredients. Developing validation methods that can account for this biological variability while maintaining reproducibility and reliability is a considerable challenge for researchers and manufacturers.
The long-term nature of many occlusive patch applications further complicates the validation process. Some patches are designed for extended wear, lasting days or even weeks. Validating the controlled release over such prolonged periods requires sophisticated in vitro models or extensive clinical trials, both of which can be time-consuming and costly. Moreover, ensuring that laboratory tests accurately predict real-world performance over these extended timeframes remains a significant technical challenge.
Regulatory requirements add another layer of complexity to patch release validation. Different regulatory bodies may have varying standards and expectations for demonstrating controlled release, necessitating the development of validation protocols that can satisfy multiple regulatory frameworks. This often requires a delicate balance between scientific rigor and practical feasibility in validation methodologies.
The selection of appropriate analytical techniques for quantifying drug release also poses challenges. Traditional dissolution testing methods may not always be suitable for occlusive patches, given their unique delivery mechanisms and the potential for interference from patch components. Developing and validating alternative analytical methods that can accurately measure drug release from patches in a manner that correlates with in vivo performance is an ongoing area of research and development.
Furthermore, the increasing trend towards personalized medicine and the development of smart patches with programmable release profiles introduces new validation challenges. These advanced patches may incorporate sensors or responsive materials that alter release rates based on patient needs or environmental cues. Validating such dynamic release systems requires innovative approaches that can assess both the baseline release profile and the patch's ability to modulate release under various conditions.
Existing Validation Methods for Patch Release
01 Occlusive dressing patches with controlled release mechanisms
These patches are designed to create a barrier over the wound while simultaneously delivering controlled amounts of therapeutic agents. The occlusive nature helps maintain a moist wound environment, promoting healing, while the controlled release system ensures a steady supply of medication over time.- Occlusive dressing patches with controlled release mechanisms: These patches are designed to provide a barrier over wounds while simultaneously delivering controlled amounts of therapeutic agents. The occlusive nature helps maintain a moist wound environment, promoting healing, while the controlled release system ensures a steady supply of medication over time.
- Multi-layered patch designs for improved drug delivery: Advanced patch designs incorporate multiple layers, each serving a specific function. These may include an adhesive layer, a drug reservoir, a rate-controlling membrane, and a protective backing. This layered approach allows for better control over drug release rates and improved patch adherence to the skin.
- Smart materials and stimuli-responsive systems in patches: Innovative patches utilize smart materials or stimuli-responsive systems to modulate drug release. These may respond to external factors such as temperature, pH, or electrical stimuli, allowing for more precise control over when and how much medication is delivered to the patient.
- Biodegradable and biocompatible materials for patch construction: The use of biodegradable and biocompatible materials in patch construction enhances patient comfort and reduces environmental impact. These materials can be designed to break down over time, eliminating the need for patch removal and potentially improving drug absorption.
- Integration of nanotechnology for enhanced drug delivery: Nanotechnology is being incorporated into occlusive dressing patches to enhance drug delivery. Nanoparticles or nanostructured surfaces can improve drug permeation through the skin, allow for higher drug loading capacity, and provide more precise control over release kinetics.
02 Multi-layered patch designs for controlled drug delivery
These patches incorporate multiple layers, each serving a specific function such as drug reservoir, rate-controlling membrane, and adhesive layer. This design allows for precise control over the release rate of active ingredients, enhancing the efficacy of treatment.Expand Specific Solutions03 Smart occlusive patches with responsive release mechanisms
Advanced patches that can respond to external stimuli or changes in the wound environment to adjust the release of therapeutic agents. These may include pH-responsive, temperature-sensitive, or enzyme-activated release systems, allowing for more targeted and efficient drug delivery.Expand Specific Solutions04 Biodegradable and biocompatible occlusive patches
Patches made from materials that can be safely absorbed by the body over time, eliminating the need for removal and reducing the risk of further tissue damage. These patches can be designed to degrade at a rate that complements the wound healing process.Expand Specific Solutions05 Nanotech-enhanced occlusive patches for controlled release
Incorporation of nanotechnology in patch design, such as nanoparticles or nanofibers, to enhance drug loading capacity, control release kinetics, and improve overall patch performance. These advanced materials can provide more precise control over drug delivery and potentially improve wound healing outcomes.Expand Specific Solutions
Key Players in Occlusive Dressing Industry
The validation of controlled release from occlusive dressing patches is a critical aspect in the advanced wound care market, which is currently in a growth phase. The global market for advanced wound dressings is expanding, driven by an aging population and increasing prevalence of chronic wounds. Technologically, the field is evolving rapidly, with companies like LTS LOHMANN Therapie-Systeme AG and Coloplast A/S leading innovation in transdermal drug delivery systems. The technology's maturity varies, with established players like Hisamitsu Pharmaceutical Co., Inc. offering well-developed products, while newer entrants like Zynerba Pharmaceuticals, Inc. are exploring novel approaches. The competitive landscape is diverse, including pharmaceutical giants like Novo Nordisk A/S and specialized medical device companies such as B. Braun Melsungen AG, indicating a dynamic and multifaceted market.
Hisamitsu Pharmaceutical Co., Inc.
Technical Solution: Hisamitsu Pharmaceutical has developed a novel approach for validating controlled release from occlusive dressing patches. Their method involves using in vitro dissolution testing with specialized Franz diffusion cells[1]. This setup mimics the skin barrier and allows for precise measurement of drug release rates over time. They have also implemented advanced analytical techniques such as high-performance liquid chromatography (HPLC) to quantify the released drug concentrations accurately[2]. Additionally, Hisamitsu has developed a proprietary mathematical model that correlates in vitro release profiles with in vivo pharmacokinetic data, enabling more accurate predictions of drug absorption and efficacy[3].
Strengths: Highly accurate and reproducible method for measuring controlled release. Correlation with in vivo data improves predictive power. Weaknesses: Requires specialized equipment and expertise, potentially limiting widespread adoption.
KCI Licensing, Inc.
Technical Solution: KCI Licensing has pioneered a multi-faceted approach to validate controlled release from occlusive dressing patches. Their method combines advanced imaging techniques with real-time monitoring of wound environments. They utilize hyperspectral imaging to visualize drug distribution within the patch and surrounding tissue[4]. This is complemented by embedded microsensors in the dressing that continuously measure pH, temperature, and moisture levels, providing insights into factors affecting drug release[5]. KCI has also developed a proprietary software platform that integrates these data streams to create dynamic models of drug release kinetics, allowing for real-time adjustments to optimize therapeutic outcomes[6].
Strengths: Comprehensive approach that considers multiple factors affecting drug release. Real-time monitoring enables adaptive treatment strategies. Weaknesses: Complex system may be costly to implement and maintain in clinical settings.
Core Innovations in Release Testing Techniques
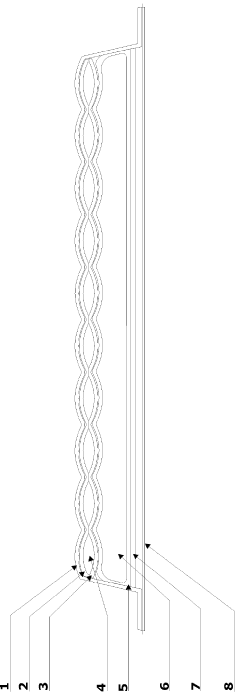
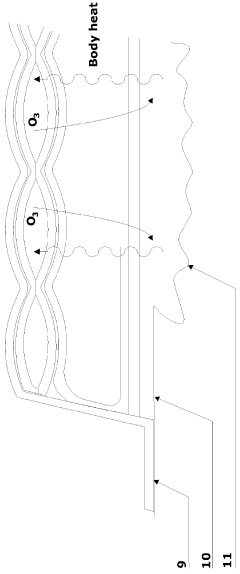
An ozone releasing wound dressing
PatentInactiveGB2508237A
Innovation
- A wound dressing with a multi-cellular sachet design that contains a non-aqueous solvent dissolving ozone, which is released at a controlled concentration through a gas-permeable but liquid-impermeable pad, utilizing body heat to maintain ozone levels within the wound site headspace, and includes a second pad for exudate absorption, ensuring effective ozone delivery and comfort.
Regulatory Requirements for Occlusive Patches
The regulatory landscape for occlusive dressing patches is complex and multifaceted, requiring manufacturers to navigate a series of stringent requirements to ensure product safety and efficacy. In the United States, the Food and Drug Administration (FDA) classifies most occlusive patches as Class II medical devices, subject to premarket notification 510(k) clearance. This process necessitates demonstrating substantial equivalence to a legally marketed predicate device.
Key regulatory considerations include biocompatibility testing, which is crucial to assess the potential for adverse reactions when the patch is in contact with skin. Manufacturers must conduct tests in accordance with ISO 10993 standards, evaluating cytotoxicity, sensitization, and irritation potential. Additionally, sterility assurance is paramount, with validation of sterilization processes required to meet ISO 11137 or equivalent standards.
Controlled release validation is a critical aspect of regulatory compliance for occlusive patches. Manufacturers must provide comprehensive data on the release kinetics of active ingredients, typically through in vitro dissolution studies. These studies should demonstrate consistent and predictable release profiles over the intended duration of use. The FDA may also require in vivo pharmacokinetic studies to correlate in vitro performance with actual drug absorption.
Stability testing is another crucial regulatory requirement. Manufacturers must provide data supporting the proposed shelf life of the product, including accelerated and real-time stability studies. These studies should evaluate the physical, chemical, and microbiological attributes of the patch over time, ensuring that the controlled release properties remain within specified limits throughout the product's shelf life.
Labeling and packaging regulations are equally important. Clear, accurate, and comprehensive labeling is essential, including information on proper application, duration of use, and potential side effects. The packaging must maintain the integrity of the patch and prevent contamination, with child-resistant packaging often required for patches containing active pharmaceutical ingredients.
Post-market surveillance is an ongoing regulatory obligation. Manufacturers must implement systems to monitor and report adverse events, conduct periodic safety reviews, and update product information as new data becomes available. This continuous vigilance ensures the long-term safety and efficacy of occlusive patches in real-world use.
Key regulatory considerations include biocompatibility testing, which is crucial to assess the potential for adverse reactions when the patch is in contact with skin. Manufacturers must conduct tests in accordance with ISO 10993 standards, evaluating cytotoxicity, sensitization, and irritation potential. Additionally, sterility assurance is paramount, with validation of sterilization processes required to meet ISO 11137 or equivalent standards.
Controlled release validation is a critical aspect of regulatory compliance for occlusive patches. Manufacturers must provide comprehensive data on the release kinetics of active ingredients, typically through in vitro dissolution studies. These studies should demonstrate consistent and predictable release profiles over the intended duration of use. The FDA may also require in vivo pharmacokinetic studies to correlate in vitro performance with actual drug absorption.
Stability testing is another crucial regulatory requirement. Manufacturers must provide data supporting the proposed shelf life of the product, including accelerated and real-time stability studies. These studies should evaluate the physical, chemical, and microbiological attributes of the patch over time, ensuring that the controlled release properties remain within specified limits throughout the product's shelf life.
Labeling and packaging regulations are equally important. Clear, accurate, and comprehensive labeling is essential, including information on proper application, duration of use, and potential side effects. The packaging must maintain the integrity of the patch and prevent contamination, with child-resistant packaging often required for patches containing active pharmaceutical ingredients.
Post-market surveillance is an ongoing regulatory obligation. Manufacturers must implement systems to monitor and report adverse events, conduct periodic safety reviews, and update product information as new data becomes available. This continuous vigilance ensures the long-term safety and efficacy of occlusive patches in real-world use.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when developing and validating controlled release from occlusive dressing patches. These factors directly impact patient well-being and regulatory approval, making them critical aspects of the validation process.
The biocompatibility of materials used in occlusive dressing patches must be thoroughly evaluated to ensure they do not cause adverse reactions when in contact with skin or wounds. This involves assessing potential cytotoxicity, sensitization, irritation, and systemic toxicity. In vitro and in vivo studies are typically conducted to evaluate these aspects, following established guidelines such as ISO 10993.
Material selection plays a crucial role in biocompatibility. Polymers commonly used in occlusive dressing patches, such as polyurethane, silicone, and hydrocolloids, should be carefully chosen based on their known biocompatibility profiles and intended use. Additionally, any additives, adhesives, or active ingredients incorporated into the patch must be evaluated for their individual and combined effects on biocompatibility.
Safety considerations extend beyond biocompatibility to include the controlled release mechanism itself. The rate and duration of drug release must be carefully validated to ensure therapeutic efficacy while avoiding potential toxicity from overexposure. This requires robust analytical methods to quantify drug release profiles under various conditions, including simulated wear time and environmental factors.
Stability testing is another critical aspect of safety validation. The patch and its active ingredients must maintain their integrity and efficacy throughout the intended shelf life and usage period. This involves accelerated aging studies and real-time stability testing to assess potential degradation or changes in release kinetics over time.
The occlusive nature of these patches introduces additional safety considerations. Moisture accumulation and potential microbial growth under the patch must be evaluated, particularly for extended-wear applications. Breathability and moisture vapor transmission rates should be optimized to balance occlusion with skin health.
Adhesive properties also factor into safety considerations. The patch must adhere securely to prevent premature detachment while allowing for atraumatic removal. Skin adhesion studies and peel tests are typically conducted to validate these properties and ensure minimal risk of skin damage or irritation during use and removal.
Regulatory requirements for biocompatibility and safety validation vary depending on the intended use and classification of the occlusive dressing patch. Developers must navigate these requirements, which may include specific testing protocols, documentation, and risk assessment procedures. Engaging with regulatory bodies early in the development process can help ensure that all necessary safety and biocompatibility data are collected efficiently.
The biocompatibility of materials used in occlusive dressing patches must be thoroughly evaluated to ensure they do not cause adverse reactions when in contact with skin or wounds. This involves assessing potential cytotoxicity, sensitization, irritation, and systemic toxicity. In vitro and in vivo studies are typically conducted to evaluate these aspects, following established guidelines such as ISO 10993.
Material selection plays a crucial role in biocompatibility. Polymers commonly used in occlusive dressing patches, such as polyurethane, silicone, and hydrocolloids, should be carefully chosen based on their known biocompatibility profiles and intended use. Additionally, any additives, adhesives, or active ingredients incorporated into the patch must be evaluated for their individual and combined effects on biocompatibility.
Safety considerations extend beyond biocompatibility to include the controlled release mechanism itself. The rate and duration of drug release must be carefully validated to ensure therapeutic efficacy while avoiding potential toxicity from overexposure. This requires robust analytical methods to quantify drug release profiles under various conditions, including simulated wear time and environmental factors.
Stability testing is another critical aspect of safety validation. The patch and its active ingredients must maintain their integrity and efficacy throughout the intended shelf life and usage period. This involves accelerated aging studies and real-time stability testing to assess potential degradation or changes in release kinetics over time.
The occlusive nature of these patches introduces additional safety considerations. Moisture accumulation and potential microbial growth under the patch must be evaluated, particularly for extended-wear applications. Breathability and moisture vapor transmission rates should be optimized to balance occlusion with skin health.
Adhesive properties also factor into safety considerations. The patch must adhere securely to prevent premature detachment while allowing for atraumatic removal. Skin adhesion studies and peel tests are typically conducted to validate these properties and ensure minimal risk of skin damage or irritation during use and removal.
Regulatory requirements for biocompatibility and safety validation vary depending on the intended use and classification of the occlusive dressing patch. Developers must navigate these requirements, which may include specific testing protocols, documentation, and risk assessment procedures. Engaging with regulatory bodies early in the development process can help ensure that all necessary safety and biocompatibility data are collected efficiently.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!