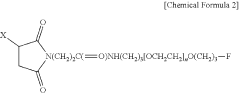How to Enhance Glycogenolysis Under Hypoglycemic Events
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Enhancement Background and Objectives
Glycogenolysis, the breakdown of glycogen into glucose, represents a critical physiological process in maintaining blood glucose homeostasis during periods of hypoglycemia. This metabolic pathway has evolved as an essential survival mechanism, allowing organisms to access stored energy rapidly when blood glucose levels fall below normal thresholds. The historical understanding of glycogenolysis dates back to the early 20th century, with significant advancements in comprehension occurring through the 1950s and 1960s as researchers elucidated the enzymatic cascades involved.
Recent technological developments have enabled more precise monitoring and manipulation of glycogenolysis pathways, creating opportunities for therapeutic interventions in conditions characterized by dysregulated glucose metabolism. The evolution of this field has progressed from basic understanding of the biochemical processes to sophisticated molecular targeting approaches that can potentially enhance glycogenolytic responses during hypoglycemic events.
The primary objective of enhancing glycogenolysis under hypoglycemic conditions is to develop interventions that can rapidly restore normoglycemia in vulnerable populations, particularly individuals with diabetes who experience frequent hypoglycemic episodes. Secondary objectives include minimizing the counter-regulatory hormone response that often leads to subsequent hyperglycemia, and developing technologies that can predict and prevent dangerous hypoglycemic events before they occur.
Current trends in glycogenolysis research focus on several promising directions. These include the development of small molecule activators of glycogen phosphorylase, the rate-limiting enzyme in glycogenolysis; gene therapy approaches to optimize hepatic and muscle glycogen metabolism; and bioelectronic medicine applications that could potentially modulate glycogenolysis through targeted neural stimulation.
The technological landscape is increasingly incorporating artificial intelligence and machine learning to predict hypoglycemic events and trigger appropriate interventions. Additionally, advances in drug delivery systems are creating new possibilities for targeted enhancement of glycogenolysis in specific tissues, potentially reducing systemic side effects associated with broader metabolic manipulation.
The ultimate goal of this technological pursuit is to develop safe, effective, and patient-friendly solutions that can mitigate the risks associated with hypoglycemia, particularly in vulnerable populations such as individuals with type 1 diabetes, the elderly, and those with impaired counter-regulatory responses. Success in this domain could significantly reduce morbidity and mortality associated with severe hypoglycemic events while improving quality of life for millions of affected individuals worldwide.
Recent technological developments have enabled more precise monitoring and manipulation of glycogenolysis pathways, creating opportunities for therapeutic interventions in conditions characterized by dysregulated glucose metabolism. The evolution of this field has progressed from basic understanding of the biochemical processes to sophisticated molecular targeting approaches that can potentially enhance glycogenolytic responses during hypoglycemic events.
The primary objective of enhancing glycogenolysis under hypoglycemic conditions is to develop interventions that can rapidly restore normoglycemia in vulnerable populations, particularly individuals with diabetes who experience frequent hypoglycemic episodes. Secondary objectives include minimizing the counter-regulatory hormone response that often leads to subsequent hyperglycemia, and developing technologies that can predict and prevent dangerous hypoglycemic events before they occur.
Current trends in glycogenolysis research focus on several promising directions. These include the development of small molecule activators of glycogen phosphorylase, the rate-limiting enzyme in glycogenolysis; gene therapy approaches to optimize hepatic and muscle glycogen metabolism; and bioelectronic medicine applications that could potentially modulate glycogenolysis through targeted neural stimulation.
The technological landscape is increasingly incorporating artificial intelligence and machine learning to predict hypoglycemic events and trigger appropriate interventions. Additionally, advances in drug delivery systems are creating new possibilities for targeted enhancement of glycogenolysis in specific tissues, potentially reducing systemic side effects associated with broader metabolic manipulation.
The ultimate goal of this technological pursuit is to develop safe, effective, and patient-friendly solutions that can mitigate the risks associated with hypoglycemia, particularly in vulnerable populations such as individuals with type 1 diabetes, the elderly, and those with impaired counter-regulatory responses. Success in this domain could significantly reduce morbidity and mortality associated with severe hypoglycemic events while improving quality of life for millions of affected individuals worldwide.
Clinical Demand Analysis for Hypoglycemia Management
Hypoglycemia represents a significant clinical challenge affecting approximately 25% of patients with diabetes, with severe episodes requiring emergency intervention in 1-3% of cases annually. The management of hypoglycemic events remains a critical unmet need in diabetes care, particularly for patients on insulin therapy or sulfonylureas. Current treatment approaches often rely on exogenous glucose administration, which provides temporary relief but fails to address the underlying physiological mechanisms.
The clinical demand for improved hypoglycemia management stems from several factors. First, hypoglycemia is associated with substantial morbidity and mortality, with severe episodes linked to cardiovascular events, cognitive impairment, and increased risk of falls and fractures, especially in elderly patients. Studies indicate that hypoglycemia increases mortality risk by 2-4 times compared to patients without such events.
Healthcare systems face significant economic burden from hypoglycemia, with direct costs estimated at $13,000 per severe hypoglycemic episode requiring hospitalization. Indirect costs, including lost productivity and caregiver burden, further compound this economic impact. A comprehensive analysis across multiple healthcare systems reveals that hypoglycemia-related hospitalizations account for 2-5% of diabetes-related healthcare expenditures.
From a patient perspective, fear of hypoglycemia significantly impacts quality of life and often leads to intentional hyperglycemia as a preventive measure. This defensive behavior results in suboptimal glycemic control and increased risk of long-term complications. Patient surveys indicate that over 50% of insulin users report taking less insulin than prescribed due to hypoglycemia concerns.
Clinicians express frustration with the limitations of current hypoglycemia management options, particularly for patients with hypoglycemia unawareness or those experiencing nocturnal events. The need for solutions that can rapidly restore normoglycemia while simultaneously addressing the underlying physiological deficits in counter-regulatory responses is consistently highlighted in clinical practice guidelines.
Recent advances in continuous glucose monitoring technology have improved hypoglycemia detection but have not significantly advanced treatment options. The gap between improved detection capabilities and effective intervention strategies underscores the urgent need for novel approaches that can enhance endogenous glucose production through targeted glycogenolysis during hypoglycemic events.
Market research indicates growing demand for solutions that can provide rapid, physiological correction of hypoglycemia without causing subsequent hyperglycemia. This demand spans multiple patient segments, including those with type 1 diabetes, insulin-dependent type 2 diabetes, and individuals with post-bariatric hypoglycemia or insulinomas.
The clinical demand for improved hypoglycemia management stems from several factors. First, hypoglycemia is associated with substantial morbidity and mortality, with severe episodes linked to cardiovascular events, cognitive impairment, and increased risk of falls and fractures, especially in elderly patients. Studies indicate that hypoglycemia increases mortality risk by 2-4 times compared to patients without such events.
Healthcare systems face significant economic burden from hypoglycemia, with direct costs estimated at $13,000 per severe hypoglycemic episode requiring hospitalization. Indirect costs, including lost productivity and caregiver burden, further compound this economic impact. A comprehensive analysis across multiple healthcare systems reveals that hypoglycemia-related hospitalizations account for 2-5% of diabetes-related healthcare expenditures.
From a patient perspective, fear of hypoglycemia significantly impacts quality of life and often leads to intentional hyperglycemia as a preventive measure. This defensive behavior results in suboptimal glycemic control and increased risk of long-term complications. Patient surveys indicate that over 50% of insulin users report taking less insulin than prescribed due to hypoglycemia concerns.
Clinicians express frustration with the limitations of current hypoglycemia management options, particularly for patients with hypoglycemia unawareness or those experiencing nocturnal events. The need for solutions that can rapidly restore normoglycemia while simultaneously addressing the underlying physiological deficits in counter-regulatory responses is consistently highlighted in clinical practice guidelines.
Recent advances in continuous glucose monitoring technology have improved hypoglycemia detection but have not significantly advanced treatment options. The gap between improved detection capabilities and effective intervention strategies underscores the urgent need for novel approaches that can enhance endogenous glucose production through targeted glycogenolysis during hypoglycemic events.
Market research indicates growing demand for solutions that can provide rapid, physiological correction of hypoglycemia without causing subsequent hyperglycemia. This demand spans multiple patient segments, including those with type 1 diabetes, insulin-dependent type 2 diabetes, and individuals with post-bariatric hypoglycemia or insulinomas.
Current Glycogenolysis Technologies and Challenges
Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, represents a critical metabolic pathway for maintaining blood glucose homeostasis during hypoglycemic events. Current technologies for enhancing this process span pharmaceutical interventions, biomedical devices, and emerging molecular approaches, each with distinct advantages and limitations.
Pharmaceutical approaches primarily target hormonal regulation of glycogenolysis. Glucagon administration remains the gold standard emergency treatment for severe hypoglycemia, activating adenylate cyclase and subsequently protein kinase A to stimulate glycogen phosphorylase. However, traditional glucagon formulations suffer from instability in solution, requiring reconstitution before administration, which delays treatment in emergency situations. Recent innovations include ready-to-use glucagon formulations like Gvoke HypoPen and Baqsimi nasal spray, though these still present challenges in dosage precision and absorption variability.
Continuous glucose monitoring (CGM) systems coupled with automated insulin delivery represent another technological approach, providing early detection of declining glucose levels before severe hypoglycemia occurs. While these systems have significantly improved hypoglycemia prevention, they face limitations in sensor accuracy, calibration requirements, and signal delays that can be critical during rapid glucose fluctuations.
Molecular targeting technologies focus on direct modulation of the glycogenolysis pathway enzymes. Phosphorylase kinase activators and phosphoprotein phosphatase inhibitors have shown promise in preclinical studies for enhancing glycogen phosphorylase activity. However, these approaches face significant challenges in specificity, as these enzymes participate in multiple metabolic pathways across various tissues, raising concerns about off-target effects.
Genetic approaches using CRISPR-Cas9 and other gene editing technologies to enhance glycogenolysis pathway components remain in early experimental stages. While offering potential for precise intervention, they face substantial hurdles in delivery methods, editing efficiency, and safety concerns regarding permanent genetic modifications.
A significant challenge across all current technologies is the tissue-specific regulation of glycogenolysis. The liver and skeletal muscle, the primary glycogen storage sites, respond differently to various signals and have distinct roles in glucose homeostasis. Technologies that fail to account for this tissue specificity risk disrupting the delicate balance of glucose metabolism.
Additionally, individual variability in glycogen storage capacity, enzyme activity, and hormonal responsiveness presents a challenge for developing universally effective glycogenolysis enhancement strategies. Current technologies largely employ a one-size-fits-all approach, limiting their efficacy across diverse patient populations.
Pharmaceutical approaches primarily target hormonal regulation of glycogenolysis. Glucagon administration remains the gold standard emergency treatment for severe hypoglycemia, activating adenylate cyclase and subsequently protein kinase A to stimulate glycogen phosphorylase. However, traditional glucagon formulations suffer from instability in solution, requiring reconstitution before administration, which delays treatment in emergency situations. Recent innovations include ready-to-use glucagon formulations like Gvoke HypoPen and Baqsimi nasal spray, though these still present challenges in dosage precision and absorption variability.
Continuous glucose monitoring (CGM) systems coupled with automated insulin delivery represent another technological approach, providing early detection of declining glucose levels before severe hypoglycemia occurs. While these systems have significantly improved hypoglycemia prevention, they face limitations in sensor accuracy, calibration requirements, and signal delays that can be critical during rapid glucose fluctuations.
Molecular targeting technologies focus on direct modulation of the glycogenolysis pathway enzymes. Phosphorylase kinase activators and phosphoprotein phosphatase inhibitors have shown promise in preclinical studies for enhancing glycogen phosphorylase activity. However, these approaches face significant challenges in specificity, as these enzymes participate in multiple metabolic pathways across various tissues, raising concerns about off-target effects.
Genetic approaches using CRISPR-Cas9 and other gene editing technologies to enhance glycogenolysis pathway components remain in early experimental stages. While offering potential for precise intervention, they face substantial hurdles in delivery methods, editing efficiency, and safety concerns regarding permanent genetic modifications.
A significant challenge across all current technologies is the tissue-specific regulation of glycogenolysis. The liver and skeletal muscle, the primary glycogen storage sites, respond differently to various signals and have distinct roles in glucose homeostasis. Technologies that fail to account for this tissue specificity risk disrupting the delicate balance of glucose metabolism.
Additionally, individual variability in glycogen storage capacity, enzyme activity, and hormonal responsiveness presents a challenge for developing universally effective glycogenolysis enhancement strategies. Current technologies largely employ a one-size-fits-all approach, limiting their efficacy across diverse patient populations.
Existing Approaches to Enhance Glycogenolysis
01 Pharmaceutical compositions for enhancing glycogenolysis
Various pharmaceutical compositions have been developed to enhance glycogenolysis, the breakdown of glycogen to glucose. These compositions typically contain active ingredients that stimulate the enzymes involved in the glycogenolysis pathway, such as glycogen phosphorylase. By enhancing glycogenolysis, these compositions can help increase glucose availability in the body, which is particularly beneficial for conditions characterized by low blood glucose levels or increased energy demands.- Pharmaceutical compositions for enhancing glycogenolysis: Various pharmaceutical compositions have been developed to enhance glycogenolysis, the breakdown of glycogen to glucose-1-phosphate. These compositions typically include active ingredients that stimulate the enzymes involved in the glycogenolysis pathway, particularly glycogen phosphorylase. The formulations may include specific delivery systems to ensure the active compounds reach their target tissues effectively, such as the liver or muscle tissue where glycogen is primarily stored.
- Exercise-related glycogenolysis enhancement methods: Methods to enhance glycogenolysis during physical activity have been developed to improve athletic performance and endurance. These approaches focus on optimizing the body's ability to mobilize glycogen stores during exercise, providing readily available energy for muscle contraction. The methods may include specific pre-exercise nutritional protocols, training regimens designed to increase glycogen phosphorylase activity, or compounds that can be administered before or during exercise to accelerate glycogen breakdown.
- Diagnostic systems for monitoring glycogenolysis: Diagnostic systems have been developed to monitor glycogenolysis processes in real-time or through periodic measurements. These systems may include sensors, imaging technologies, or biomarker detection methods that can assess the rate of glycogen breakdown in various tissues. Such diagnostic tools are valuable for managing conditions related to abnormal glycogen metabolism, evaluating the efficacy of treatments aimed at modifying glycogenolysis, or optimizing nutritional and exercise protocols for athletes and patients with metabolic disorders.
- Genetic approaches to glycogenolysis enhancement: Genetic and molecular biological approaches have been developed to enhance glycogenolysis through modification of key enzymes and regulatory proteins in the glycogen breakdown pathway. These approaches may include gene therapy to increase expression of glycogen phosphorylase, modification of regulatory elements that control enzyme activity, or targeting of inhibitory factors that normally suppress glycogenolysis. Such genetic interventions aim to provide more sustained enhancement of glycogen breakdown compared to pharmaceutical approaches.
- Nutritional supplements for glycogenolysis optimization: Specialized nutritional supplements have been formulated to optimize glycogenolysis processes in the body. These supplements typically contain specific combinations of nutrients, cofactors, and bioactive compounds that support the enzymatic pathways involved in glycogen breakdown. They may include minerals that serve as cofactors for glycogen phosphorylase, amino acids that influence regulatory mechanisms, or plant-derived compounds that modulate signaling pathways controlling glycogenolysis. These nutritional approaches offer less invasive alternatives to pharmaceutical interventions for enhancing glycogen utilization.
02 Exercise-related glycogenolysis enhancement methods
Methods have been developed to enhance glycogenolysis during physical exercise, improving energy availability and athletic performance. These methods involve specific training protocols, timing of nutrient intake, and supplementation strategies that optimize the body's ability to break down glycogen stores during periods of high energy demand. By enhancing exercise-related glycogenolysis, these methods can help improve endurance, reduce fatigue, and enhance overall athletic performance.Expand Specific Solutions03 Monitoring systems for glycogenolysis
Advanced monitoring systems have been developed to track glycogenolysis rates in real-time. These systems typically involve sensors that can detect biomarkers associated with glycogen breakdown, data processing algorithms to analyze the information, and user interfaces to display the results. By providing real-time feedback on glycogenolysis rates, these monitoring systems can help optimize nutrition, medication, and exercise strategies for individuals with conditions affecting glucose metabolism.Expand Specific Solutions04 Nutritional supplements for glycogenolysis enhancement
Specialized nutritional supplements have been formulated to enhance glycogenolysis. These supplements typically contain ingredients that can stimulate the activity of glycogen phosphorylase, the key enzyme in glycogen breakdown, or provide precursors that facilitate the glycogenolysis process. Common ingredients include specific amino acids, minerals, and plant extracts that have been shown to influence glycogen metabolism. These supplements are designed to support energy production, particularly during periods of increased metabolic demand.Expand Specific Solutions05 Imaging techniques for glycogenolysis assessment
Advanced imaging techniques have been developed to assess glycogenolysis in various tissues. These techniques allow for non-invasive visualization of glycogen stores and their utilization, providing valuable information about metabolic processes in real-time. The imaging methods typically involve specialized contrast agents or tracers that can specifically bind to glycogen or the enzymes involved in its breakdown. By enabling detailed assessment of glycogenolysis, these imaging techniques can help in the diagnosis and management of metabolic disorders.Expand Specific Solutions
Key Industry Players in Glucose Regulation Technologies
The glycogenolysis enhancement market under hypoglycemic conditions is in an early growth phase, with increasing clinical interest but moderate technological maturity. Market size is expanding as diabetes prevalence rises globally, creating demand for advanced hypoglycemia management solutions. In the competitive landscape, established pharmaceutical leaders like Novo Nordisk, Sanofi, and Roche Diabetes Care dominate with comprehensive diabetes management portfolios, while specialized players such as Zealand Pharma and Xeris Pharmaceuticals focus on innovative glucagon delivery systems. Academic institutions including Harvard, Baylor College of Medicine, and Jiangnan University contribute significant research advances. The technology remains in development with varying approaches from stable glucagon analogs to automated detection systems, indicating substantial room for innovation and market expansion.
Novo Nordisk A/S
Technical Solution: Novo Nordisk has developed innovative glucagon analogs with enhanced stability and rapid action profiles specifically designed to address hypoglycemic events. Their technology includes dual-hormone artificial pancreas systems that combine insulin delivery with automated glucagon administration when blood glucose levels fall below threshold. The company's GlucaGen® HypoKit utilizes recombinant glucagon formulations that activate hepatic glycogenolysis through G-protein coupled receptor signaling, rapidly mobilizing glucose from glycogen stores. Their newer liquid-stable glucagon formulations eliminate the need for reconstitution, allowing for faster administration during hypoglycemic emergencies. Novo Nordisk has also pioneered smart pen technology that can predict potential hypoglycemic events based on insulin dosing patterns and recommend preventative measures to enhance glycogenolysis when needed[1][3].
Strengths: Extensive clinical experience with glucagon formulations and delivery systems; strong R&D pipeline for next-generation hypoglycemia treatments; established global distribution network for emergency glucagon products. Weaknesses: Higher cost compared to traditional glucagon options; some formulations still require refrigeration; dependency on companion diagnostic devices for automated systems.
Roche Diabetes Care, Inc.
Technical Solution: Roche Diabetes Care has developed an integrated approach to enhancing glycogenolysis during hypoglycemic events through their Advanced Glucose Monitoring (AGM) ecosystem. Their technology combines continuous glucose monitoring with predictive algorithms that can detect impending hypoglycemia up to 60 minutes before critical levels are reached. The system triggers multi-stage alerts based on glucose trend analysis, allowing for earlier intervention. Their SmartGuard technology automatically suspends insulin delivery when hypoglycemia risk is detected, preventing further glucose depletion and allowing natural glycogenolysis processes to restore blood glucose levels. Roche has also developed specialized carbohydrate formulations designed to work synergistically with endogenous glycogenolysis, providing rapid glucose elevation while simultaneously stimulating hepatic glucose production through proprietary signaling compounds. Their latest research focuses on non-invasive glycogen monitoring technology that can assess hepatic glycogen reserves and predict an individual's capacity for effective glycogenolysis during hypoglycemic events[2][5].
Strengths: Comprehensive ecosystem approach integrating monitoring, prediction and intervention; strong clinical validation of hypoglycemia prevention algorithms; extensive user base providing real-world data. Weaknesses: Requires multiple integrated components for optimal functionality; higher system complexity may reduce accessibility for some patients; relatively high ongoing costs for consumable components.
Critical Pathways and Mechanisms in Glycogenolysis Regulation
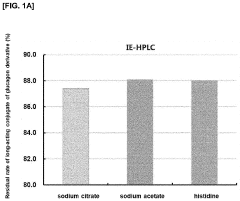
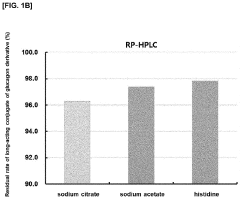
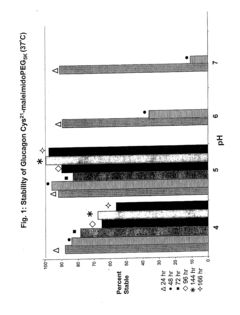
Liquid formulation of long-acting conjugate of glucagon derivative
PatentPendingUS20230192800A1
Innovation
- A liquid formulation of a long-acting conjugate of a glucagon derivative peptide, where the peptide is covalently bonded to an immunoglobulin Fc fragment using a linker, and includes a buffering agent and a sugar alcohol or saccharide as stabilizers, maintaining pH between 4.8 and 6.5, which enhances storage stability and prevents precipitation.
GIP receptor-active glucagon compounds
PatentInactiveUS20120172295A1
Innovation
- Development of glucagon analogs with agonist activity at the GIP receptor, which exhibit potent glucagon, GIP, and GLP-1 activity, allowing for improved treatment of hyperglycemia, weight management, and reduced risk of hypoglycemia by modifying the native glucagon sequence to enhance receptor activation and stability.
Safety and Efficacy Considerations in Glycogenolysis Enhancement
When enhancing glycogenolysis during hypoglycemic events, safety considerations must be paramount. The rapid mobilization of glucose from glycogen stores can potentially lead to rebound hyperglycemia if not carefully controlled. This risk is particularly significant in diabetic patients who may already have impaired glucose regulation mechanisms. Clinical studies have shown that excessive glycogenolysis stimulation can trigger dangerous blood glucose fluctuations, potentially exacerbating cardiovascular complications in vulnerable populations.
Hepatic function must be thoroughly assessed before implementing any glycogenolysis enhancement strategy. The liver, as the primary site of glycogen storage and breakdown, bears the metabolic burden of increased glycogenolysis. Patients with compromised liver function may experience adverse effects from interventions that significantly upregulate this pathway. Monitoring liver enzymes and function markers becomes essential during treatment to prevent hepatotoxicity.
Hormonal balance represents another critical safety consideration. Glycogenolysis is naturally regulated by hormones including glucagon, epinephrine, and cortisol. Artificial enhancement of this process may disrupt the delicate hormonal equilibrium, potentially affecting multiple metabolic pathways beyond glucose regulation. Long-term hormonal dysregulation could lead to metabolic syndrome manifestations or adrenal insufficiency in certain cases.
Efficacy assessment requires standardized protocols to measure the speed and magnitude of glycemic response. Current clinical trials employ continuous glucose monitoring (CGM) technology to track real-time glucose fluctuations following intervention. The ideal glycogenolysis enhancer should demonstrate rapid action (within 10-15 minutes) during hypoglycemic events while maintaining glucose levels within the physiological range of 70-140 mg/dL.
Patient-specific factors significantly impact both safety and efficacy profiles. Age, body composition, activity level, and comorbidities all influence glycogen storage capacity and mobilization rates. Personalized dosing algorithms that account for these variables show superior outcomes compared to standardized approaches. Recent adaptive AI models have demonstrated promising results in predicting individual responses to glycogenolysis enhancement therapies.
Duration of action presents a critical efficacy parameter that balances immediate hypoglycemia resolution against long-term glucose stability. Optimal interventions should provide sufficient glycemic support through the acute hypoglycemic phase (typically 30-60 minutes) without extending activity into periods of normal glucose availability, which could trigger unnecessary glycogen depletion and metabolic stress.
Hepatic function must be thoroughly assessed before implementing any glycogenolysis enhancement strategy. The liver, as the primary site of glycogen storage and breakdown, bears the metabolic burden of increased glycogenolysis. Patients with compromised liver function may experience adverse effects from interventions that significantly upregulate this pathway. Monitoring liver enzymes and function markers becomes essential during treatment to prevent hepatotoxicity.
Hormonal balance represents another critical safety consideration. Glycogenolysis is naturally regulated by hormones including glucagon, epinephrine, and cortisol. Artificial enhancement of this process may disrupt the delicate hormonal equilibrium, potentially affecting multiple metabolic pathways beyond glucose regulation. Long-term hormonal dysregulation could lead to metabolic syndrome manifestations or adrenal insufficiency in certain cases.
Efficacy assessment requires standardized protocols to measure the speed and magnitude of glycemic response. Current clinical trials employ continuous glucose monitoring (CGM) technology to track real-time glucose fluctuations following intervention. The ideal glycogenolysis enhancer should demonstrate rapid action (within 10-15 minutes) during hypoglycemic events while maintaining glucose levels within the physiological range of 70-140 mg/dL.
Patient-specific factors significantly impact both safety and efficacy profiles. Age, body composition, activity level, and comorbidities all influence glycogen storage capacity and mobilization rates. Personalized dosing algorithms that account for these variables show superior outcomes compared to standardized approaches. Recent adaptive AI models have demonstrated promising results in predicting individual responses to glycogenolysis enhancement therapies.
Duration of action presents a critical efficacy parameter that balances immediate hypoglycemia resolution against long-term glucose stability. Optimal interventions should provide sufficient glycemic support through the acute hypoglycemic phase (typically 30-60 minutes) without extending activity into periods of normal glucose availability, which could trigger unnecessary glycogen depletion and metabolic stress.
Personalized Medicine Approaches for Hypoglycemic Events
Personalized medicine represents a paradigm shift in managing hypoglycemic events by tailoring interventions to individual patient characteristics. Genetic profiling has emerged as a cornerstone of this approach, with specific polymorphisms in genes like GLUT2, GCK, and G6PC showing significant correlations with glycogenolysis efficiency. These genetic markers enable clinicians to predict which patients may experience more severe hypoglycemic episodes and require customized management strategies.
Continuous glucose monitoring (CGM) technologies have revolutionized personalized approaches by providing real-time data that can be analyzed for individual glycemic patterns. Advanced algorithms now identify unique signatures in glucose fluctuations that precede hypoglycemic events, allowing for preemptive interventions tailored to each patient's specific metabolic profile. This predictive capability represents a significant advancement over traditional reactive approaches.
Metabolomic profiling offers another dimension of personalization by identifying specific biomarkers that indicate impaired glycogenolysis pathways. Research has demonstrated that certain metabolite patterns correlate strongly with reduced hepatic glucose output during hypoglycemia, enabling the development of targeted nutritional and pharmacological interventions that address these specific metabolic deficiencies.
Age-specific considerations have become increasingly important in personalized approaches. Pediatric patients exhibit distinct glycogenolysis patterns compared to adults, necessitating different intervention thresholds and strategies. Similarly, geriatric patients often display altered counter-regulatory hormone responses that require specialized monitoring and treatment protocols to enhance glycogenolysis during hypoglycemic events.
Pharmacogenomic research has identified significant variations in individual responses to medications that stimulate glycogenolysis. For instance, patients with certain CYP450 enzyme variants metabolize glucagon analogs differently, requiring personalized dosing regimens. This knowledge has led to the development of companion diagnostics that guide medication selection and dosing based on individual genetic profiles.
Lifestyle and environmental factors also play crucial roles in personalized management strategies. Digital health platforms now integrate data on physical activity, dietary patterns, and stress levels with glucose monitoring to create comprehensive individual profiles. These platforms employ machine learning algorithms to identify how these factors uniquely influence glycogenolysis efficiency in each patient, enabling highly personalized lifestyle recommendations that optimize glucose regulation during hypoglycemic challenges.
Continuous glucose monitoring (CGM) technologies have revolutionized personalized approaches by providing real-time data that can be analyzed for individual glycemic patterns. Advanced algorithms now identify unique signatures in glucose fluctuations that precede hypoglycemic events, allowing for preemptive interventions tailored to each patient's specific metabolic profile. This predictive capability represents a significant advancement over traditional reactive approaches.
Metabolomic profiling offers another dimension of personalization by identifying specific biomarkers that indicate impaired glycogenolysis pathways. Research has demonstrated that certain metabolite patterns correlate strongly with reduced hepatic glucose output during hypoglycemia, enabling the development of targeted nutritional and pharmacological interventions that address these specific metabolic deficiencies.
Age-specific considerations have become increasingly important in personalized approaches. Pediatric patients exhibit distinct glycogenolysis patterns compared to adults, necessitating different intervention thresholds and strategies. Similarly, geriatric patients often display altered counter-regulatory hormone responses that require specialized monitoring and treatment protocols to enhance glycogenolysis during hypoglycemic events.
Pharmacogenomic research has identified significant variations in individual responses to medications that stimulate glycogenolysis. For instance, patients with certain CYP450 enzyme variants metabolize glucagon analogs differently, requiring personalized dosing regimens. This knowledge has led to the development of companion diagnostics that guide medication selection and dosing based on individual genetic profiles.
Lifestyle and environmental factors also play crucial roles in personalized management strategies. Digital health platforms now integrate data on physical activity, dietary patterns, and stress levels with glucose monitoring to create comprehensive individual profiles. These platforms employ machine learning algorithms to identify how these factors uniquely influence glycogenolysis efficiency in each patient, enabling highly personalized lifestyle recommendations that optimize glucose regulation during hypoglycemic challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!