How to Develop Cost-Effective Magnesium Nitride Synthesis Methods?
AUG 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg3N2 Synthesis Background and Objectives
Magnesium nitride (Mg3N2) synthesis has gained significant attention in recent years due to its potential applications in various fields, including energy storage, catalysis, and advanced materials. The development of cost-effective synthesis methods for Mg3N2 is crucial for its widespread adoption and commercialization. This technical research report aims to provide a comprehensive overview of the background and objectives related to Mg3N2 synthesis, with a focus on cost-effective approaches.
The history of Mg3N2 synthesis dates back to the early 20th century when it was first prepared by direct nitridation of magnesium metal. However, traditional synthesis methods often require high temperatures and pressures, leading to energy-intensive and costly production processes. As a result, the development of more efficient and economical synthesis routes has become a primary focus for researchers and industry professionals.
In recent years, the growing demand for sustainable energy solutions and advanced materials has driven the need for innovative Mg3N2 synthesis techniques. The compound's unique properties, such as its high theoretical hydrogen storage capacity and potential as a precursor for other magnesium-based materials, have further fueled research efforts in this area.
The primary objective of developing cost-effective Mg3N2 synthesis methods is to overcome the limitations of conventional approaches. This includes reducing energy consumption, minimizing the use of expensive precursors, and simplifying the production process. By achieving these goals, researchers aim to make Mg3N2 more accessible for large-scale applications and enhance its economic viability.
Another crucial objective is to improve the purity and quality of the synthesized Mg3N2. Cost-effective methods should not compromise the material's performance or introduce impurities that could hinder its functionality in various applications. Achieving a balance between cost reduction and product quality is essential for the successful implementation of new synthesis techniques.
Furthermore, the development of scalable production methods is a key objective in the pursuit of cost-effective Mg3N2 synthesis. Laboratory-scale processes often face challenges when transitioning to industrial-scale production. Therefore, researchers are focusing on designing synthesis methods that can be easily scaled up without significant loss of efficiency or increase in costs.
Environmental considerations also play a crucial role in shaping the objectives of Mg3N2 synthesis research. Cost-effective methods should aim to minimize waste generation, reduce the use of hazardous chemicals, and lower overall environmental impact. This aligns with the growing emphasis on sustainable manufacturing practices and green chemistry principles in the chemical industry.
As the field of Mg3N2 synthesis continues to evolve, researchers are exploring various innovative approaches to achieve these objectives. These include the development of novel precursors, the use of alternative energy sources, and the application of advanced catalysts to enhance reaction kinetics and reduce energy requirements. By addressing these challenges and pursuing these objectives, the scientific community aims to unlock the full potential of Mg3N2 and pave the way for its widespread adoption in diverse technological applications.
The history of Mg3N2 synthesis dates back to the early 20th century when it was first prepared by direct nitridation of magnesium metal. However, traditional synthesis methods often require high temperatures and pressures, leading to energy-intensive and costly production processes. As a result, the development of more efficient and economical synthesis routes has become a primary focus for researchers and industry professionals.
In recent years, the growing demand for sustainable energy solutions and advanced materials has driven the need for innovative Mg3N2 synthesis techniques. The compound's unique properties, such as its high theoretical hydrogen storage capacity and potential as a precursor for other magnesium-based materials, have further fueled research efforts in this area.
The primary objective of developing cost-effective Mg3N2 synthesis methods is to overcome the limitations of conventional approaches. This includes reducing energy consumption, minimizing the use of expensive precursors, and simplifying the production process. By achieving these goals, researchers aim to make Mg3N2 more accessible for large-scale applications and enhance its economic viability.
Another crucial objective is to improve the purity and quality of the synthesized Mg3N2. Cost-effective methods should not compromise the material's performance or introduce impurities that could hinder its functionality in various applications. Achieving a balance between cost reduction and product quality is essential for the successful implementation of new synthesis techniques.
Furthermore, the development of scalable production methods is a key objective in the pursuit of cost-effective Mg3N2 synthesis. Laboratory-scale processes often face challenges when transitioning to industrial-scale production. Therefore, researchers are focusing on designing synthesis methods that can be easily scaled up without significant loss of efficiency or increase in costs.
Environmental considerations also play a crucial role in shaping the objectives of Mg3N2 synthesis research. Cost-effective methods should aim to minimize waste generation, reduce the use of hazardous chemicals, and lower overall environmental impact. This aligns with the growing emphasis on sustainable manufacturing practices and green chemistry principles in the chemical industry.
As the field of Mg3N2 synthesis continues to evolve, researchers are exploring various innovative approaches to achieve these objectives. These include the development of novel precursors, the use of alternative energy sources, and the application of advanced catalysts to enhance reaction kinetics and reduce energy requirements. By addressing these challenges and pursuing these objectives, the scientific community aims to unlock the full potential of Mg3N2 and pave the way for its widespread adoption in diverse technological applications.
Market Analysis for Mg3N2 Applications
The market for magnesium nitride (Mg3N2) applications is experiencing significant growth driven by its unique properties and versatile uses across various industries. Mg3N2 finds applications in advanced ceramics, semiconductor manufacturing, hydrogen storage, and as a precursor for other magnesium compounds. The global market for Mg3N2 is projected to expand steadily over the next decade, with a compound annual growth rate (CAGR) estimated in the mid-single digits.
In the advanced ceramics sector, Mg3N2 is utilized as a sintering aid and grain growth inhibitor, enhancing the mechanical properties and thermal stability of ceramic materials. This application is particularly relevant in the aerospace and automotive industries, where high-performance materials are crucial. The growing demand for lightweight and durable components in these sectors is expected to drive the consumption of Mg3N2.
The semiconductor industry represents another significant market for Mg3N2. It is used in the production of gallium nitride (GaN) semiconductors, which are gaining traction in power electronics, RF devices, and LED applications. As the demand for high-efficiency electronic devices continues to rise, the market for GaN semiconductors is expected to grow, consequently boosting the demand for Mg3N2 as a precursor material.
Hydrogen storage is an emerging application area for Mg3N2. Research has shown that magnesium nitride can potentially store hydrogen more efficiently than pure magnesium, making it a promising material for hydrogen-based energy systems. As the global focus on clean energy intensifies, this application could become a significant driver of Mg3N2 demand in the future.
The chemical industry also utilizes Mg3N2 as a precursor for synthesizing various magnesium compounds, including magnesium oxide and magnesium hydroxide. These compounds have wide-ranging applications in industries such as pharmaceuticals, environmental remediation, and flame retardants. The growth in these end-use industries is expected to contribute to the increasing demand for Mg3N2.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for Mg3N2 applications, driven by the rapid industrialization and technological advancements in countries like China, Japan, and South Korea. North America and Europe are also significant markets, particularly in high-tech industries and research applications.
However, the market faces challenges related to the high cost of Mg3N2 production, which is primarily due to the energy-intensive synthesis methods currently employed. This cost factor limits the widespread adoption of Mg3N2 in price-sensitive applications. Developing cost-effective synthesis methods is crucial for expanding the market potential of Mg3N2 and unlocking new application areas.
In the advanced ceramics sector, Mg3N2 is utilized as a sintering aid and grain growth inhibitor, enhancing the mechanical properties and thermal stability of ceramic materials. This application is particularly relevant in the aerospace and automotive industries, where high-performance materials are crucial. The growing demand for lightweight and durable components in these sectors is expected to drive the consumption of Mg3N2.
The semiconductor industry represents another significant market for Mg3N2. It is used in the production of gallium nitride (GaN) semiconductors, which are gaining traction in power electronics, RF devices, and LED applications. As the demand for high-efficiency electronic devices continues to rise, the market for GaN semiconductors is expected to grow, consequently boosting the demand for Mg3N2 as a precursor material.
Hydrogen storage is an emerging application area for Mg3N2. Research has shown that magnesium nitride can potentially store hydrogen more efficiently than pure magnesium, making it a promising material for hydrogen-based energy systems. As the global focus on clean energy intensifies, this application could become a significant driver of Mg3N2 demand in the future.
The chemical industry also utilizes Mg3N2 as a precursor for synthesizing various magnesium compounds, including magnesium oxide and magnesium hydroxide. These compounds have wide-ranging applications in industries such as pharmaceuticals, environmental remediation, and flame retardants. The growth in these end-use industries is expected to contribute to the increasing demand for Mg3N2.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for Mg3N2 applications, driven by the rapid industrialization and technological advancements in countries like China, Japan, and South Korea. North America and Europe are also significant markets, particularly in high-tech industries and research applications.
However, the market faces challenges related to the high cost of Mg3N2 production, which is primarily due to the energy-intensive synthesis methods currently employed. This cost factor limits the widespread adoption of Mg3N2 in price-sensitive applications. Developing cost-effective synthesis methods is crucial for expanding the market potential of Mg3N2 and unlocking new application areas.
Current Challenges in Mg3N2 Production
The production of magnesium nitride (Mg3N2) faces several significant challenges that hinder its widespread adoption and cost-effective synthesis. One of the primary obstacles is the high reactivity of magnesium with oxygen and moisture, which necessitates stringent reaction conditions. This reactivity often leads to the formation of undesired byproducts, such as magnesium oxide or hydroxide, reducing the overall yield and purity of the final product.
Another major challenge is the energy-intensive nature of current synthesis methods. Traditional approaches, including direct nitridation of magnesium metal with nitrogen gas, require high temperatures (typically above 600°C) and extended reaction times. These conditions not only increase production costs but also pose safety concerns due to the potential for rapid exothermic reactions.
The scalability of Mg3N2 production processes presents a significant hurdle for industrial applications. Many existing methods are limited to small-scale laboratory synthesis, making it difficult to meet the growing demand for magnesium nitride in various sectors. The transition from bench-scale to industrial-scale production often encounters issues related to heat transfer, reaction kinetics, and product uniformity.
Raw material costs and availability also contribute to the challenges in Mg3N2 production. High-purity magnesium metal, often used as a starting material, can be expensive and subject to market fluctuations. Additionally, the need for ultra-high purity nitrogen gas further adds to the production expenses, especially when considering large-scale manufacturing.
The handling and storage of Mg3N2 pose additional difficulties due to its moisture sensitivity. Exposure to air or humidity can lead to rapid degradation of the product, necessitating specialized packaging and storage solutions. This sensitivity not only complicates the production process but also increases the overall cost of handling and transportation.
Furthermore, the lack of standardized quality control methods for Mg3N2 production creates challenges in ensuring consistent product quality across different batches and manufacturers. The development of reliable analytical techniques for assessing purity, particle size distribution, and surface properties is crucial for advancing the field and meeting industry standards.
Environmental and safety concerns associated with Mg3N2 production also present challenges. The potential release of ammonia gas during synthesis or hydrolysis of the product requires careful consideration of emission control and worker safety measures. Addressing these issues is essential for developing sustainable and environmentally friendly production methods.
Another major challenge is the energy-intensive nature of current synthesis methods. Traditional approaches, including direct nitridation of magnesium metal with nitrogen gas, require high temperatures (typically above 600°C) and extended reaction times. These conditions not only increase production costs but also pose safety concerns due to the potential for rapid exothermic reactions.
The scalability of Mg3N2 production processes presents a significant hurdle for industrial applications. Many existing methods are limited to small-scale laboratory synthesis, making it difficult to meet the growing demand for magnesium nitride in various sectors. The transition from bench-scale to industrial-scale production often encounters issues related to heat transfer, reaction kinetics, and product uniformity.
Raw material costs and availability also contribute to the challenges in Mg3N2 production. High-purity magnesium metal, often used as a starting material, can be expensive and subject to market fluctuations. Additionally, the need for ultra-high purity nitrogen gas further adds to the production expenses, especially when considering large-scale manufacturing.
The handling and storage of Mg3N2 pose additional difficulties due to its moisture sensitivity. Exposure to air or humidity can lead to rapid degradation of the product, necessitating specialized packaging and storage solutions. This sensitivity not only complicates the production process but also increases the overall cost of handling and transportation.
Furthermore, the lack of standardized quality control methods for Mg3N2 production creates challenges in ensuring consistent product quality across different batches and manufacturers. The development of reliable analytical techniques for assessing purity, particle size distribution, and surface properties is crucial for advancing the field and meeting industry standards.
Environmental and safety concerns associated with Mg3N2 production also present challenges. The potential release of ammonia gas during synthesis or hydrolysis of the product requires careful consideration of emission control and worker safety measures. Addressing these issues is essential for developing sustainable and environmentally friendly production methods.
Existing Cost-Effective Synthesis Approaches
01 Cost-effective production methods
Various methods have been developed to produce magnesium nitride more cost-effectively. These include optimizing reaction conditions, using alternative precursors, and improving synthesis processes. Such advancements have led to reduced production costs and increased efficiency in magnesium nitride manufacturing.- Cost-effective production methods: Various methods have been developed to produce magnesium nitride more cost-effectively. These include optimizing reaction conditions, using alternative precursors, and improving synthesis processes. Such advancements help reduce production costs and increase the economic viability of magnesium nitride for various applications.
- Energy-efficient synthesis techniques: Research has focused on developing energy-efficient synthesis techniques for magnesium nitride. These methods aim to reduce energy consumption during production, thereby lowering overall costs. Techniques such as low-temperature synthesis and microwave-assisted processes have shown promise in improving the cost-effectiveness of magnesium nitride production.
- Applications in renewable energy: Magnesium nitride has found applications in renewable energy technologies, particularly in hydrogen storage and production. Its use in these areas can potentially offset production costs through improved energy efficiency and sustainability. The cost-effectiveness of magnesium nitride in renewable energy applications is an active area of research and development.
- Recycling and resource recovery: To improve cost-effectiveness, methods for recycling and recovering magnesium nitride from waste streams have been developed. These processes aim to reduce raw material costs and minimize environmental impact. Efficient recycling techniques contribute to the overall economic viability of magnesium nitride use in various industries.
- Economic analysis and market strategies: Economic analyses and market strategies have been conducted to assess and improve the cost-effectiveness of magnesium nitride. These studies consider factors such as production costs, market demand, and potential applications to optimize the economic value of magnesium nitride. Such analyses help in developing strategies for more competitive pricing and market positioning.
02 Applications in semiconductor industry
Magnesium nitride has found cost-effective applications in the semiconductor industry. It is used in the production of various electronic components and devices, offering improved performance and reduced manufacturing costs compared to alternative materials.Expand Specific Solutions03 Energy storage and conversion
Magnesium nitride has shown promise in energy storage and conversion applications. Its use in batteries and fuel cells has been explored, potentially offering a cost-effective alternative to existing materials. Research in this area aims to improve energy efficiency and reduce overall costs in energy-related technologies.Expand Specific Solutions04 Agricultural and fertilizer applications
The use of magnesium nitride in agricultural applications, particularly as a component in fertilizers, has been investigated. Its potential to provide both magnesium and nitrogen to plants in a cost-effective manner could lead to improved crop yields and reduced fertilizer costs for farmers.Expand Specific Solutions05 Cost analysis and economic evaluation
Various studies and methods have been developed to analyze the cost-effectiveness of magnesium nitride production and applications. These include economic evaluations, market analyses, and comparison with alternative materials to determine the most cost-effective solutions for different industries and applications.Expand Specific Solutions
Key Players in Mg3N2 Manufacturing
The development of cost-effective magnesium nitride synthesis methods is in an emerging phase, with growing market potential due to increasing applications in electronics and energy storage. The global market size is expanding, driven by demand in advanced materials sectors. Technologically, the field is progressing but still maturing, with various approaches being explored. Companies like Taiheiyo Cement Corp., SE Corp., and China Petroleum & Chemical Corp. are actively involved in research and development, while academic institutions such as Shanghai Jiao Tong University and Wuhan University of Science & Technology are contributing to fundamental advancements. The competitive landscape is diverse, with both established chemical companies and specialized materials firms vying for breakthroughs in synthesis efficiency and scalability.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute has developed a novel cost-effective method for magnesium nitride synthesis using a mechanochemical approach. This process involves ball milling magnesium powder with nitrogen gas at room temperature, eliminating the need for high-temperature reactions[1]. The method utilizes a specially designed planetary ball mill with a gas-tight milling vial, allowing for continuous nitrogen flow during the milling process[2]. The reaction is initiated by the mechanical energy input, creating fresh magnesium surfaces that readily react with nitrogen. The process can be optimized by controlling milling parameters such as ball-to-powder ratio, milling speed, and duration[3]. This method has shown to produce high-purity magnesium nitride with a yield of over 95% in less than 4 hours of milling time[4].
Strengths: Low energy consumption, room temperature operation, high yield, and purity. Weaknesses: Potential for contamination from milling media, scalability challenges for large-scale production.
The Regents of the University of California
Technical Solution: Researchers at the University of California have developed a plasma-assisted synthesis method for magnesium nitride. This approach uses a microwave-induced nitrogen plasma to react with magnesium vapor at relatively low temperatures (600-800°C)[5]. The process involves generating magnesium vapor through resistive heating of a magnesium source, which is then exposed to the nitrogen plasma. The highly reactive nitrogen species in the plasma facilitate rapid nitridation of magnesium, resulting in the formation of magnesium nitride[6]. The method allows for precise control over the reaction parameters, including plasma power, substrate temperature, and magnesium vapor pressure. This technique has demonstrated the ability to produce high-quality magnesium nitride thin films and nanostructures with controlled morphology and composition[7].
Strengths: Lower reaction temperatures compared to conventional methods, ability to produce nanostructured materials, high purity. Weaknesses: Requires specialized plasma equipment, potential challenges in scaling up for bulk production.
Innovative Mg3N2 Production Techniques
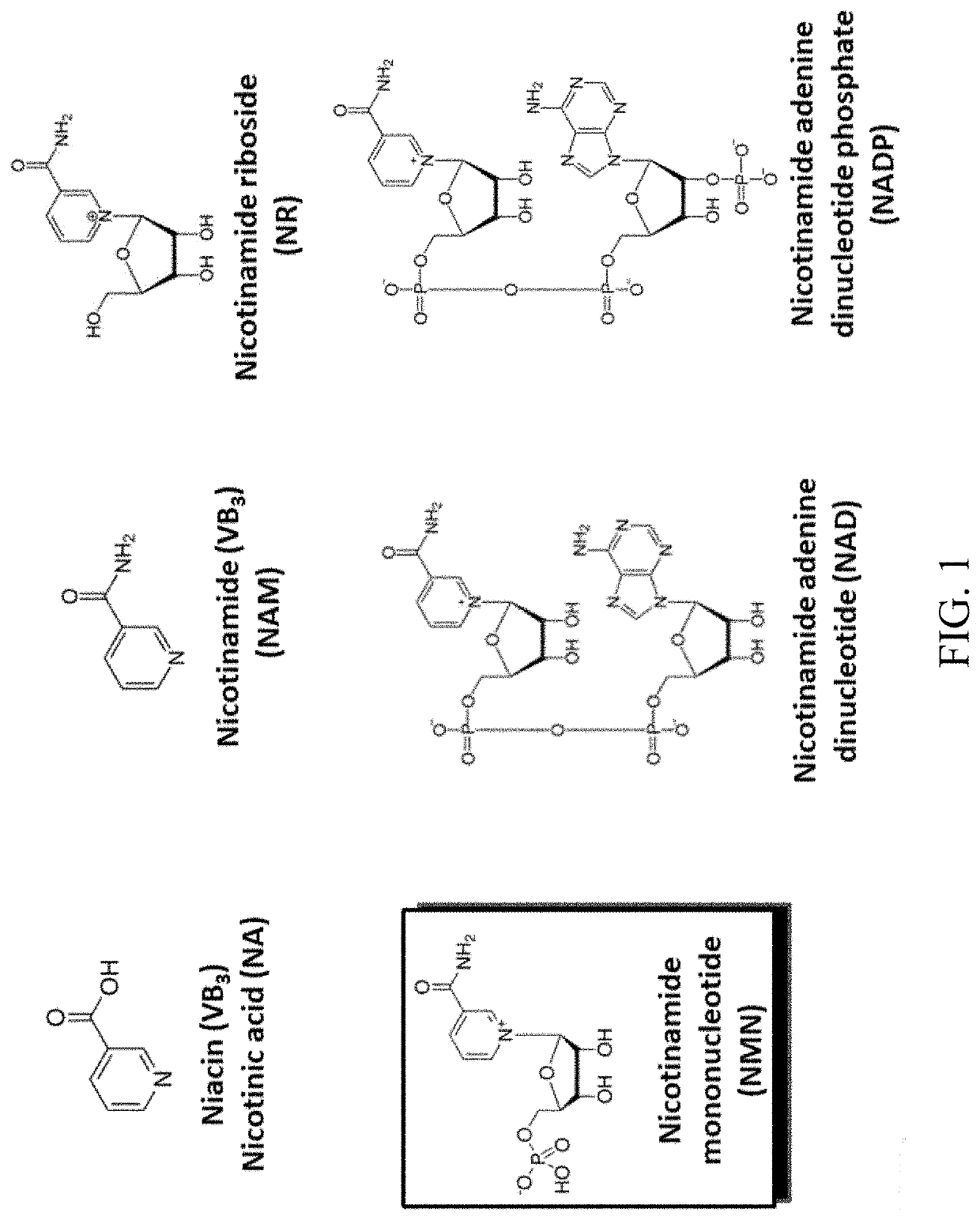
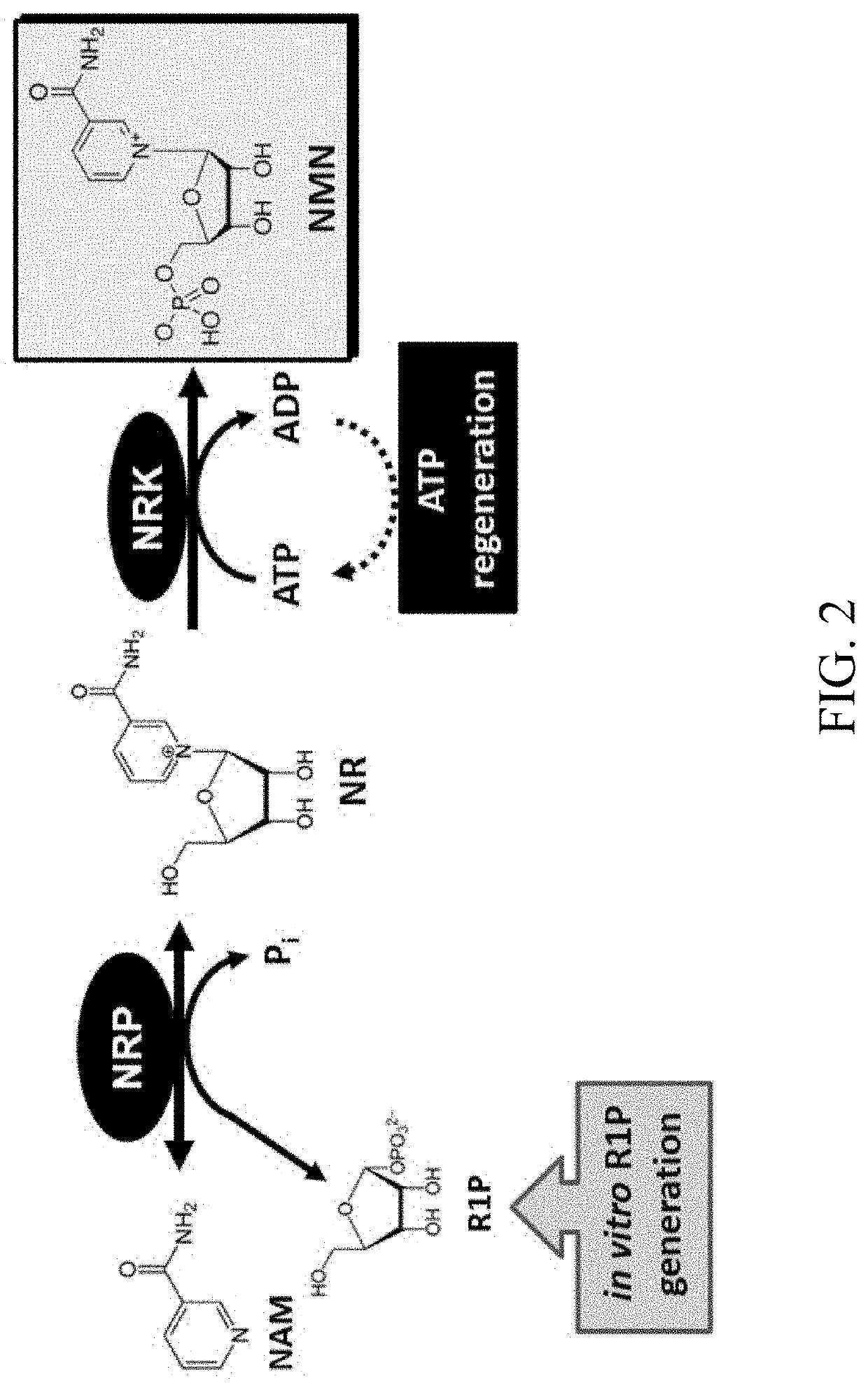
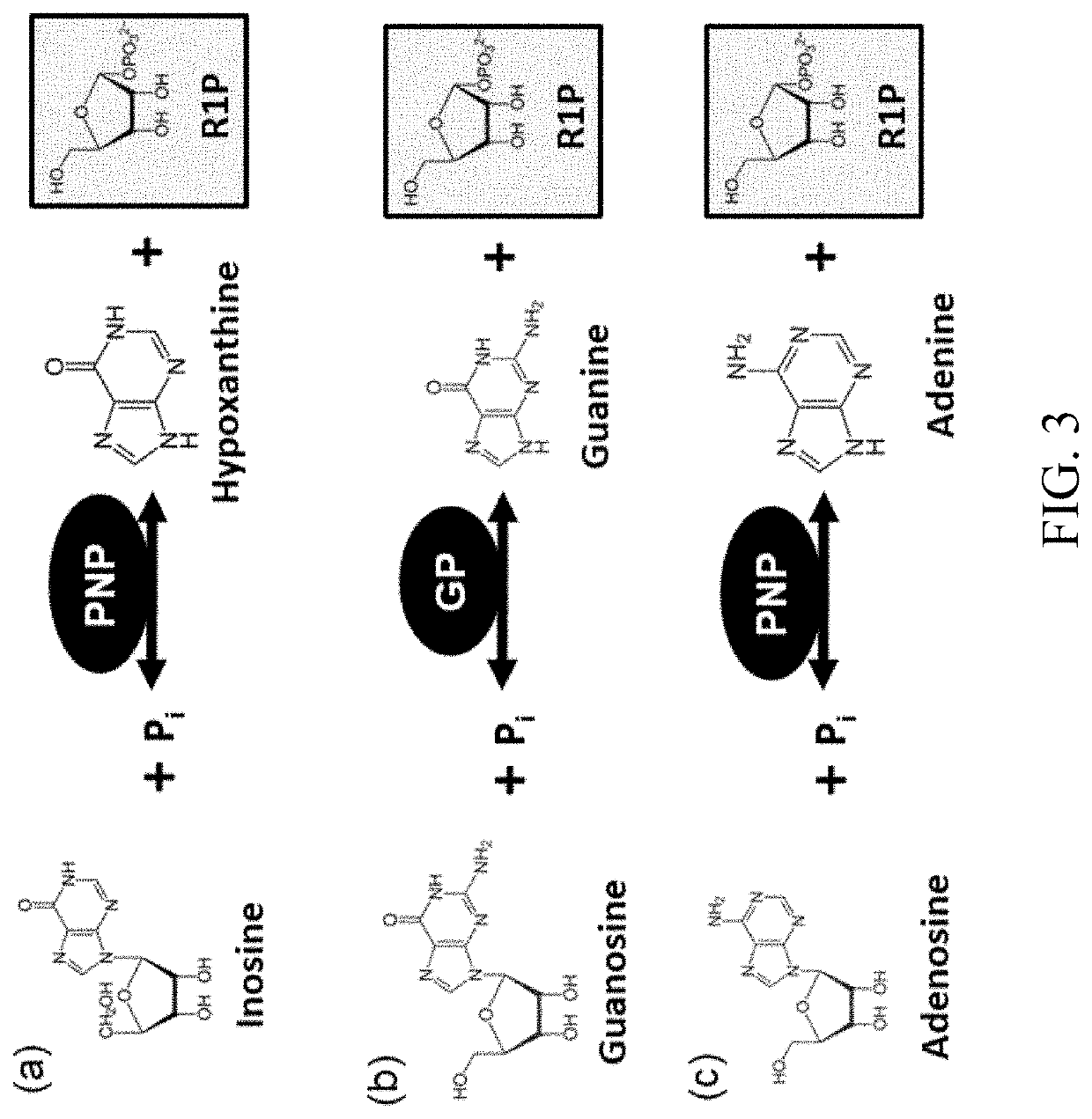
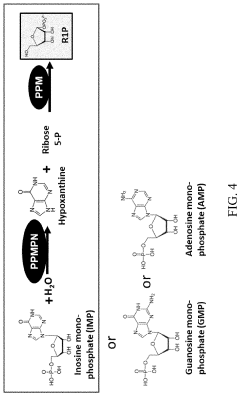
Biosynthesis of preparing nicotinamide mononucleotide and derivatives thereof
PatentInactiveUS20210246476A1
Innovation
- The use of low-cost starting materials, isolated enzymes, and microorganisms to create integrated artificial enzymatic pathways that regenerate ATP and produce NMN from nicotinamide (NAM) and D-ribose-1-phosphate through multiple enzyme reactions, optimizing reaction conditions to achieve high yields.
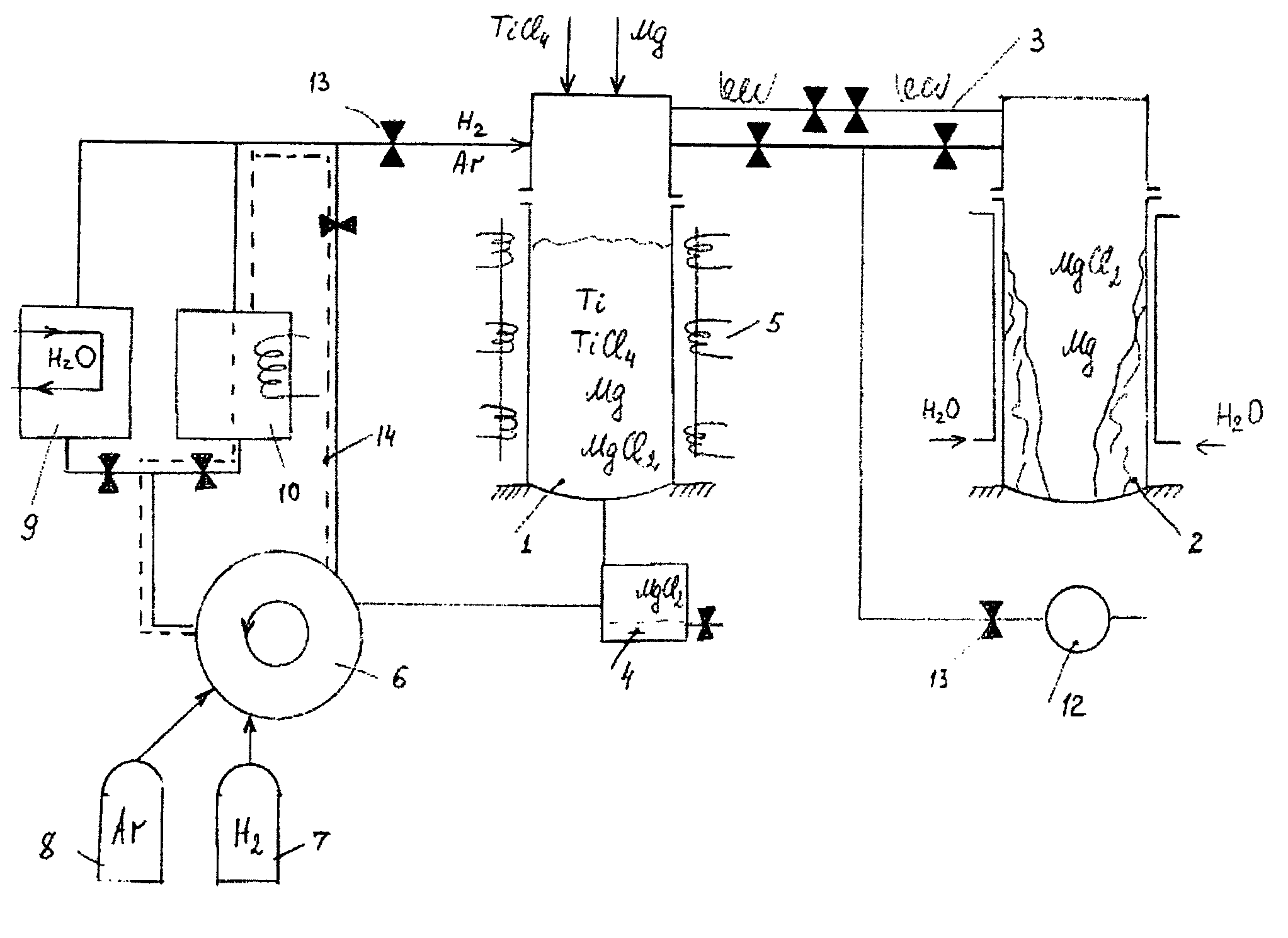
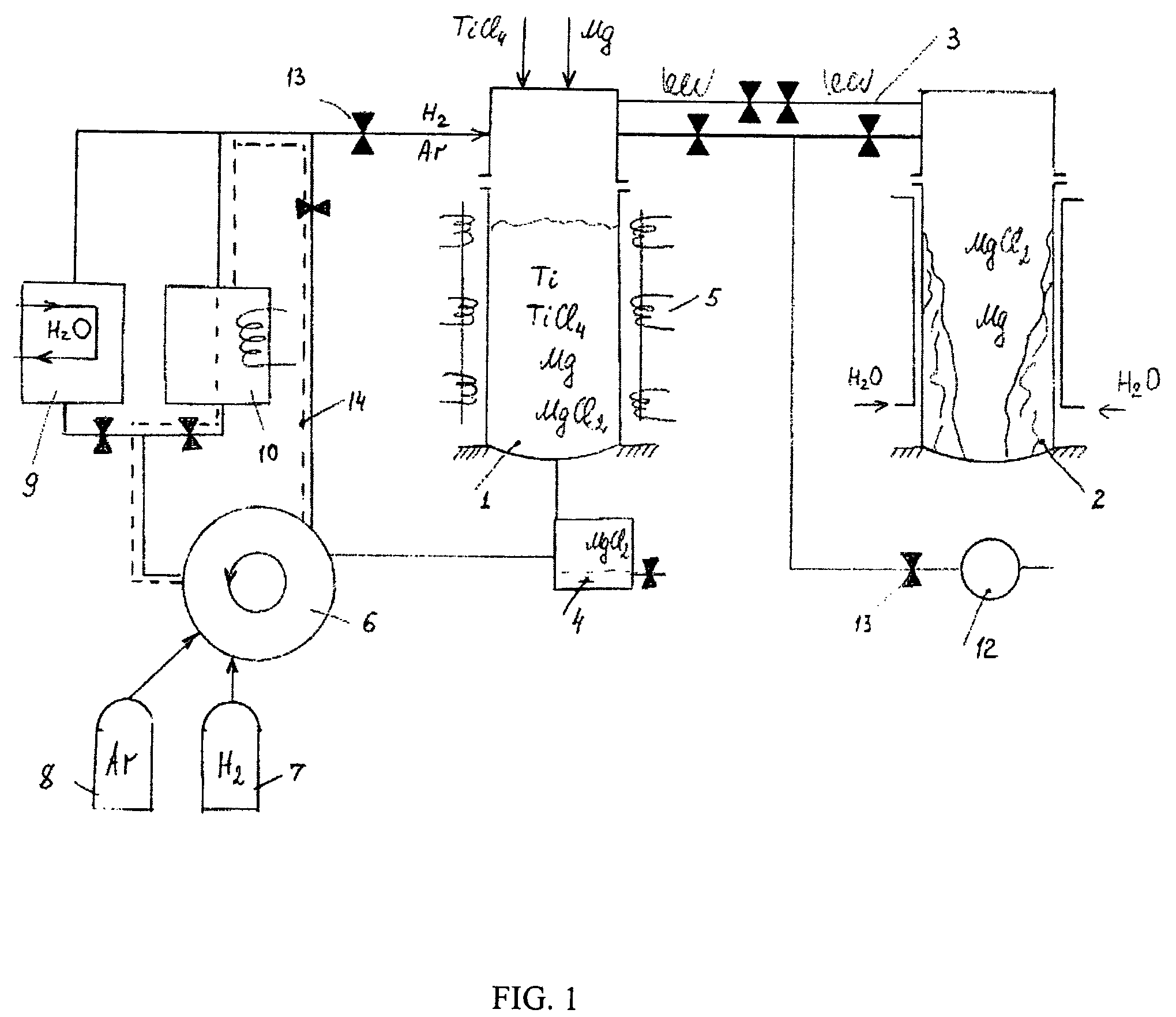
Semi-continuous magnesium-hydrogen reduction process for manufacturing of hydrogenated, purified titanium powder
PatentInactiveUS8007562B2
Innovation
- A semi-continuous process involving magnesium-thermic reduction of titanium chlorides at 830-880°C in a hydrogen atmosphere, followed by full thermal-vacuum separation and hydrogenation, which allows for the removal of magnesium and magnesium chloride within the same production cycle, resulting in a purified hydrogenated titanium powder with reduced impurity levels and increased productivity.
Economic Feasibility Assessment
The economic feasibility of developing cost-effective magnesium nitride synthesis methods is a critical factor in determining the viability of large-scale production and application. Current synthesis methods often involve high energy consumption and expensive precursors, making magnesium nitride production costly. To assess the economic feasibility, it is essential to consider various factors that influence the overall cost structure.
Raw material costs play a significant role in the economic assessment. Magnesium metal, the primary precursor, is relatively abundant but requires energy-intensive extraction processes. Exploring alternative magnesium sources, such as magnesium-rich minerals or recycled materials, could potentially reduce raw material costs. Additionally, the nitrogen source, typically high-purity nitrogen gas, contributes to the overall expenses. Investigating more cost-effective nitrogen sources or optimizing nitrogen utilization efficiency could lead to substantial cost reductions.
Energy consumption is another crucial aspect of the economic feasibility analysis. Traditional synthesis methods often require high temperatures and pressures, resulting in significant energy expenditures. Developing low-temperature synthesis routes or utilizing renewable energy sources could greatly improve the economic viability of magnesium nitride production. Furthermore, exploring catalytic processes that lower activation energies and reaction times may lead to reduced energy requirements and improved cost-effectiveness.
Equipment and infrastructure costs must also be considered in the economic assessment. The need for specialized reactors, high-pressure vessels, or precise temperature control systems can significantly impact the initial investment and ongoing operational expenses. Designing scalable and modular production systems that can be easily integrated into existing manufacturing facilities could help minimize infrastructure costs and improve overall economic feasibility.
Process efficiency and yield are critical factors affecting the economic viability of magnesium nitride synthesis. Developing methods that maximize conversion rates and minimize by-product formation can significantly reduce production costs. Additionally, implementing efficient separation and purification techniques to recover unreacted precursors and valuable by-products could further enhance the economic feasibility of the process.
Market demand and potential applications play a crucial role in determining the economic viability of magnesium nitride production. As an emerging material with promising properties, magnesium nitride has potential applications in various fields, including energy storage, catalysis, and electronics. Assessing the market size, growth potential, and competitive landscape for these applications is essential in evaluating the long-term economic feasibility of developing cost-effective synthesis methods.
Raw material costs play a significant role in the economic assessment. Magnesium metal, the primary precursor, is relatively abundant but requires energy-intensive extraction processes. Exploring alternative magnesium sources, such as magnesium-rich minerals or recycled materials, could potentially reduce raw material costs. Additionally, the nitrogen source, typically high-purity nitrogen gas, contributes to the overall expenses. Investigating more cost-effective nitrogen sources or optimizing nitrogen utilization efficiency could lead to substantial cost reductions.
Energy consumption is another crucial aspect of the economic feasibility analysis. Traditional synthesis methods often require high temperatures and pressures, resulting in significant energy expenditures. Developing low-temperature synthesis routes or utilizing renewable energy sources could greatly improve the economic viability of magnesium nitride production. Furthermore, exploring catalytic processes that lower activation energies and reaction times may lead to reduced energy requirements and improved cost-effectiveness.
Equipment and infrastructure costs must also be considered in the economic assessment. The need for specialized reactors, high-pressure vessels, or precise temperature control systems can significantly impact the initial investment and ongoing operational expenses. Designing scalable and modular production systems that can be easily integrated into existing manufacturing facilities could help minimize infrastructure costs and improve overall economic feasibility.
Process efficiency and yield are critical factors affecting the economic viability of magnesium nitride synthesis. Developing methods that maximize conversion rates and minimize by-product formation can significantly reduce production costs. Additionally, implementing efficient separation and purification techniques to recover unreacted precursors and valuable by-products could further enhance the economic feasibility of the process.
Market demand and potential applications play a crucial role in determining the economic viability of magnesium nitride production. As an emerging material with promising properties, magnesium nitride has potential applications in various fields, including energy storage, catalysis, and electronics. Assessing the market size, growth potential, and competitive landscape for these applications is essential in evaluating the long-term economic feasibility of developing cost-effective synthesis methods.
Environmental Impact of Mg3N2 Production
The environmental impact of Mg3N2 production is a critical consideration in developing cost-effective magnesium nitride synthesis methods. The manufacturing process of Mg3N2 involves several stages that can potentially affect the environment, necessitating a comprehensive assessment of its ecological footprint.
One of the primary environmental concerns in Mg3N2 production is energy consumption. The synthesis of magnesium nitride typically requires high temperatures, often exceeding 700°C, which demands significant energy input. This energy-intensive process contributes to greenhouse gas emissions, particularly if the energy source is not renewable. Efforts to optimize energy efficiency in the production process are crucial for minimizing the carbon footprint associated with Mg3N2 synthesis.
Raw material extraction and processing also play a significant role in the environmental impact of Mg3N2 production. Magnesium, the primary component, is often obtained through mining operations or seawater extraction, both of which can lead to habitat disruption and potential soil and water contamination. The production of nitrogen gas, another key ingredient, typically involves the energy-intensive process of air separation, further contributing to the overall environmental burden.
Waste management is another critical aspect of Mg3N2 production. The synthesis process may generate by-products and unreacted materials that require proper disposal or recycling. Improper handling of these waste products can lead to soil and water pollution, potentially affecting local ecosystems and human health. Implementing efficient recycling systems and waste reduction strategies is essential for mitigating these environmental risks.
Air emissions from Mg3N2 production facilities are also a concern. The high-temperature reactions involved in the synthesis process can release particulate matter and potentially harmful gases into the atmosphere. These emissions may contribute to local air quality issues and have broader implications for atmospheric pollution. Installing and maintaining effective air filtration and scrubbing systems is crucial for minimizing these impacts.
Water usage and potential contamination are additional environmental factors to consider. The production process may require significant amounts of water for cooling and cleaning purposes. Ensuring proper water treatment and recycling systems are in place is essential for conserving water resources and preventing the release of contaminated effluents into local water bodies.
The transportation of raw materials and finished products also contributes to the environmental footprint of Mg3N2 production. Long-distance shipping and transportation can result in increased fuel consumption and associated emissions. Optimizing supply chains and considering local sourcing options where possible can help reduce these transportation-related impacts.
In conclusion, developing cost-effective magnesium nitride synthesis methods must take into account the multifaceted environmental impacts of Mg3N2 production. Addressing these challenges requires a holistic approach that considers energy efficiency, raw material sourcing, waste management, emission control, water conservation, and transportation optimization. By implementing sustainable practices and innovative technologies throughout the production process, it is possible to minimize the environmental footprint of Mg3N2 synthesis while maintaining cost-effectiveness.
One of the primary environmental concerns in Mg3N2 production is energy consumption. The synthesis of magnesium nitride typically requires high temperatures, often exceeding 700°C, which demands significant energy input. This energy-intensive process contributes to greenhouse gas emissions, particularly if the energy source is not renewable. Efforts to optimize energy efficiency in the production process are crucial for minimizing the carbon footprint associated with Mg3N2 synthesis.
Raw material extraction and processing also play a significant role in the environmental impact of Mg3N2 production. Magnesium, the primary component, is often obtained through mining operations or seawater extraction, both of which can lead to habitat disruption and potential soil and water contamination. The production of nitrogen gas, another key ingredient, typically involves the energy-intensive process of air separation, further contributing to the overall environmental burden.
Waste management is another critical aspect of Mg3N2 production. The synthesis process may generate by-products and unreacted materials that require proper disposal or recycling. Improper handling of these waste products can lead to soil and water pollution, potentially affecting local ecosystems and human health. Implementing efficient recycling systems and waste reduction strategies is essential for mitigating these environmental risks.
Air emissions from Mg3N2 production facilities are also a concern. The high-temperature reactions involved in the synthesis process can release particulate matter and potentially harmful gases into the atmosphere. These emissions may contribute to local air quality issues and have broader implications for atmospheric pollution. Installing and maintaining effective air filtration and scrubbing systems is crucial for minimizing these impacts.
Water usage and potential contamination are additional environmental factors to consider. The production process may require significant amounts of water for cooling and cleaning purposes. Ensuring proper water treatment and recycling systems are in place is essential for conserving water resources and preventing the release of contaminated effluents into local water bodies.
The transportation of raw materials and finished products also contributes to the environmental footprint of Mg3N2 production. Long-distance shipping and transportation can result in increased fuel consumption and associated emissions. Optimizing supply chains and considering local sourcing options where possible can help reduce these transportation-related impacts.
In conclusion, developing cost-effective magnesium nitride synthesis methods must take into account the multifaceted environmental impacts of Mg3N2 production. Addressing these challenges requires a holistic approach that considers energy efficiency, raw material sourcing, waste management, emission control, water conservation, and transportation optimization. By implementing sustainable practices and innovative technologies throughout the production process, it is possible to minimize the environmental footprint of Mg3N2 synthesis while maintaining cost-effectiveness.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!