How to Design Glycogenolysis Experiments in Vitro
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Research Background and Objectives
Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, represents a critical metabolic pathway for maintaining blood glucose homeostasis. This process has been extensively studied since the early 20th century, with significant milestones including the discovery of glycogen structure by Claude Bernard in 1857 and the elucidation of the glycogenolysis pathway by Carl and Gerty Cori in the 1930s. The field has evolved from basic understanding of enzymatic mechanisms to sophisticated molecular and cellular approaches for studying regulatory networks.
Recent technological advancements have enabled more precise investigation of glycogenolysis, including real-time monitoring of enzyme activities, high-resolution imaging of glycogen particles, and systems biology approaches to understand pathway integration. The emergence of CRISPR-Cas9 gene editing has further revolutionized our ability to manipulate key enzymes and regulatory proteins involved in this process.
The primary objective of in vitro glycogenolysis experimental design is to establish controlled systems that accurately mimic physiological conditions while allowing precise manipulation of individual variables. These experiments aim to elucidate the molecular mechanisms governing glycogen breakdown, including enzyme kinetics, allosteric regulation, and post-translational modifications that modulate pathway activity.
Current research focuses on several key areas: understanding tissue-specific differences in glycogenolysis regulation, elucidating the role of glycogen phosphorylase isoforms, investigating the impact of glycogen structure on degradation rates, and exploring the interplay between glycogenolysis and other metabolic pathways. Additionally, there is growing interest in how glycogenolysis dysregulation contributes to metabolic disorders such as glycogen storage diseases, diabetes, and certain forms of cancer.
The technological trajectory suggests increasing integration of multi-omics approaches, including proteomics, metabolomics, and computational modeling to create comprehensive views of glycogenolysis regulation. Machine learning algorithms are increasingly being applied to predict enzyme behavior under various conditions, while microfluidic systems enable high-throughput screening of factors affecting glycogen breakdown.
Ultimately, well-designed in vitro glycogenolysis experiments serve as foundational tools for translational research, potentially leading to novel therapeutic strategies for metabolic disorders. The field is moving toward more physiologically relevant experimental systems, including organoid cultures and microfluidic "organ-on-a-chip" platforms that better recapitulate the complexity of living systems while maintaining the controllability of traditional in vitro approaches.
Recent technological advancements have enabled more precise investigation of glycogenolysis, including real-time monitoring of enzyme activities, high-resolution imaging of glycogen particles, and systems biology approaches to understand pathway integration. The emergence of CRISPR-Cas9 gene editing has further revolutionized our ability to manipulate key enzymes and regulatory proteins involved in this process.
The primary objective of in vitro glycogenolysis experimental design is to establish controlled systems that accurately mimic physiological conditions while allowing precise manipulation of individual variables. These experiments aim to elucidate the molecular mechanisms governing glycogen breakdown, including enzyme kinetics, allosteric regulation, and post-translational modifications that modulate pathway activity.
Current research focuses on several key areas: understanding tissue-specific differences in glycogenolysis regulation, elucidating the role of glycogen phosphorylase isoforms, investigating the impact of glycogen structure on degradation rates, and exploring the interplay between glycogenolysis and other metabolic pathways. Additionally, there is growing interest in how glycogenolysis dysregulation contributes to metabolic disorders such as glycogen storage diseases, diabetes, and certain forms of cancer.
The technological trajectory suggests increasing integration of multi-omics approaches, including proteomics, metabolomics, and computational modeling to create comprehensive views of glycogenolysis regulation. Machine learning algorithms are increasingly being applied to predict enzyme behavior under various conditions, while microfluidic systems enable high-throughput screening of factors affecting glycogen breakdown.
Ultimately, well-designed in vitro glycogenolysis experiments serve as foundational tools for translational research, potentially leading to novel therapeutic strategies for metabolic disorders. The field is moving toward more physiologically relevant experimental systems, including organoid cultures and microfluidic "organ-on-a-chip" platforms that better recapitulate the complexity of living systems while maintaining the controllability of traditional in vitro approaches.
Market Applications and Demand for Glycogenolysis Studies
The market for glycogenolysis research spans multiple sectors, with pharmaceutical and biotechnology industries representing the primary demand drivers. These industries actively seek improved understanding of glycogen metabolism to develop treatments for glycogen storage diseases (GSDs), diabetes, and exercise-related metabolic disorders. Current estimates indicate the global market for GSD therapeutics alone exceeds $1.5 billion annually, with a compound annual growth rate of approximately 7% through 2028.
Clinical diagnostics represents another significant market segment, where in vitro glycogenolysis assays serve as valuable tools for diagnosing metabolic disorders. The precision medicine approach to metabolic diseases has created increased demand for standardized glycogenolysis testing protocols that can be implemented in clinical laboratories. This diagnostic segment is projected to grow substantially as personalized medicine continues gaining traction in healthcare systems worldwide.
Sports nutrition and performance enhancement industries have demonstrated growing interest in glycogenolysis research. Companies developing nutritional supplements and training regimens require reliable in vitro models to test how various compounds affect glycogen breakdown during exercise. This market segment has expanded considerably as elite athletics and fitness communities increasingly adopt science-based approaches to performance optimization.
Academic and research institutions constitute a stable market for glycogenolysis experimental tools and protocols. Universities, medical schools, and independent research centers regularly conduct studies requiring reliable in vitro glycogenolysis models. This demand is reflected in publication trends, with over 2,000 research papers addressing aspects of glycogen metabolism published annually.
The pharmaceutical industry specifically seeks standardized in vitro glycogenolysis models for drug discovery and development. High-throughput screening platforms that incorporate glycogenolysis assays enable efficient identification of compounds affecting glycogen metabolism. Several major pharmaceutical companies have established dedicated metabolic disease research programs that rely on such assays.
Emerging applications in artificial organ development and tissue engineering represent a nascent but potentially significant market. Researchers developing bioartificial liver systems and muscle tissue constructs require accurate models of glycogen metabolism to ensure proper tissue function. This specialized segment, while currently small, shows promising growth potential as regenerative medicine advances.
The geographical distribution of market demand shows concentration in North America and Europe, with rapidly growing interest from Asia-Pacific regions, particularly Japan, China, and South Korea, where increasing prevalence of metabolic disorders has prompted greater research investment in glycogen metabolism.
Clinical diagnostics represents another significant market segment, where in vitro glycogenolysis assays serve as valuable tools for diagnosing metabolic disorders. The precision medicine approach to metabolic diseases has created increased demand for standardized glycogenolysis testing protocols that can be implemented in clinical laboratories. This diagnostic segment is projected to grow substantially as personalized medicine continues gaining traction in healthcare systems worldwide.
Sports nutrition and performance enhancement industries have demonstrated growing interest in glycogenolysis research. Companies developing nutritional supplements and training regimens require reliable in vitro models to test how various compounds affect glycogen breakdown during exercise. This market segment has expanded considerably as elite athletics and fitness communities increasingly adopt science-based approaches to performance optimization.
Academic and research institutions constitute a stable market for glycogenolysis experimental tools and protocols. Universities, medical schools, and independent research centers regularly conduct studies requiring reliable in vitro glycogenolysis models. This demand is reflected in publication trends, with over 2,000 research papers addressing aspects of glycogen metabolism published annually.
The pharmaceutical industry specifically seeks standardized in vitro glycogenolysis models for drug discovery and development. High-throughput screening platforms that incorporate glycogenolysis assays enable efficient identification of compounds affecting glycogen metabolism. Several major pharmaceutical companies have established dedicated metabolic disease research programs that rely on such assays.
Emerging applications in artificial organ development and tissue engineering represent a nascent but potentially significant market. Researchers developing bioartificial liver systems and muscle tissue constructs require accurate models of glycogen metabolism to ensure proper tissue function. This specialized segment, while currently small, shows promising growth potential as regenerative medicine advances.
The geographical distribution of market demand shows concentration in North America and Europe, with rapidly growing interest from Asia-Pacific regions, particularly Japan, China, and South Korea, where increasing prevalence of metabolic disorders has prompted greater research investment in glycogen metabolism.
Current Methodologies and Technical Challenges
Glycogenolysis experiments in vitro have evolved significantly over the past decades, with several established methodologies now serving as standard approaches in biochemical research. The primary techniques currently employed include enzyme-based assays, radioisotope labeling, fluorescence-based methods, and more recently, microfluidic systems. Each methodology offers distinct advantages while presenting unique challenges that researchers must navigate.
Enzyme-based assays represent the most traditional approach, typically utilizing phosphorylase a or b to catalyze glycogen breakdown under controlled conditions. These assays monitor either the consumption of inorganic phosphate or the production of glucose-1-phosphate. While reliable and well-documented, these methods often suffer from limited sensitivity and require significant sample volumes, making them less suitable for high-throughput applications or experiments with limited biological material.
Radioisotope labeling techniques, particularly those using 14C or 32P, provide exceptional sensitivity for tracking glycogenolysis pathways. However, these methods face increasing regulatory constraints, require specialized disposal protocols, and present safety concerns that limit their widespread adoption in many research settings. The technical expertise required for handling radioisotopes also creates a barrier to entry for many laboratories.
Fluorescence-based detection methods have gained popularity due to their non-radioactive nature and improved sensitivity. These approaches typically employ fluorescent probes that interact with glycogen or its breakdown products, enabling real-time monitoring of glycogenolysis. Despite these advantages, fluorescence methods can be compromised by background interference, photobleaching, and the potential for probe-induced artifacts that may skew experimental results.
A significant technical challenge across all methodologies is the accurate recreation of physiological conditions in vitro. The complex regulatory mechanisms governing glycogenolysis in vivo—including hormonal control, allosteric regulation, and compartmentalization—are difficult to replicate in test tube environments. This limitation often results in discrepancies between in vitro findings and in vivo reality.
Sample preparation represents another critical challenge, as glycogen's structural integrity must be maintained during extraction and purification. Current protocols frequently result in partial degradation or structural alterations that can affect experimental outcomes. Additionally, the heterogeneity of glycogen structures from different tissue sources introduces variability that complicates standardization efforts.
Recent technological advances have introduced microfluidic and lab-on-a-chip approaches that promise to address some of these limitations by miniaturizing reaction volumes and enabling more precise control over experimental conditions. However, these cutting-edge methods require specialized equipment and expertise that remain inaccessible to many research groups.
Enzyme-based assays represent the most traditional approach, typically utilizing phosphorylase a or b to catalyze glycogen breakdown under controlled conditions. These assays monitor either the consumption of inorganic phosphate or the production of glucose-1-phosphate. While reliable and well-documented, these methods often suffer from limited sensitivity and require significant sample volumes, making them less suitable for high-throughput applications or experiments with limited biological material.
Radioisotope labeling techniques, particularly those using 14C or 32P, provide exceptional sensitivity for tracking glycogenolysis pathways. However, these methods face increasing regulatory constraints, require specialized disposal protocols, and present safety concerns that limit their widespread adoption in many research settings. The technical expertise required for handling radioisotopes also creates a barrier to entry for many laboratories.
Fluorescence-based detection methods have gained popularity due to their non-radioactive nature and improved sensitivity. These approaches typically employ fluorescent probes that interact with glycogen or its breakdown products, enabling real-time monitoring of glycogenolysis. Despite these advantages, fluorescence methods can be compromised by background interference, photobleaching, and the potential for probe-induced artifacts that may skew experimental results.
A significant technical challenge across all methodologies is the accurate recreation of physiological conditions in vitro. The complex regulatory mechanisms governing glycogenolysis in vivo—including hormonal control, allosteric regulation, and compartmentalization—are difficult to replicate in test tube environments. This limitation often results in discrepancies between in vitro findings and in vivo reality.
Sample preparation represents another critical challenge, as glycogen's structural integrity must be maintained during extraction and purification. Current protocols frequently result in partial degradation or structural alterations that can affect experimental outcomes. Additionally, the heterogeneity of glycogen structures from different tissue sources introduces variability that complicates standardization efforts.
Recent technological advances have introduced microfluidic and lab-on-a-chip approaches that promise to address some of these limitations by miniaturizing reaction volumes and enabling more precise control over experimental conditions. However, these cutting-edge methods require specialized equipment and expertise that remain inaccessible to many research groups.
Established In Vitro Glycogenolysis Experimental Protocols
01 Inhibitors of glycogenolysis for treating metabolic disorders
Various compounds have been developed as inhibitors of glycogenolysis to treat metabolic disorders such as diabetes and obesity. These inhibitors work by preventing the breakdown of glycogen into glucose, thereby helping to regulate blood glucose levels. The inhibition of glycogenolysis represents a therapeutic approach for managing conditions characterized by dysregulated glucose metabolism.- Inhibitors of glycogenolysis for therapeutic applications: Various compounds have been developed as inhibitors of glycogenolysis for treating conditions related to abnormal glucose metabolism. These inhibitors target enzymes involved in the breakdown of glycogen, such as glycogen phosphorylase, to help regulate blood glucose levels. Such compounds are particularly useful in treating diabetes, obesity, and other metabolic disorders by preventing excessive glucose release from glycogen stores in the liver.
- Diagnostic methods for monitoring glycogenolysis: Diagnostic techniques have been developed to monitor glycogenolysis activity in biological samples. These methods include assays for measuring glycogen phosphorylase activity, glucose release rates, and other biomarkers associated with glycogen breakdown. Such diagnostic approaches are valuable for assessing metabolic disorders, liver function, and the efficacy of treatments targeting glycogenolysis pathways.
- Exercise-induced glycogenolysis regulation: Research has focused on understanding and modulating glycogenolysis during physical exercise. Various compounds and methods have been developed to optimize glycogen utilization during exercise, enhance athletic performance, and improve recovery. These approaches target the signaling pathways that regulate glycogen breakdown in muscle tissue during physical activity, aiming to improve energy availability and reduce fatigue.
- Glycogenolysis in neurological function and disorders: Studies have investigated the role of glycogenolysis in brain function and neurological disorders. Glycogen serves as an important energy reserve in astrocytes, and its breakdown through glycogenolysis provides energy substrates for neurons during periods of high activity or metabolic stress. Modulation of brain glycogenolysis has been explored as a potential therapeutic approach for conditions such as epilepsy, stroke, and neurodegenerative diseases.
- Pharmaceutical compositions targeting glycogenolysis pathways: Novel pharmaceutical compositions have been developed to target specific aspects of glycogenolysis pathways. These formulations include various delivery systems, combination therapies, and targeted approaches to modulate glycogen breakdown in specific tissues. The compositions are designed to improve efficacy, reduce side effects, and enhance patient compliance in treating conditions associated with dysregulated glycogenolysis, such as diabetes, glycogen storage diseases, and certain forms of hypoglycemia.
02 Glycogenolysis regulation in muscle tissue
Research has focused on understanding and controlling glycogenolysis in muscle tissue, particularly during exercise and recovery. Compounds that modulate glycogenolysis in skeletal muscle can influence energy availability, physical performance, and muscle fatigue. These developments have applications in sports medicine, treatment of muscle disorders, and management of exercise-related metabolic responses.Expand Specific Solutions03 Diagnostic methods for glycogenolysis disorders
Novel diagnostic techniques have been developed to identify and characterize disorders related to glycogenolysis pathways. These methods include biomarkers, genetic testing, and imaging techniques that can detect abnormalities in glycogen metabolism. Early and accurate diagnosis of glycogenolysis disorders enables timely intervention and appropriate management strategies for affected individuals.Expand Specific Solutions04 Glycogenolysis modulation for liver diseases
Targeting glycogenolysis pathways in the liver has emerged as a therapeutic strategy for various hepatic conditions. Compounds that regulate hepatic glycogenolysis can help manage liver diseases characterized by abnormal glucose production and metabolism. These approaches aim to restore normal liver function by controlling the rate at which stored glycogen is converted to glucose.Expand Specific Solutions05 Combined approaches targeting glycogenolysis and related metabolic pathways
Integrated therapeutic strategies have been developed that simultaneously target glycogenolysis and related metabolic pathways such as gluconeogenesis or glycolysis. These multi-target approaches provide more comprehensive control of glucose metabolism and energy homeostasis. By addressing multiple aspects of carbohydrate metabolism, these combined interventions may offer superior efficacy in treating complex metabolic disorders.Expand Specific Solutions
Key Enzymatic Mechanisms and Regulatory Pathways
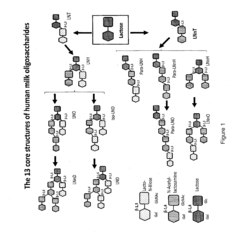
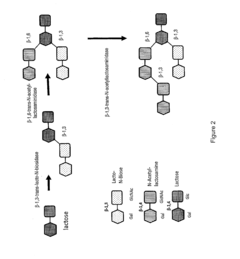
DIVERSIFICATION OF HUMAN MILK OLIGOSACCHARIDES (HMOs) OR PRECURSORS THEREOF
PatentActiveUS20160289721A1
Innovation
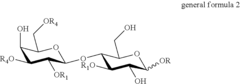
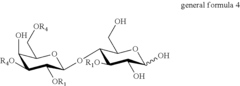
- A method involving the use of sialylated and fucosylated lactose derivatives and specific enzymes like transglycosidases to synthesize HMOs, allowing for the production of diverse oligosaccharides with 4-12 saccharide units through a series of incubation and hydrogenolysis steps.
Methods and polynucleotides involved in glycosylation of proteins
PatentWO2022207676A3
Innovation
- Development of an in vitro method for modifying glycosylation patterns of glycoproteins using immobilized and biotinylated enzymes, enabling more controlled and efficient glycan modification.
- Creation of a novel expression system combining solubility tag, catalytic domain, linker, and AviTag with co-expression of BirA enzyme for efficient production of biotinylated glycosylation enzymes.
- Design of a modular approach for glycan modification that separates the catalytic domain from immobilization components, potentially allowing for greater flexibility in glycoengineering applications.
Translational Potential from In Vitro to In Vivo Systems
The translation of glycogenolysis experimental findings from in vitro systems to in vivo applications represents a critical bridge between laboratory research and clinical relevance. In vitro glycogenolysis experiments provide controlled environments for studying enzymatic mechanisms, regulatory pathways, and molecular interactions, but their ultimate value lies in their ability to inform in vivo applications and therapeutic strategies.
The primary challenge in translational research for glycogenolysis lies in accounting for the complex physiological context absent in vitro. In living systems, glycogenolysis operates within intricate hormonal regulation networks, responds to metabolic demands across multiple tissues, and functions under dynamic conditions that are difficult to replicate in laboratory settings. Successful translation requires systematic validation across increasingly complex models, progressing from cell cultures to organoids, ex vivo tissue preparations, and finally animal models before clinical applications.
Recent advances in microfluidic systems and organ-on-chip technologies have significantly improved translational potential by creating more physiologically relevant in vitro environments. These platforms incorporate multiple cell types, fluid flow, and mechanical forces that better mimic in vivo conditions, allowing researchers to observe glycogenolysis in contexts that more accurately reflect living systems. Such intermediate models serve as valuable bridges between traditional in vitro experiments and animal studies.
Biomarker development represents another crucial aspect of translational research. Identifying reliable indicators of glycogenolysis activity that can be measured both in vitro and in vivo enables researchers to establish correlations between laboratory findings and physiological outcomes. Metabolomic profiles, enzyme activity signatures, and glycogen-derived metabolites serve as translational biomarkers that can be tracked across experimental platforms.
The pharmaceutical industry has successfully leveraged in vitro glycogenolysis research for drug development targeting metabolic disorders, glycogen storage diseases, and diabetes. Compounds that demonstrate efficacy in modulating glycogenolysis pathways in vitro undergo progressive testing in more complex systems, with careful attention to pharmacokinetics, tissue distribution, and systemic effects that cannot be fully predicted from initial laboratory studies.
Mathematical modeling and computational approaches further enhance translational potential by integrating data from multiple experimental systems. These models can predict how molecular mechanisms observed in vitro might manifest under various physiological conditions, accounting for factors like tissue heterogeneity, temporal dynamics, and whole-body metabolic integration that influence glycogenolysis in living organisms.
The primary challenge in translational research for glycogenolysis lies in accounting for the complex physiological context absent in vitro. In living systems, glycogenolysis operates within intricate hormonal regulation networks, responds to metabolic demands across multiple tissues, and functions under dynamic conditions that are difficult to replicate in laboratory settings. Successful translation requires systematic validation across increasingly complex models, progressing from cell cultures to organoids, ex vivo tissue preparations, and finally animal models before clinical applications.
Recent advances in microfluidic systems and organ-on-chip technologies have significantly improved translational potential by creating more physiologically relevant in vitro environments. These platforms incorporate multiple cell types, fluid flow, and mechanical forces that better mimic in vivo conditions, allowing researchers to observe glycogenolysis in contexts that more accurately reflect living systems. Such intermediate models serve as valuable bridges between traditional in vitro experiments and animal studies.
Biomarker development represents another crucial aspect of translational research. Identifying reliable indicators of glycogenolysis activity that can be measured both in vitro and in vivo enables researchers to establish correlations between laboratory findings and physiological outcomes. Metabolomic profiles, enzyme activity signatures, and glycogen-derived metabolites serve as translational biomarkers that can be tracked across experimental platforms.
The pharmaceutical industry has successfully leveraged in vitro glycogenolysis research for drug development targeting metabolic disorders, glycogen storage diseases, and diabetes. Compounds that demonstrate efficacy in modulating glycogenolysis pathways in vitro undergo progressive testing in more complex systems, with careful attention to pharmacokinetics, tissue distribution, and systemic effects that cannot be fully predicted from initial laboratory studies.
Mathematical modeling and computational approaches further enhance translational potential by integrating data from multiple experimental systems. These models can predict how molecular mechanisms observed in vitro might manifest under various physiological conditions, accounting for factors like tissue heterogeneity, temporal dynamics, and whole-body metabolic integration that influence glycogenolysis in living organisms.
Reproducibility and Standardization Considerations
The reproducibility crisis in scientific research has significantly impacted the field of glycogenolysis experimentation. Establishing robust standardization protocols is essential for ensuring that in vitro glycogenolysis experiments yield consistent, reliable, and comparable results across different laboratories. This consideration becomes particularly critical when studying complex enzymatic cascades involved in glycogen breakdown pathways.
A fundamental aspect of reproducibility in glycogenolysis experiments involves the standardization of biological materials. The source, purity, and preparation methods of glycogen samples significantly influence experimental outcomes. Commercial glycogen preparations often contain varying degrees of branching and molecular weights, which can substantially affect enzyme accessibility and reaction kinetics. Implementing standardized protocols for glycogen preparation or utilizing certified reference materials can mitigate these variations.
Enzyme preparations represent another critical variable requiring standardization. Phosphorylase kinase, glycogen phosphorylase, and debranching enzymes should be characterized for specific activity, purity, and stability before experimentation. Batch-to-batch variations in enzyme preparations can lead to inconsistent results, necessitating thorough quality control measures and detailed reporting of enzyme sources and preparation methods.
Buffer composition, pH, temperature, and ionic strength significantly impact glycogenolysis reactions. Minor variations in these parameters can dramatically alter enzyme activity and substrate accessibility. Detailed documentation of reaction conditions, including temperature control methods and buffer preparation protocols, is essential for experimental reproducibility. The use of automated systems for maintaining consistent reaction conditions can further enhance reproducibility.
Analytical methods for measuring glycogenolysis products require standardization as well. Whether utilizing colorimetric assays, HPLC, mass spectrometry, or enzymatic coupled assays, the sensitivity, specificity, and calibration of detection methods should be thoroughly validated and reported. Interlaboratory comparisons using identical samples can help identify methodological discrepancies and establish consensus protocols.
Data reporting standards represent a final critical consideration. Comprehensive documentation should include raw data, statistical analyses, and detailed methodological descriptions. The adoption of minimum information guidelines specific to glycogenolysis experiments would facilitate meaningful comparison of results across studies. Additionally, repositories for standardized protocols and experimental data would enhance transparency and reproducibility in the field.
Implementation of these standardization measures requires collaborative efforts across the research community, potentially through consensus workshops or specialized working groups focused on glycogenolysis methodologies. Such initiatives would accelerate progress in understanding glycogen metabolism while ensuring that experimental findings translate effectively from bench to clinical applications.
A fundamental aspect of reproducibility in glycogenolysis experiments involves the standardization of biological materials. The source, purity, and preparation methods of glycogen samples significantly influence experimental outcomes. Commercial glycogen preparations often contain varying degrees of branching and molecular weights, which can substantially affect enzyme accessibility and reaction kinetics. Implementing standardized protocols for glycogen preparation or utilizing certified reference materials can mitigate these variations.
Enzyme preparations represent another critical variable requiring standardization. Phosphorylase kinase, glycogen phosphorylase, and debranching enzymes should be characterized for specific activity, purity, and stability before experimentation. Batch-to-batch variations in enzyme preparations can lead to inconsistent results, necessitating thorough quality control measures and detailed reporting of enzyme sources and preparation methods.
Buffer composition, pH, temperature, and ionic strength significantly impact glycogenolysis reactions. Minor variations in these parameters can dramatically alter enzyme activity and substrate accessibility. Detailed documentation of reaction conditions, including temperature control methods and buffer preparation protocols, is essential for experimental reproducibility. The use of automated systems for maintaining consistent reaction conditions can further enhance reproducibility.
Analytical methods for measuring glycogenolysis products require standardization as well. Whether utilizing colorimetric assays, HPLC, mass spectrometry, or enzymatic coupled assays, the sensitivity, specificity, and calibration of detection methods should be thoroughly validated and reported. Interlaboratory comparisons using identical samples can help identify methodological discrepancies and establish consensus protocols.
Data reporting standards represent a final critical consideration. Comprehensive documentation should include raw data, statistical analyses, and detailed methodological descriptions. The adoption of minimum information guidelines specific to glycogenolysis experiments would facilitate meaningful comparison of results across studies. Additionally, repositories for standardized protocols and experimental data would enhance transparency and reproducibility in the field.
Implementation of these standardization measures requires collaborative efforts across the research community, potentially through consensus workshops or specialized working groups focused on glycogenolysis methodologies. Such initiatives would accelerate progress in understanding glycogen metabolism while ensuring that experimental findings translate effectively from bench to clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!