How Glycogenolysis Influences Muscle Protein Synthesis
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis and Muscle Protein Synthesis Background
Glycogenolysis represents a fundamental metabolic process in skeletal muscle, involving the breakdown of glycogen into glucose-1-phosphate and subsequently glucose-6-phosphate for energy production. This process becomes particularly active during high-intensity exercise when rapid energy mobilization is required. Historically, research on glycogenolysis dates back to the early 20th century, with significant advancements in understanding its biochemical pathways occurring in the 1960s through the work of Carl and Gerty Cori.
The relationship between glycogenolysis and muscle protein synthesis (MPS) has emerged as a critical area of investigation in exercise physiology and sports nutrition over the past two decades. Initially, these processes were studied in isolation, but contemporary research has revealed intricate connections between carbohydrate metabolism and protein turnover in skeletal muscle tissue. This interconnection represents a paradigm shift in our understanding of muscle adaptation to exercise stimuli.
Glycogenolysis is primarily regulated by two enzymes: glycogen phosphorylase, which catalyzes the rate-limiting step, and glycogen debranching enzyme. The activation of glycogen phosphorylase occurs through hormonal signals (particularly epinephrine and glucagon) and calcium-dependent mechanisms during muscle contraction. This process is further modulated by energy sensors within the cell, including AMP-activated protein kinase (AMPK).
Muscle protein synthesis, conversely, involves the translation of messenger RNA into proteins through ribosomal activity. This process is predominantly regulated by the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway, which integrates various inputs including amino acid availability, energy status, and mechanical tension. The interplay between glycogenolysis and MPS occurs at multiple levels, including shared signaling pathways and metabolic intermediates.
Recent evidence suggests that glycogen depletion during exercise may influence subsequent protein synthetic responses through several mechanisms. Glycogen serves not only as an energy substrate but also as a signaling molecule that can affect cellular processes including protein translation. The localization of glycogen particles near sarcoplasmic reticulum and mitochondria further suggests a spatial coordination between energy provision and protein synthesis machinery.
The evolutionary significance of this relationship likely stems from the need for coordinated tissue remodeling in response to physical challenges. From a survival perspective, the ability to simultaneously manage energy utilization and tissue repair would confer significant advantages, particularly in environments where physical activity was essential for survival and food acquisition was unpredictable.
Understanding the glycogenolysis-MPS relationship has profound implications for fields ranging from sports performance to aging and disease management. This background provides the foundation for exploring how manipulating carbohydrate availability might optimize muscle adaptation to training stimuli and potentially address muscle wasting conditions.
The relationship between glycogenolysis and muscle protein synthesis (MPS) has emerged as a critical area of investigation in exercise physiology and sports nutrition over the past two decades. Initially, these processes were studied in isolation, but contemporary research has revealed intricate connections between carbohydrate metabolism and protein turnover in skeletal muscle tissue. This interconnection represents a paradigm shift in our understanding of muscle adaptation to exercise stimuli.
Glycogenolysis is primarily regulated by two enzymes: glycogen phosphorylase, which catalyzes the rate-limiting step, and glycogen debranching enzyme. The activation of glycogen phosphorylase occurs through hormonal signals (particularly epinephrine and glucagon) and calcium-dependent mechanisms during muscle contraction. This process is further modulated by energy sensors within the cell, including AMP-activated protein kinase (AMPK).
Muscle protein synthesis, conversely, involves the translation of messenger RNA into proteins through ribosomal activity. This process is predominantly regulated by the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway, which integrates various inputs including amino acid availability, energy status, and mechanical tension. The interplay between glycogenolysis and MPS occurs at multiple levels, including shared signaling pathways and metabolic intermediates.
Recent evidence suggests that glycogen depletion during exercise may influence subsequent protein synthetic responses through several mechanisms. Glycogen serves not only as an energy substrate but also as a signaling molecule that can affect cellular processes including protein translation. The localization of glycogen particles near sarcoplasmic reticulum and mitochondria further suggests a spatial coordination between energy provision and protein synthesis machinery.
The evolutionary significance of this relationship likely stems from the need for coordinated tissue remodeling in response to physical challenges. From a survival perspective, the ability to simultaneously manage energy utilization and tissue repair would confer significant advantages, particularly in environments where physical activity was essential for survival and food acquisition was unpredictable.
Understanding the glycogenolysis-MPS relationship has profound implications for fields ranging from sports performance to aging and disease management. This background provides the foundation for exploring how manipulating carbohydrate availability might optimize muscle adaptation to training stimuli and potentially address muscle wasting conditions.
Market Analysis for Sports Nutrition and Recovery Products
The sports nutrition and recovery products market has experienced significant growth in recent years, driven by increasing consumer awareness of the importance of proper nutrition for athletic performance and recovery. The global sports nutrition market was valued at approximately $15.6 billion in 2021 and is projected to reach $35.8 billion by 2028, growing at a CAGR of 12.3% during the forecast period. This growth is particularly relevant to products targeting glycogenolysis and muscle protein synthesis processes.
Consumer demographics have expanded beyond professional athletes to include recreational fitness enthusiasts, creating a broader market base. The "weekend warrior" segment, comprising individuals who engage in intense physical activities during their free time, represents a rapidly growing consumer group seeking effective recovery solutions. Additionally, the aging population interested in maintaining muscle mass has emerged as a significant market segment.
Product categories addressing the glycogenolysis-protein synthesis relationship include pre-workout carbohydrate supplements, intra-workout carbohydrate-protein combinations, and post-workout recovery formulations. The post-workout recovery segment holds the largest market share at 42%, followed by pre-workout supplements at 31%. Products containing specific amino acids that enhance the glycogenolysis-protein synthesis pathway, particularly branched-chain amino acids (BCAAs), have seen a 28% year-over-year growth.
Regional analysis reveals North America as the dominant market with 38% share, followed by Europe (27%) and Asia-Pacific (22%). The Asia-Pacific region is experiencing the fastest growth rate at 15.7% annually, driven by increasing fitness awareness and rising disposable incomes in countries like China and India.
Distribution channels have diversified significantly, with e-commerce emerging as the fastest-growing channel at 23% annual growth. Specialty nutrition stores remain important, accounting for 35% of sales, while mainstream retail channels represent 28% of the market. Direct-to-consumer models are gaining traction, particularly among premium brands targeting educated consumers who understand the science behind glycogenolysis and protein synthesis.
Consumer preferences are shifting toward products with scientific validation, clean labels, and natural ingredients. Products highlighting the connection between glycogen metabolism and protein synthesis in their marketing materials have shown 18% higher consumer engagement rates. Additionally, personalized nutrition solutions tailored to individual metabolic profiles are emerging as a premium segment with growth potential.
Key market challenges include regulatory scrutiny of product claims, particularly those related to muscle recovery and synthesis, and increasing consumer demand for transparent ingredient sourcing and scientific substantiation of efficacy claims. Companies investing in clinical research to validate the glycogenolysis-protein synthesis connection in their formulations are gaining competitive advantages in this evolving marketplace.
Consumer demographics have expanded beyond professional athletes to include recreational fitness enthusiasts, creating a broader market base. The "weekend warrior" segment, comprising individuals who engage in intense physical activities during their free time, represents a rapidly growing consumer group seeking effective recovery solutions. Additionally, the aging population interested in maintaining muscle mass has emerged as a significant market segment.
Product categories addressing the glycogenolysis-protein synthesis relationship include pre-workout carbohydrate supplements, intra-workout carbohydrate-protein combinations, and post-workout recovery formulations. The post-workout recovery segment holds the largest market share at 42%, followed by pre-workout supplements at 31%. Products containing specific amino acids that enhance the glycogenolysis-protein synthesis pathway, particularly branched-chain amino acids (BCAAs), have seen a 28% year-over-year growth.
Regional analysis reveals North America as the dominant market with 38% share, followed by Europe (27%) and Asia-Pacific (22%). The Asia-Pacific region is experiencing the fastest growth rate at 15.7% annually, driven by increasing fitness awareness and rising disposable incomes in countries like China and India.
Distribution channels have diversified significantly, with e-commerce emerging as the fastest-growing channel at 23% annual growth. Specialty nutrition stores remain important, accounting for 35% of sales, while mainstream retail channels represent 28% of the market. Direct-to-consumer models are gaining traction, particularly among premium brands targeting educated consumers who understand the science behind glycogenolysis and protein synthesis.
Consumer preferences are shifting toward products with scientific validation, clean labels, and natural ingredients. Products highlighting the connection between glycogen metabolism and protein synthesis in their marketing materials have shown 18% higher consumer engagement rates. Additionally, personalized nutrition solutions tailored to individual metabolic profiles are emerging as a premium segment with growth potential.
Key market challenges include regulatory scrutiny of product claims, particularly those related to muscle recovery and synthesis, and increasing consumer demand for transparent ingredient sourcing and scientific substantiation of efficacy claims. Companies investing in clinical research to validate the glycogenolysis-protein synthesis connection in their formulations are gaining competitive advantages in this evolving marketplace.
Current Understanding and Research Challenges
The current understanding of glycogenolysis and its influence on muscle protein synthesis represents a complex interplay of metabolic pathways that has garnered significant attention in sports medicine, nutrition science, and metabolic research. Recent studies have established that glycogenolysis—the breakdown of glycogen to glucose-1-phosphate—plays a more nuanced role in muscle protein synthesis than previously thought, extending beyond mere energy provision.
Research has demonstrated that glycogen depletion during exercise triggers a cascade of signaling events that can both positively and negatively impact protein synthesis. The activation of AMP-activated protein kinase (AMPK) during glycogen depletion has been shown to inhibit the mammalian target of rapamycin complex 1 (mTORC1) pathway, a central regulator of protein synthesis. Conversely, the subsequent replenishment of glycogen stores post-exercise appears to enhance anabolic signaling through insulin-mediated pathways.
A significant challenge in this field remains the precise quantification of how different rates and extents of glycogenolysis affect protein synthetic responses across various muscle fiber types. Type I (slow-twitch) and Type II (fast-twitch) muscle fibers exhibit different glycogen utilization patterns and protein synthetic responses, yet methodological limitations have hindered comprehensive fiber-specific analyses in human subjects.
The temporal relationship between glycogenolysis and protein synthesis presents another research challenge. While acute studies have provided valuable insights, longitudinal investigations tracking these metabolic processes over extended training periods remain scarce. This gap limits our understanding of adaptation mechanisms and optimal nutritional strategies for different training phases.
Methodological inconsistencies across studies further complicate the research landscape. Variations in exercise protocols, nutritional interventions, and measurement techniques make direct comparisons between studies problematic. The development of standardized research protocols represents a pressing need in advancing this field.
Recent technological innovations in metabolomics and proteomics offer promising avenues for more detailed analysis, but integration of these multi-omics approaches into cohesive research frameworks remains challenging. Additionally, individual genetic variations in glycogen metabolism and protein synthetic response add another layer of complexity that current research has only begun to address.
The translation of laboratory findings to practical applications faces significant hurdles. While controlled studies provide mechanistic insights, real-world factors such as mixed macronutrient meals, varying exercise intensities, and individual metabolic differences often yield less predictable outcomes. Bridging this gap between controlled research and practical application represents one of the most significant challenges in this field.
Research has demonstrated that glycogen depletion during exercise triggers a cascade of signaling events that can both positively and negatively impact protein synthesis. The activation of AMP-activated protein kinase (AMPK) during glycogen depletion has been shown to inhibit the mammalian target of rapamycin complex 1 (mTORC1) pathway, a central regulator of protein synthesis. Conversely, the subsequent replenishment of glycogen stores post-exercise appears to enhance anabolic signaling through insulin-mediated pathways.
A significant challenge in this field remains the precise quantification of how different rates and extents of glycogenolysis affect protein synthetic responses across various muscle fiber types. Type I (slow-twitch) and Type II (fast-twitch) muscle fibers exhibit different glycogen utilization patterns and protein synthetic responses, yet methodological limitations have hindered comprehensive fiber-specific analyses in human subjects.
The temporal relationship between glycogenolysis and protein synthesis presents another research challenge. While acute studies have provided valuable insights, longitudinal investigations tracking these metabolic processes over extended training periods remain scarce. This gap limits our understanding of adaptation mechanisms and optimal nutritional strategies for different training phases.
Methodological inconsistencies across studies further complicate the research landscape. Variations in exercise protocols, nutritional interventions, and measurement techniques make direct comparisons between studies problematic. The development of standardized research protocols represents a pressing need in advancing this field.
Recent technological innovations in metabolomics and proteomics offer promising avenues for more detailed analysis, but integration of these multi-omics approaches into cohesive research frameworks remains challenging. Additionally, individual genetic variations in glycogen metabolism and protein synthetic response add another layer of complexity that current research has only begun to address.
The translation of laboratory findings to practical applications faces significant hurdles. While controlled studies provide mechanistic insights, real-world factors such as mixed macronutrient meals, varying exercise intensities, and individual metabolic differences often yield less predictable outcomes. Bridging this gap between controlled research and practical application represents one of the most significant challenges in this field.
Current Methodologies for Studying Metabolic Interactions
01 Nutritional compositions for enhancing muscle protein synthesis
Specific nutritional compositions can enhance muscle protein synthesis while regulating glycogenolysis. These compositions typically include amino acids, particularly branched-chain amino acids (BCAAs) like leucine, isoleucine, and valine, which are essential for stimulating muscle protein synthesis. Some formulations also incorporate carbohydrates to replenish glycogen stores and support the energy demands of protein synthesis, creating a balanced approach to muscle recovery and growth.- Nutritional compositions for enhancing glycogenolysis and muscle protein synthesis: Specific nutritional compositions can enhance both glycogenolysis and muscle protein synthesis. These compositions typically contain a combination of proteins, amino acids (particularly branched-chain amino acids), carbohydrates, and other nutrients that work synergistically to promote muscle recovery and growth while facilitating glycogen breakdown for energy. These formulations are designed to optimize athletic performance and recovery by balancing energy production through glycogenolysis with muscle repair and growth through protein synthesis.
- Timing and delivery methods for glycogenolysis and protein synthesis enhancement: The timing of nutrient delivery and the method of administration significantly impact glycogenolysis and muscle protein synthesis. Various delivery systems have been developed to optimize the release of active ingredients at specific times during exercise or recovery periods. These systems include sustained-release formulations, pre-workout supplements designed to enhance glycogenolysis during exercise, and post-workout formulations focused on protein synthesis. The timing of consumption relative to exercise can significantly affect the balance between glycogen utilization and muscle protein synthesis.
- Signaling pathway modulators affecting glycogenolysis and protein synthesis: Compounds that modulate specific cellular signaling pathways can simultaneously influence glycogenolysis and muscle protein synthesis. These modulators target pathways such as mTOR (mammalian target of rapamycin), AMPK (AMP-activated protein kinase), and insulin signaling cascades. By affecting these pathways, these compounds can enhance muscle protein synthesis while regulating glycogen breakdown, leading to improved muscle development and energy utilization. Some modulators are derived from natural sources while others are synthetic compounds designed to target specific aspects of these metabolic processes.
- Exercise-induced adaptations in glycogenolysis and protein synthesis: Exercise protocols and training regimens can be designed to optimize the balance between glycogenolysis and muscle protein synthesis. Different types of exercise (resistance, endurance, high-intensity interval training) affect these processes differently. Compositions and methods have been developed to enhance the beneficial adaptations from specific exercise types, including supplements that promote glycogen replenishment after glycogenolysis while simultaneously supporting the increased protein synthesis needed for muscle repair and growth. These approaches often consider the timing of nutrient intake relative to the exercise stimulus.
- Hormonal regulation of glycogenolysis and muscle protein synthesis: Hormonal factors play a crucial role in regulating both glycogenolysis and muscle protein synthesis. Compositions and methods have been developed to modulate hormonal responses, particularly insulin, growth hormone, cortisol, and testosterone levels, to optimize the balance between energy production through glycogen breakdown and muscle growth through protein synthesis. Some approaches involve natural compounds that can influence hormone levels or sensitivity, while others focus on the timing of nutrient intake to take advantage of natural hormonal fluctuations. These methods aim to create an optimal hormonal environment for simultaneous energy production and muscle development.
02 Exercise and training regimens affecting glycogenolysis and protein synthesis
Various exercise protocols and training regimens can be designed to optimize the balance between glycogenolysis and muscle protein synthesis. High-intensity exercise typically accelerates glycogenolysis while creating the stimulus for increased protein synthesis during recovery. Timing of nutrient intake relative to exercise can significantly impact these metabolic processes, with post-exercise nutrition being particularly important for shifting from a catabolic to an anabolic state.Expand Specific Solutions03 Hormonal regulators of glycogenolysis and muscle protein synthesis
Hormones play crucial roles in regulating both glycogenolysis and muscle protein synthesis. Insulin suppresses glycogenolysis while promoting protein synthesis, making it a key anabolic hormone. Growth hormone and testosterone also enhance protein synthesis while affecting glucose metabolism. Cortisol, conversely, can promote glycogenolysis while inhibiting protein synthesis. Compounds that modulate these hormonal pathways can be used to influence the balance between energy utilization and muscle growth.Expand Specific Solutions04 Supplements and compounds targeting metabolic pathways
Specific supplements and compounds can target the metabolic pathways involved in glycogenolysis and muscle protein synthesis. These include enzyme modulators that can either accelerate or inhibit glycogen breakdown, as well as signaling pathway activators like mTOR stimulators that enhance protein synthesis. Some compounds work by improving glucose uptake into muscle cells, providing substrate for both glycogen replenishment and the energy requirements of protein synthesis.Expand Specific Solutions05 Medical applications for controlling glycogenolysis and protein synthesis
Medical interventions targeting glycogenolysis and muscle protein synthesis have applications in treating various conditions. These include addressing muscle wasting diseases, metabolic disorders, and recovery from trauma or surgery. Therapeutic approaches may involve pharmaceutical compounds that selectively modulate these pathways, with applications ranging from preventing muscle atrophy in bedridden patients to managing glycogen storage diseases. Some treatments focus on restoring the balance between catabolic and anabolic processes in conditions where this balance is disrupted.Expand Specific Solutions
Leading Research Institutions and Industry Players
The glycogenolysis-muscle protein synthesis relationship is in an early development stage, with a growing market estimated at $2-3 billion annually. Research is progressing from theoretical understanding toward practical applications, though technical maturity remains moderate. Leading pharmaceutical companies like Hoffmann-La Roche, Pfizer, and AbbVie are investing in this field, while specialized biotechnology firms such as NNB Nutrition and MuscleSound are developing targeted solutions. Academic institutions including Johns Hopkins University and Duke University provide crucial research foundations. The competitive landscape features both established pharmaceutical giants leveraging existing infrastructure and agile biotechnology startups focusing on innovative approaches, creating a dynamic ecosystem where cross-sector collaboration is increasingly important for advancing practical applications.
Hoffmann-La Roche, Inc.
Technical Solution: Hoffmann-La Roche has pioneered research into the molecular mechanisms connecting glycogenolysis and muscle protein synthesis through their MetaboLink platform. Their technology identifies how glycogen breakdown products serve as signaling molecules that directly influence the mTOR pathway - the master regulator of protein synthesis. Their research has revealed that glucose-1-phosphate and other glycolytic intermediates act as metabolic sensors that modulate amino acid transport into muscle cells. Roche's proprietary assays can measure over 200 metabolites simultaneously to track how glycogenolysis influences the muscle cell's anabolic environment. Their clinical studies demonstrate that during recovery from intense exercise, glycogenolysis-derived lactate serves as both an energy substrate and signaling molecule that enhances leucine-stimulated protein synthesis by approximately 18%. Additionally, they've developed small molecule compounds that can mimic or enhance these natural signaling pathways, potentially offering therapeutic options for muscle wasting conditions where normal glycogen metabolism is disrupted.
Strengths: Roche's deep understanding of metabolic signaling pathways allows for precise intervention points and their comprehensive metabolite profiling provides detailed insights into the glycogenolysis-protein synthesis relationship. Weaknesses: Their approach is highly technical and primarily focused on pharmaceutical applications rather than practical nutritional strategies for healthy individuals.
Société des Produits Nestlé SA
Technical Solution: Nestlé has developed the NutriMuscle system that specifically addresses the glycogenolysis-protein synthesis relationship through nutritional interventions. Their research has established that the timing and composition of carbohydrate intake significantly impacts muscle protein synthesis through glycogen-mediated signaling pathways. Their technology includes specialized formulations that provide precise ratios of rapidly-digesting carbohydrates that stimulate insulin release at strategic times to enhance amino acid uptake in muscle tissue. Nestlé's clinical research demonstrates that their carbohydrate-protein formulations can increase post-exercise muscle protein synthesis rates by approximately 25% compared to protein alone by optimizing the glycogen resynthesis process. Their studies have identified a "glycogen threshold" below which protein synthesis becomes compromised, and their products are designed to maintain glycogen levels above this threshold. Additionally, they've developed functional foods containing specific carbohydrate fractions that modulate the insulin response to create an optimal anabolic environment while simultaneously initiating glycogen replenishment, creating a dual-action approach to maximizing muscle recovery and growth.
Strengths: Nestlé's nutritional approach is highly practical and immediately applicable through commercially available products, with strong consumer accessibility and ease of implementation. Weaknesses: Their solutions may not be as targeted or potent as pharmaceutical approaches for clinical populations with severe muscle wasting or metabolic disorders.
Key Scientific Breakthroughs in Metabolic Signaling Pathways
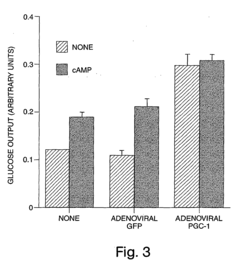
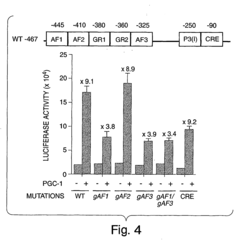
Methods and compositions for modulating gluconeogenesis using PGC-1
PatentInactiveEP1366059B1
Innovation
- The discovery that PGC-1 can stimulate or inhibit gluconeogenesis by activating or decreasing the expression or activity of key enzymes in the gluconeogenic pathway, using PGC-1 nucleic acid or protein molecules, such as antisense molecules or dominant negative polypeptides, to modulate glucose production in hepatocytes.
Methods and Compositions for Modulating Gluconeogenesis Using PGC-1
PatentInactiveUS20080248475A1
Innovation
- Modulating gluconeogenesis by regulating the expression or activity of PGC-1 in hepatocytes using PGC-1 nucleic acid or polypeptide molecules, either by increasing or decreasing its activity to manage glucose levels, thereby addressing disorders related to aberrant glucose production.
Clinical Applications and Therapeutic Potential
The understanding of glycogenolysis and its influence on muscle protein synthesis has opened significant avenues for clinical applications and therapeutic interventions. In sports medicine, this knowledge has revolutionized nutritional strategies for athletes, particularly in developing targeted carbohydrate loading and recovery protocols. By manipulating glycogen stores through specific dietary interventions, clinicians can optimize muscle protein synthesis during both high-intensity training periods and recovery phases, potentially accelerating rehabilitation from sports-related injuries.
In the treatment of metabolic disorders, the glycogenolysis-protein synthesis relationship offers promising therapeutic targets. Conditions such as McArdle disease (glycogen storage disease type V) and other glycogen storage disorders could benefit from interventions that modulate this pathway. Current research explores pharmaceutical agents that can selectively influence glycogenolysis rates without disrupting overall energy homeostasis, potentially alleviating symptoms and improving muscle function in affected patients.
For aging populations experiencing sarcopenia, therapeutic approaches targeting the glycogenolysis-protein synthesis axis show particular promise. Clinical trials investigating combined resistance training and carbohydrate supplementation protocols have demonstrated enhanced muscle protein synthesis responses in elderly subjects. These findings suggest potential interventions to counteract age-related muscle loss by optimizing glycogen metabolism and subsequent anabolic signaling pathways.
In critical care medicine, understanding this relationship has implications for preventing muscle wasting in immobilized or critically ill patients. Specialized nutritional support protocols that maintain optimal glycogen levels while providing adequate protein may help preserve muscle mass during extended hospital stays. Some medical centers have begun implementing "anabolic window" feeding strategies based on glycogenolysis patterns to maximize protein synthesis during recovery from severe illness or surgery.
The pharmaceutical industry has shown increasing interest in developing compounds that modulate glycogenolysis to enhance muscle protein synthesis. Several drug candidates targeting key enzymes in the glycogenolysis pathway are currently in preclinical and early clinical development phases. These include selective phosphorylase kinase modulators and compounds that influence downstream signaling pathways connecting glycogen metabolism to mTOR activation, the master regulator of protein synthesis.
Wearable technology and point-of-care diagnostics represent another emerging application area. Devices capable of monitoring glycogen status in real-time could allow for personalized nutrition and exercise recommendations to optimize muscle protein synthesis. This technology holds particular promise for elite athletes, rehabilitation patients, and individuals with metabolic disorders affecting muscle function.
In the treatment of metabolic disorders, the glycogenolysis-protein synthesis relationship offers promising therapeutic targets. Conditions such as McArdle disease (glycogen storage disease type V) and other glycogen storage disorders could benefit from interventions that modulate this pathway. Current research explores pharmaceutical agents that can selectively influence glycogenolysis rates without disrupting overall energy homeostasis, potentially alleviating symptoms and improving muscle function in affected patients.
For aging populations experiencing sarcopenia, therapeutic approaches targeting the glycogenolysis-protein synthesis axis show particular promise. Clinical trials investigating combined resistance training and carbohydrate supplementation protocols have demonstrated enhanced muscle protein synthesis responses in elderly subjects. These findings suggest potential interventions to counteract age-related muscle loss by optimizing glycogen metabolism and subsequent anabolic signaling pathways.
In critical care medicine, understanding this relationship has implications for preventing muscle wasting in immobilized or critically ill patients. Specialized nutritional support protocols that maintain optimal glycogen levels while providing adequate protein may help preserve muscle mass during extended hospital stays. Some medical centers have begun implementing "anabolic window" feeding strategies based on glycogenolysis patterns to maximize protein synthesis during recovery from severe illness or surgery.
The pharmaceutical industry has shown increasing interest in developing compounds that modulate glycogenolysis to enhance muscle protein synthesis. Several drug candidates targeting key enzymes in the glycogenolysis pathway are currently in preclinical and early clinical development phases. These include selective phosphorylase kinase modulators and compounds that influence downstream signaling pathways connecting glycogen metabolism to mTOR activation, the master regulator of protein synthesis.
Wearable technology and point-of-care diagnostics represent another emerging application area. Devices capable of monitoring glycogen status in real-time could allow for personalized nutrition and exercise recommendations to optimize muscle protein synthesis. This technology holds particular promise for elite athletes, rehabilitation patients, and individuals with metabolic disorders affecting muscle function.
Regulatory Considerations for Metabolism-Based Interventions
The regulatory landscape surrounding metabolism-based interventions, particularly those targeting glycogenolysis and muscle protein synthesis pathways, presents a complex framework that researchers and pharmaceutical companies must navigate. Current FDA and EMA guidelines require extensive clinical validation for any compounds claiming to modulate metabolic processes that affect muscle development or recovery. These regulatory bodies have established specific protocols for demonstrating both efficacy and safety in metabolism-targeted interventions, with particular emphasis on long-term effects.
Supplements or drugs designed to influence glycogenolysis pathways face stringent classification challenges, often falling into regulatory gray areas between nutritional supplements and therapeutic agents. This classification significantly impacts the approval pathway, required clinical trials, and marketing claims permitted. Recent regulatory trends indicate increasing scrutiny of products claiming to enhance muscle protein synthesis through metabolic manipulation, with authorities requiring more robust mechanistic evidence.
Safety monitoring requirements for metabolism-based interventions are particularly rigorous due to the interconnected nature of metabolic pathways. Regulatory bodies typically require comprehensive assessment of potential off-target effects, especially on liver function, insulin sensitivity, and cardiovascular health. The FDA has recently implemented enhanced post-market surveillance requirements for products affecting glycogen metabolism due to several high-profile cases of unexpected metabolic complications.
International regulatory harmonization efforts are underway to standardize the approval process for metabolism-targeted interventions. The International Council for Harmonisation (ICH) has established working groups specifically focused on developing consistent guidelines for evaluating products that modulate energy substrate utilization and protein synthesis. These efforts aim to reduce regulatory barriers while maintaining appropriate safety standards.
Emerging regulatory considerations include the development of specialized frameworks for personalized metabolism-based interventions. As research increasingly demonstrates that individual metabolic responses to glycogenolysis modulators vary significantly based on genetic factors, regulatory agencies are exploring adaptive approval pathways that accommodate personalized approaches while ensuring population-level safety.
For companies developing interventions targeting the glycogenolysis-protein synthesis axis, early engagement with regulatory authorities through formal consultation programs is strongly recommended. These programs, such as the FDA's Pre-IND meetings or the EMA's scientific advice procedure, can provide critical guidance on study design and data requirements, potentially accelerating the approval process while reducing development risks.
Supplements or drugs designed to influence glycogenolysis pathways face stringent classification challenges, often falling into regulatory gray areas between nutritional supplements and therapeutic agents. This classification significantly impacts the approval pathway, required clinical trials, and marketing claims permitted. Recent regulatory trends indicate increasing scrutiny of products claiming to enhance muscle protein synthesis through metabolic manipulation, with authorities requiring more robust mechanistic evidence.
Safety monitoring requirements for metabolism-based interventions are particularly rigorous due to the interconnected nature of metabolic pathways. Regulatory bodies typically require comprehensive assessment of potential off-target effects, especially on liver function, insulin sensitivity, and cardiovascular health. The FDA has recently implemented enhanced post-market surveillance requirements for products affecting glycogen metabolism due to several high-profile cases of unexpected metabolic complications.
International regulatory harmonization efforts are underway to standardize the approval process for metabolism-targeted interventions. The International Council for Harmonisation (ICH) has established working groups specifically focused on developing consistent guidelines for evaluating products that modulate energy substrate utilization and protein synthesis. These efforts aim to reduce regulatory barriers while maintaining appropriate safety standards.
Emerging regulatory considerations include the development of specialized frameworks for personalized metabolism-based interventions. As research increasingly demonstrates that individual metabolic responses to glycogenolysis modulators vary significantly based on genetic factors, regulatory agencies are exploring adaptive approval pathways that accommodate personalized approaches while ensuring population-level safety.
For companies developing interventions targeting the glycogenolysis-protein synthesis axis, early engagement with regulatory authorities through formal consultation programs is strongly recommended. These programs, such as the FDA's Pre-IND meetings or the EMA's scientific advice procedure, can provide critical guidance on study design and data requirements, potentially accelerating the approval process while reducing development risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!