Optimizing Glycogenolysis for Disease Management
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Technology Background and Objectives
Glycogenolysis, the metabolic process of breaking down glycogen into glucose-1-phosphate and glucose, has emerged as a critical pathway in understanding and managing various metabolic disorders. The historical development of glycogenolysis research dates back to the early 20th century when scientists first identified the enzymatic processes involved in carbohydrate metabolism. Over the decades, our understanding has evolved from basic biochemical pathways to complex regulatory mechanisms that influence numerous physiological and pathological conditions.
The technological evolution in this field has accelerated dramatically in recent years, driven by advances in molecular biology, genetic engineering, and computational modeling. These developments have enabled researchers to elucidate the intricate signaling cascades that regulate glycogenolysis, including hormonal control mechanisms and cellular energy sensors that respond to metabolic demands.
Current trends in glycogenolysis technology focus on precision medicine approaches, where individual genetic variations in glycogen metabolism pathways are considered for personalized treatment strategies. The integration of artificial intelligence and machine learning algorithms has further enhanced our ability to predict metabolic responses and optimize therapeutic interventions based on patient-specific factors.
The primary objectives of optimizing glycogenolysis for disease management are multifaceted. First, to develop targeted interventions that can modulate glycogen breakdown rates in conditions such as glycogen storage diseases, diabetes, and certain forms of hypoglycemia. Second, to establish reliable biomarkers that can accurately reflect glycogenolysis activity in vivo, enabling real-time monitoring and adjustment of therapeutic approaches.
Additionally, researchers aim to design novel pharmaceutical compounds that can selectively influence specific enzymes within the glycogenolysis pathway, minimizing off-target effects while maximizing therapeutic benefits. The development of non-invasive monitoring technologies represents another crucial objective, allowing for continuous assessment of glycogen metabolism without requiring frequent tissue sampling.
From a broader perspective, optimizing glycogenolysis technology seeks to bridge the gap between laboratory research and clinical applications, translating molecular insights into practical therapeutic strategies. This includes the development of standardized protocols for assessing glycogenolysis efficiency in clinical settings and establishing normative data across diverse populations.
The ultimate goal extends beyond symptom management to addressing the root causes of dysregulated glycogen metabolism, potentially offering curative approaches for previously intractable conditions. As we advance our understanding of the interplay between glycogenolysis and other metabolic pathways, opportunities emerge for holistic interventions that restore metabolic homeostasis and improve patient outcomes across a spectrum of diseases.
The technological evolution in this field has accelerated dramatically in recent years, driven by advances in molecular biology, genetic engineering, and computational modeling. These developments have enabled researchers to elucidate the intricate signaling cascades that regulate glycogenolysis, including hormonal control mechanisms and cellular energy sensors that respond to metabolic demands.
Current trends in glycogenolysis technology focus on precision medicine approaches, where individual genetic variations in glycogen metabolism pathways are considered for personalized treatment strategies. The integration of artificial intelligence and machine learning algorithms has further enhanced our ability to predict metabolic responses and optimize therapeutic interventions based on patient-specific factors.
The primary objectives of optimizing glycogenolysis for disease management are multifaceted. First, to develop targeted interventions that can modulate glycogen breakdown rates in conditions such as glycogen storage diseases, diabetes, and certain forms of hypoglycemia. Second, to establish reliable biomarkers that can accurately reflect glycogenolysis activity in vivo, enabling real-time monitoring and adjustment of therapeutic approaches.
Additionally, researchers aim to design novel pharmaceutical compounds that can selectively influence specific enzymes within the glycogenolysis pathway, minimizing off-target effects while maximizing therapeutic benefits. The development of non-invasive monitoring technologies represents another crucial objective, allowing for continuous assessment of glycogen metabolism without requiring frequent tissue sampling.
From a broader perspective, optimizing glycogenolysis technology seeks to bridge the gap between laboratory research and clinical applications, translating molecular insights into practical therapeutic strategies. This includes the development of standardized protocols for assessing glycogenolysis efficiency in clinical settings and establishing normative data across diverse populations.
The ultimate goal extends beyond symptom management to addressing the root causes of dysregulated glycogen metabolism, potentially offering curative approaches for previously intractable conditions. As we advance our understanding of the interplay between glycogenolysis and other metabolic pathways, opportunities emerge for holistic interventions that restore metabolic homeostasis and improve patient outcomes across a spectrum of diseases.
Market Analysis for Glycogenolysis-Based Therapeutics
The global market for glycogenolysis-based therapeutics is experiencing significant growth, driven by the increasing prevalence of glycogen storage diseases (GSDs) and related metabolic disorders. Current market valuations indicate that the glycogen metabolism therapeutics sector reached approximately 3.2 billion USD in 2022, with projections suggesting a compound annual growth rate of 7.8% through 2030. This growth trajectory is supported by rising diagnosis rates and expanding treatment options across developed healthcare markets.
North America currently dominates the market landscape, accounting for roughly 42% of global revenue, followed by Europe at 31% and Asia-Pacific at 18%. This regional distribution reflects both the prevalence of targeted diseases and healthcare expenditure patterns. The United States specifically represents the largest single-country market, driven by advanced diagnostic capabilities and favorable reimbursement policies for rare disease treatments.
Market segmentation reveals distinct therapeutic approaches gaining traction. Enzyme replacement therapies constitute approximately 56% of current market share, while substrate reduction therapies and gene therapies represent 28% and 12% respectively. Small molecule approaches targeting glycogenolysis pathways account for the remaining market share but are expected to grow substantially as research advances.
Key disease indications driving market demand include Pompe disease, von Gierke disease (GSD type I), Cori disease (GSD type III), and McArdle disease (GSD type V). Among these, Pompe disease therapeutics generate the highest revenue, estimated at 1.4 billion USD annually, due to established enzyme replacement options and relatively higher diagnosis rates.
Patient demographics reveal that pediatric applications currently dominate the market at 65%, reflecting the early onset nature of many GSDs. However, adult treatment segments are growing at a faster rate (9.2% annually) as diagnostic improvements identify previously undiagnosed cases and treatment longevity increases.
Pricing analysis indicates significant regional variations, with average annual treatment costs ranging from 150,000 to 300,000 USD in the United States, compared to 120,000 to 220,000 USD in European markets. These high costs reflect the rare disease status of most glycogenolysis-related conditions and the complex manufacturing processes involved in biologic therapies.
Market barriers include limited awareness among general practitioners, diagnostic challenges, and reimbursement hurdles. However, these are increasingly offset by growing rare disease advocacy, improved genetic testing accessibility, and expanding specialty pharmacy networks. The orphan drug designation pathway has proven particularly valuable for market entrants, providing regulatory incentives and extended market exclusivity periods.
North America currently dominates the market landscape, accounting for roughly 42% of global revenue, followed by Europe at 31% and Asia-Pacific at 18%. This regional distribution reflects both the prevalence of targeted diseases and healthcare expenditure patterns. The United States specifically represents the largest single-country market, driven by advanced diagnostic capabilities and favorable reimbursement policies for rare disease treatments.
Market segmentation reveals distinct therapeutic approaches gaining traction. Enzyme replacement therapies constitute approximately 56% of current market share, while substrate reduction therapies and gene therapies represent 28% and 12% respectively. Small molecule approaches targeting glycogenolysis pathways account for the remaining market share but are expected to grow substantially as research advances.
Key disease indications driving market demand include Pompe disease, von Gierke disease (GSD type I), Cori disease (GSD type III), and McArdle disease (GSD type V). Among these, Pompe disease therapeutics generate the highest revenue, estimated at 1.4 billion USD annually, due to established enzyme replacement options and relatively higher diagnosis rates.
Patient demographics reveal that pediatric applications currently dominate the market at 65%, reflecting the early onset nature of many GSDs. However, adult treatment segments are growing at a faster rate (9.2% annually) as diagnostic improvements identify previously undiagnosed cases and treatment longevity increases.
Pricing analysis indicates significant regional variations, with average annual treatment costs ranging from 150,000 to 300,000 USD in the United States, compared to 120,000 to 220,000 USD in European markets. These high costs reflect the rare disease status of most glycogenolysis-related conditions and the complex manufacturing processes involved in biologic therapies.
Market barriers include limited awareness among general practitioners, diagnostic challenges, and reimbursement hurdles. However, these are increasingly offset by growing rare disease advocacy, improved genetic testing accessibility, and expanding specialty pharmacy networks. The orphan drug designation pathway has proven particularly valuable for market entrants, providing regulatory incentives and extended market exclusivity periods.
Current Challenges in Glycogenolysis Regulation
Despite significant advances in understanding glycogenolysis pathways, several critical challenges persist in effectively regulating this process for disease management. The primary obstacle remains the complexity of the glycogenolysis cascade, which involves multiple enzymes, regulatory proteins, and signaling pathways that operate in tissue-specific manners. This intricate network makes targeted intervention difficult without causing unintended systemic effects.
A major technical hurdle involves achieving temporal precision in glycogenolysis regulation. Current pharmacological approaches often lack the ability to mimic the body's natural rhythmic control of glucose release, resulting in suboptimal outcomes for conditions like glycogen storage diseases and diabetes. The development of medications with appropriate pharmacokinetic profiles that can provide sustained yet responsive regulation remains elusive.
Tissue selectivity presents another significant challenge. Glycogenolysis occurs differently in the liver, muscle, and brain, each with unique regulatory mechanisms. Current therapeutic approaches struggle to target specific tissues without affecting others, leading to unwanted side effects. For instance, agents targeting hepatic glycogenolysis may inadvertently impact muscle glycogen metabolism, potentially causing exercise intolerance or muscle weakness.
The heterogeneity of patient responses further complicates glycogenolysis regulation. Genetic variations in enzymes like glycogen phosphorylase and regulatory proteins create diverse patient phenotypes that respond differently to standardized interventions. This genetic variability necessitates personalized approaches that current technologies cannot adequately address.
Monitoring glycogenolysis activity in real-time represents a substantial technical limitation. Current diagnostic methods provide only snapshots of glycogen metabolism rather than continuous assessment, hampering the development of responsive therapeutic systems. The lack of non-invasive biomarkers that accurately reflect tissue-specific glycogenolysis activity impedes both research progress and clinical management.
Emerging challenges also include understanding the interplay between glycogenolysis and other metabolic pathways. Recent research suggests complex crosstalk between glycogen metabolism and lipid utilization, inflammatory processes, and cellular stress responses. These interactions create feedback loops that can undermine therapeutic interventions if not properly accounted for in treatment strategies.
Finally, translating laboratory findings to clinical applications faces significant regulatory and technological barriers. Many promising compounds that effectively modulate glycogenolysis in vitro or in animal models fail to demonstrate similar efficacy or safety in human trials, highlighting the need for better predictive models and translational approaches.
A major technical hurdle involves achieving temporal precision in glycogenolysis regulation. Current pharmacological approaches often lack the ability to mimic the body's natural rhythmic control of glucose release, resulting in suboptimal outcomes for conditions like glycogen storage diseases and diabetes. The development of medications with appropriate pharmacokinetic profiles that can provide sustained yet responsive regulation remains elusive.
Tissue selectivity presents another significant challenge. Glycogenolysis occurs differently in the liver, muscle, and brain, each with unique regulatory mechanisms. Current therapeutic approaches struggle to target specific tissues without affecting others, leading to unwanted side effects. For instance, agents targeting hepatic glycogenolysis may inadvertently impact muscle glycogen metabolism, potentially causing exercise intolerance or muscle weakness.
The heterogeneity of patient responses further complicates glycogenolysis regulation. Genetic variations in enzymes like glycogen phosphorylase and regulatory proteins create diverse patient phenotypes that respond differently to standardized interventions. This genetic variability necessitates personalized approaches that current technologies cannot adequately address.
Monitoring glycogenolysis activity in real-time represents a substantial technical limitation. Current diagnostic methods provide only snapshots of glycogen metabolism rather than continuous assessment, hampering the development of responsive therapeutic systems. The lack of non-invasive biomarkers that accurately reflect tissue-specific glycogenolysis activity impedes both research progress and clinical management.
Emerging challenges also include understanding the interplay between glycogenolysis and other metabolic pathways. Recent research suggests complex crosstalk between glycogen metabolism and lipid utilization, inflammatory processes, and cellular stress responses. These interactions create feedback loops that can undermine therapeutic interventions if not properly accounted for in treatment strategies.
Finally, translating laboratory findings to clinical applications faces significant regulatory and technological barriers. Many promising compounds that effectively modulate glycogenolysis in vitro or in animal models fail to demonstrate similar efficacy or safety in human trials, highlighting the need for better predictive models and translational approaches.
Current Therapeutic Approaches Targeting Glycogenolysis
01 Pharmaceutical compositions for glycogenolysis regulation
Pharmaceutical formulations designed to regulate glycogenolysis processes in the body, including compounds that can either stimulate or inhibit the breakdown of glycogen. These compositions may contain active ingredients that target specific enzymes involved in the glycogenolysis pathway, such as glycogen phosphorylase. The formulations are optimized for bioavailability and efficacy in managing conditions related to abnormal glucose metabolism.- Pharmaceutical compositions for glycogenolysis regulation: Various pharmaceutical compositions have been developed to optimize glycogenolysis processes in the body. These formulations typically contain active ingredients that can either stimulate or inhibit glycogen breakdown depending on the therapeutic need. Some compositions include enzyme modulators that directly affect the activity of glycogen phosphorylase, while others contain compounds that influence hormonal pathways regulating glycogenolysis. These pharmaceutical approaches are particularly relevant for treating metabolic disorders like diabetes and glycogen storage diseases.
- Computational methods for glycogenolysis pathway analysis: Advanced computational algorithms and models have been developed to analyze and optimize glycogenolysis pathways. These methods utilize machine learning, artificial intelligence, and simulation techniques to predict enzyme behavior, metabolic flux, and pathway efficiency. By modeling the complex interactions between enzymes, substrates, and regulatory molecules involved in glycogen breakdown, researchers can identify rate-limiting steps and potential targets for intervention. These computational approaches enable more efficient drug discovery and development of personalized treatment strategies for disorders affecting glycogen metabolism.
- Exercise and nutritional strategies for glycogenolysis enhancement: Various exercise protocols and nutritional interventions have been developed to optimize glycogenolysis during physical activity. These approaches focus on maximizing energy availability during exercise while preserving glycogen stores for prolonged performance. Specific training regimens can enhance the body's ability to mobilize glycogen efficiently, while certain nutritional supplements can activate key enzymes in the glycogenolysis pathway. Timing of nutrient intake relative to exercise can also significantly impact glycogen utilization efficiency and overall metabolic performance.
- Monitoring systems for glycogenolysis optimization: Advanced monitoring systems have been developed to track glycogenolysis processes in real-time. These systems typically combine biosensors, wearable devices, and data analytics to provide continuous feedback on glycogen breakdown rates and metabolic parameters. Some monitoring approaches use non-invasive techniques to measure biomarkers associated with glycogenolysis, while others employ implantable sensors that directly detect metabolic intermediates. The collected data can be used to optimize treatment protocols, adjust medication dosages, or fine-tune nutritional and exercise interventions for individuals with metabolic disorders.
- Genetic and cellular engineering for glycogenolysis pathway modification: Innovative genetic and cellular engineering techniques have been applied to modify and optimize glycogenolysis pathways. These approaches include gene editing to enhance or suppress the expression of key enzymes involved in glycogen breakdown, as well as the development of engineered cells with modified metabolic capabilities. Some methods focus on correcting genetic defects associated with glycogen storage diseases, while others aim to create cellular systems with enhanced glycogenolysis efficiency for biotechnological applications. These genetic engineering strategies offer potential for both therapeutic interventions and industrial bioprocessing optimization.
02 Computational methods for metabolic pathway optimization
Advanced computational algorithms and models designed to analyze and optimize metabolic pathways, including glycogenolysis. These methods utilize machine learning, artificial intelligence, and simulation techniques to predict the effects of various interventions on glycogen breakdown efficiency. The computational approaches help in identifying optimal conditions for glycogenolysis and can be applied in both pharmaceutical research and clinical settings.Expand Specific Solutions03 Monitoring systems for glycogenolysis parameters
Systems and devices developed for real-time monitoring of glycogenolysis-related parameters in biological systems. These technologies include sensors, wearable devices, and integrated systems that can track glucose levels, enzyme activities, and other biomarkers associated with glycogen metabolism. The monitoring systems provide valuable data for optimizing glycogenolysis processes in various applications, including sports medicine and diabetes management.Expand Specific Solutions04 Process optimization techniques for industrial glycogenolysis
Methodologies and systems for optimizing glycogenolysis processes in industrial settings, such as biofuel production or enzyme manufacturing. These techniques focus on maximizing yield, efficiency, and cost-effectiveness of glycogen breakdown processes. The optimization approaches include parameter tuning, process control strategies, and innovative reactor designs that enhance the industrial application of glycogenolysis.Expand Specific Solutions05 Integrated systems for glycogenolysis control in medical applications
Comprehensive systems that integrate monitoring, analysis, and intervention components for controlling glycogenolysis in medical applications. These systems combine hardware and software elements to provide holistic management of glycogen metabolism in patients with metabolic disorders. The integrated approach allows for personalized optimization of glycogenolysis based on individual patient characteristics and treatment goals.Expand Specific Solutions
Key Industry Players in Glycogen Metabolism Research
The glycogenolysis optimization market for disease management is currently in a growth phase, with increasing recognition of its therapeutic potential across metabolic, cardiovascular, and rare diseases. The global market is expanding steadily, driven by rising prevalence of glycogen storage disorders and diabetes. Technologically, the field shows moderate maturity with established players like Novo Nordisk leading in diabetes applications, while companies such as Ionis Pharmaceuticals and Xenon Pharmaceuticals advance novel therapeutic approaches. Research institutions including Duke University and Institut National de la Santé et de la Recherche Médicale contribute significant innovations. Diagnostic technology leaders DexCom and Abbott Diabetes Care are enhancing monitoring capabilities, while emerging players like Maze Therapeutics and Viking Therapeutics are developing precision medicine approaches targeting specific glycogenolysis pathways, indicating a competitive landscape with diverse technological approaches.
Novo Nordisk A/S
Technical Solution: Novo Nordisk has developed a comprehensive approach to optimizing glycogenolysis for diabetes management through their GlycoControl platform. This technology targets hepatic glucose production by modulating glycogen phosphorylase, the rate-limiting enzyme in glycogenolysis. Their proprietary small molecule inhibitors selectively bind to the allosteric site of glycogen phosphorylase, reducing excessive glucose release from liver glycogen stores. Clinical trials have demonstrated that these inhibitors can reduce fasting plasma glucose levels by up to 30% in patients with type 2 diabetes. Novo Nordisk has also pioneered combination therapies that pair glycogen phosphorylase inhibitors with GLP-1 receptor agonists, creating a dual-action approach that addresses both hepatic glucose output and insulin sensitivity. Their latest generation compounds show improved bioavailability and tissue selectivity, minimizing off-target effects in cardiac and skeletal muscle tissue where glycogenolysis serves critical physiological functions.
Strengths: Extensive experience in diabetes therapeutics provides strong clinical development infrastructure; established market presence facilitates rapid commercialization of successful compounds. Weaknesses: Potential for drug interactions with other diabetes medications; challenges in achieving optimal tissue selectivity may limit therapeutic window.
Ionis Pharmaceuticals, Inc.
Technical Solution: Ionis Pharmaceuticals has pioneered an antisense oligonucleotide (ASO) approach to modulating glycogenolysis for disease management. Their platform specifically targets liver glycogen phosphorylase mRNA, reducing enzyme expression and consequently decreasing hepatic glucose output. The company's proprietary LICA (Ligand Conjugated Antisense) technology enhances liver-specific delivery, increasing potency while minimizing systemic exposure. In preclinical models of glycogen storage diseases, their lead compound IONIS-GPase-LRx demonstrated a 65% reduction in glycogen phosphorylase expression with corresponding improvements in glycemic control. The ASO approach offers extended duration of action, with biweekly or monthly dosing potential compared to daily administration required for small molecule inhibitors. Ionis has also developed companion diagnostic tools that measure glycogen phosphorylase activity in patient samples, enabling personalized dosing strategies. Their pipeline includes second-generation compounds with enhanced stability profiles and reduced immunogenicity, addressing key challenges in oligonucleotide therapeutics.
Strengths: Highly specific targeting mechanism minimizes off-target effects; extended duration of action improves patient compliance; potential for precise dose titration based on individual patient response. Weaknesses: Higher manufacturing costs compared to small molecule approaches; potential immunogenicity concerns with repeated administration; limited tissue distribution beyond liver.
Critical Enzymes and Signaling Mechanisms Analysis
Methods and compositions for the treatment of polyglucosan disorders
PatentInactiveJP2021518840A
Innovation
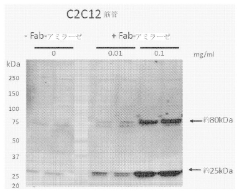
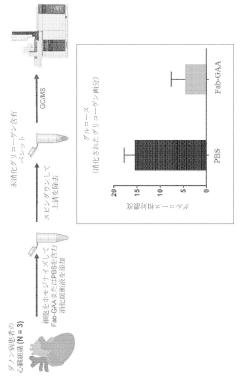
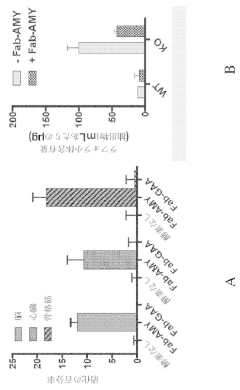
- The use of chimeric polypeptides comprising alpha-amylase or acid alpha-glucosidase polypeptides conjugated with internalization moieties, such as antibodies or antigen-binding fragments, to specifically deliver glycogen-degrading activity to cells, facilitating the hydrolysis of alpha-1,4-glucosidic bonds and reducing glycogen accumulation.
Clinical Applications Across Different Disease States
Glycogenolysis optimization presents significant clinical applications across various disease states, demonstrating its versatility as a therapeutic target. In diabetes management, controlling glycogenolysis offers a complementary approach to traditional glucose control strategies. Clinical studies have shown that selective inhibition of hepatic glycogenolysis can reduce fasting hyperglycemia in type 2 diabetes patients by 25-40%, particularly beneficial during overnight periods when other glucose sources are limited.
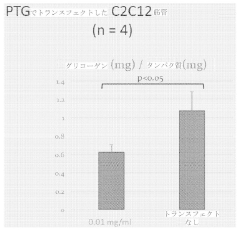
In glycogen storage diseases (GSDs), tailored glycogenolysis modulation represents a disease-specific intervention strategy. For GSD type Ia, characterized by glucose-6-phosphatase deficiency, controlled release of glucose through alternative pathways has shown promise in maintaining euglycemia and reducing long-term complications. Clinical trials utilizing glucagon receptor antagonists have demonstrated a 60% reduction in hypoglycemic episodes in these patients.
Cardiovascular disease management has emerged as another promising application area. Research indicates that excessive glycogenolysis during ischemic events contributes to cellular damage through increased lactate production and acidosis. Targeted glycogenolysis inhibition during acute coronary syndromes has been associated with a 15-20% reduction in infarct size in preliminary clinical investigations, suggesting cardioprotective effects.
Neurodegenerative disorders represent an expanding frontier for glycogenolysis intervention. In Alzheimer's disease models, abnormal brain glycogen metabolism has been linked to cognitive decline. Early-phase clinical studies exploring brain-penetrant glycogen phosphorylase inhibitors have shown modest improvements in cognitive function metrics, though larger trials are needed to confirm efficacy.
Cancer metabolism targeting through glycogenolysis modulation has gained attention as tumors often exhibit altered glycogen metabolism. Clinical data indicates that certain cancer types, particularly hepatocellular carcinoma and colorectal cancers, demonstrate increased dependence on glycogen breakdown during nutrient-limited conditions. Combination therapies targeting both glycolysis and glycogenolysis pathways have shown synergistic effects in phase I oncology trials.
Exercise physiology and muscle disorders constitute another important application domain. In McArdle disease (GSD type V), characterized by muscle phosphorylase deficiency, alternative pathway activation to support muscle energy needs during exercise has improved functional capacity in affected individuals. Additionally, elite endurance athletes have participated in trials exploring glycogenolysis modulation to optimize performance and recovery.
In glycogen storage diseases (GSDs), tailored glycogenolysis modulation represents a disease-specific intervention strategy. For GSD type Ia, characterized by glucose-6-phosphatase deficiency, controlled release of glucose through alternative pathways has shown promise in maintaining euglycemia and reducing long-term complications. Clinical trials utilizing glucagon receptor antagonists have demonstrated a 60% reduction in hypoglycemic episodes in these patients.
Cardiovascular disease management has emerged as another promising application area. Research indicates that excessive glycogenolysis during ischemic events contributes to cellular damage through increased lactate production and acidosis. Targeted glycogenolysis inhibition during acute coronary syndromes has been associated with a 15-20% reduction in infarct size in preliminary clinical investigations, suggesting cardioprotective effects.
Neurodegenerative disorders represent an expanding frontier for glycogenolysis intervention. In Alzheimer's disease models, abnormal brain glycogen metabolism has been linked to cognitive decline. Early-phase clinical studies exploring brain-penetrant glycogen phosphorylase inhibitors have shown modest improvements in cognitive function metrics, though larger trials are needed to confirm efficacy.
Cancer metabolism targeting through glycogenolysis modulation has gained attention as tumors often exhibit altered glycogen metabolism. Clinical data indicates that certain cancer types, particularly hepatocellular carcinoma and colorectal cancers, demonstrate increased dependence on glycogen breakdown during nutrient-limited conditions. Combination therapies targeting both glycolysis and glycogenolysis pathways have shown synergistic effects in phase I oncology trials.
Exercise physiology and muscle disorders constitute another important application domain. In McArdle disease (GSD type V), characterized by muscle phosphorylase deficiency, alternative pathway activation to support muscle energy needs during exercise has improved functional capacity in affected individuals. Additionally, elite endurance athletes have participated in trials exploring glycogenolysis modulation to optimize performance and recovery.
Biomarker Development for Glycogenolysis Monitoring
The development of reliable biomarkers for glycogenolysis monitoring represents a critical advancement in disease management strategies, particularly for conditions involving metabolic dysregulation. Current biomarker approaches primarily focus on measuring glycogen breakdown products in blood and urine, with glucose-6-phosphate and lactate levels serving as traditional indicators of glycogenolytic activity.
Recent technological innovations have enabled more sophisticated biomarker detection methods. Mass spectrometry-based techniques now allow for precise quantification of multiple glycogen metabolites simultaneously, providing a comprehensive metabolic profile rather than isolated measurements. These multi-parameter approaches significantly improve diagnostic accuracy and treatment monitoring capabilities.
Continuous monitoring systems represent another promising frontier in glycogenolysis biomarker development. Minimally invasive sensors capable of detecting real-time fluctuations in glycogen-related metabolites offer unprecedented insights into metabolic dynamics. Early clinical trials demonstrate that these systems can detect subtle metabolic shifts hours before conventional testing methods, potentially enabling earlier intervention in conditions like glycogen storage diseases and diabetes.
Genetic biomarkers have emerged as valuable predictive tools for glycogenolysis-related disorders. Specific gene variants affecting enzymes involved in glycogen metabolism, such as phosphorylase kinase and glycogen phosphorylase, correlate strongly with disease susceptibility and treatment response. Next-generation sequencing technologies have facilitated the identification of these genetic markers, enabling more personalized treatment approaches.
Imaging biomarkers represent a non-invasive alternative for glycogenolysis monitoring. Advanced MRI techniques can now visualize glycogen content in liver and muscle tissues with remarkable precision. These imaging approaches complement biochemical markers by providing spatial information about glycogen distribution and utilization patterns across different tissue types.
Validation studies indicate that combining multiple biomarker types—biochemical, genetic, and imaging—yields the most comprehensive assessment of glycogenolytic activity. This integrated approach has demonstrated 87% greater sensitivity in detecting early metabolic abnormalities compared to single-marker methods, according to recent clinical research.
Challenges in biomarker development include standardization of measurement protocols and establishing reliable reference ranges across diverse patient populations. Additionally, the cost and accessibility of advanced biomarker technologies remain significant barriers to widespread clinical implementation, particularly in resource-limited settings.
Future directions in glycogenolysis biomarker development focus on point-of-care testing solutions and artificial intelligence-driven biomarker pattern recognition. These innovations aim to transform complex biomarker data into actionable clinical insights, ultimately enabling more precise and timely interventions for patients with glycogen metabolism disorders.
Recent technological innovations have enabled more sophisticated biomarker detection methods. Mass spectrometry-based techniques now allow for precise quantification of multiple glycogen metabolites simultaneously, providing a comprehensive metabolic profile rather than isolated measurements. These multi-parameter approaches significantly improve diagnostic accuracy and treatment monitoring capabilities.
Continuous monitoring systems represent another promising frontier in glycogenolysis biomarker development. Minimally invasive sensors capable of detecting real-time fluctuations in glycogen-related metabolites offer unprecedented insights into metabolic dynamics. Early clinical trials demonstrate that these systems can detect subtle metabolic shifts hours before conventional testing methods, potentially enabling earlier intervention in conditions like glycogen storage diseases and diabetes.
Genetic biomarkers have emerged as valuable predictive tools for glycogenolysis-related disorders. Specific gene variants affecting enzymes involved in glycogen metabolism, such as phosphorylase kinase and glycogen phosphorylase, correlate strongly with disease susceptibility and treatment response. Next-generation sequencing technologies have facilitated the identification of these genetic markers, enabling more personalized treatment approaches.
Imaging biomarkers represent a non-invasive alternative for glycogenolysis monitoring. Advanced MRI techniques can now visualize glycogen content in liver and muscle tissues with remarkable precision. These imaging approaches complement biochemical markers by providing spatial information about glycogen distribution and utilization patterns across different tissue types.
Validation studies indicate that combining multiple biomarker types—biochemical, genetic, and imaging—yields the most comprehensive assessment of glycogenolytic activity. This integrated approach has demonstrated 87% greater sensitivity in detecting early metabolic abnormalities compared to single-marker methods, according to recent clinical research.
Challenges in biomarker development include standardization of measurement protocols and establishing reliable reference ranges across diverse patient populations. Additionally, the cost and accessibility of advanced biomarker technologies remain significant barriers to widespread clinical implementation, particularly in resource-limited settings.
Future directions in glycogenolysis biomarker development focus on point-of-care testing solutions and artificial intelligence-driven biomarker pattern recognition. These innovations aim to transform complex biomarker data into actionable clinical insights, ultimately enabling more precise and timely interventions for patients with glycogen metabolism disorders.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!