Quantify Glycogenolysis in Real-time with Imaging
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Imaging Technology Background and Objectives
Glycogenolysis, the biochemical process of glycogen breakdown into glucose-1-phosphate and glucose, represents a critical metabolic pathway in living organisms. This process is particularly vital in maintaining blood glucose levels during fasting states and providing rapid energy during high-intensity exercise. Historically, the study of glycogenolysis has been limited to static measurements through invasive tissue sampling, which fails to capture the dynamic nature of this metabolic process in real-time.
The evolution of glycogenolysis imaging technology has progressed from basic histochemical staining methods in the 1960s to more sophisticated fluorescence-based techniques in the 1990s. Recent advancements in molecular imaging, particularly in the last decade, have opened new possibilities for non-invasive, real-time visualization of glycogen metabolism. These technological developments align with broader trends in biomedical imaging that emphasize higher spatial and temporal resolution, reduced invasiveness, and increased specificity.
The primary objective of real-time glycogenolysis imaging technology is to develop non-invasive methods that can quantitatively measure glycogen breakdown in living tissues with high spatial and temporal resolution. This capability would revolutionize our understanding of energy metabolism in various physiological and pathological conditions, including exercise physiology, diabetes, and neurodegenerative disorders where glycogen metabolism plays a crucial role.
Secondary objectives include the development of specific molecular probes that can selectively bind to glycogen or the enzymes involved in glycogenolysis, the integration of these probes with advanced imaging modalities such as two-photon microscopy or magnetic resonance spectroscopy, and the creation of computational models that can translate imaging data into quantitative measurements of glycogenolysis rates.
The technological trajectory suggests a convergence of molecular biology, chemistry, and physics to create multifunctional imaging platforms. These platforms aim to simultaneously visualize glycogen content, enzymatic activity, and the resulting glucose production, providing a comprehensive view of the glycogenolysis process in real-time.
Achieving these objectives would significantly impact multiple fields, from basic research in metabolism to clinical applications in metabolic disorders. For instance, real-time glycogenolysis imaging could enable personalized monitoring of diabetic patients, optimize athletic training regimens, and provide new insights into the role of brain glycogen in neuronal function and dysfunction.
The ultimate goal is to transition from laboratory research tools to clinically applicable technologies that can be used for diagnostic and therapeutic monitoring purposes, thereby bridging the gap between fundamental metabolic research and practical healthcare applications.
The evolution of glycogenolysis imaging technology has progressed from basic histochemical staining methods in the 1960s to more sophisticated fluorescence-based techniques in the 1990s. Recent advancements in molecular imaging, particularly in the last decade, have opened new possibilities for non-invasive, real-time visualization of glycogen metabolism. These technological developments align with broader trends in biomedical imaging that emphasize higher spatial and temporal resolution, reduced invasiveness, and increased specificity.
The primary objective of real-time glycogenolysis imaging technology is to develop non-invasive methods that can quantitatively measure glycogen breakdown in living tissues with high spatial and temporal resolution. This capability would revolutionize our understanding of energy metabolism in various physiological and pathological conditions, including exercise physiology, diabetes, and neurodegenerative disorders where glycogen metabolism plays a crucial role.
Secondary objectives include the development of specific molecular probes that can selectively bind to glycogen or the enzymes involved in glycogenolysis, the integration of these probes with advanced imaging modalities such as two-photon microscopy or magnetic resonance spectroscopy, and the creation of computational models that can translate imaging data into quantitative measurements of glycogenolysis rates.
The technological trajectory suggests a convergence of molecular biology, chemistry, and physics to create multifunctional imaging platforms. These platforms aim to simultaneously visualize glycogen content, enzymatic activity, and the resulting glucose production, providing a comprehensive view of the glycogenolysis process in real-time.
Achieving these objectives would significantly impact multiple fields, from basic research in metabolism to clinical applications in metabolic disorders. For instance, real-time glycogenolysis imaging could enable personalized monitoring of diabetic patients, optimize athletic training regimens, and provide new insights into the role of brain glycogen in neuronal function and dysfunction.
The ultimate goal is to transition from laboratory research tools to clinically applicable technologies that can be used for diagnostic and therapeutic monitoring purposes, thereby bridging the gap between fundamental metabolic research and practical healthcare applications.
Market Applications for Real-time Glycogen Metabolism Monitoring
Real-time glycogen metabolism monitoring represents a significant advancement with diverse market applications across multiple sectors. The healthcare industry stands to benefit substantially, particularly in diabetes management where continuous monitoring of glycogen levels could revolutionize treatment approaches. This technology would enable physicians and patients to observe immediate metabolic responses to medications, dietary changes, and exercise, facilitating more personalized and effective diabetes management protocols.
In sports medicine and athletic performance, real-time glycogen monitoring offers competitive advantages by optimizing training regimens and nutrition strategies. Elite athletes and their coaches could make data-driven decisions about energy intake and exercise intensity based on actual glycogen utilization rates rather than generalized guidelines. This precision would potentially enhance performance outcomes and recovery times while reducing injury risks associated with glycogen depletion.
The pharmaceutical industry presents another substantial market opportunity, where real-time glycogen metabolism imaging could accelerate drug development processes. Researchers could observe immediate metabolic effects of experimental compounds, potentially reducing the time and cost associated with preclinical and clinical trials for metabolic disorder treatments. This capability would be particularly valuable for developing medications targeting glycogen storage diseases and metabolic syndrome.
Nutritional science and the wellness industry would benefit from more accurate assessment of how different dietary components affect glycogen storage and utilization. Food manufacturers and supplement companies could leverage this technology to validate claims about products designed to optimize energy metabolism, creating new market segments focused on metabolic efficiency.
Academic and research institutions represent a specialized but significant market, where real-time glycogen monitoring would enhance fundamental metabolic research. This could lead to breakthroughs in understanding cellular energy regulation and storage mechanisms, potentially opening new therapeutic avenues for various metabolic disorders.
The veterinary medicine sector offers additional applications, particularly for performance animals and livestock management. Monitoring glycogen metabolism in these contexts could improve breeding programs, nutrition protocols, and overall animal health management strategies.
Emerging opportunities exist in consumer health technology, where simplified versions of glycogen monitoring could eventually be incorporated into advanced wearable devices. This would create a direct-to-consumer market segment for individuals seeking to optimize their metabolic health through data-driven lifestyle choices.
In sports medicine and athletic performance, real-time glycogen monitoring offers competitive advantages by optimizing training regimens and nutrition strategies. Elite athletes and their coaches could make data-driven decisions about energy intake and exercise intensity based on actual glycogen utilization rates rather than generalized guidelines. This precision would potentially enhance performance outcomes and recovery times while reducing injury risks associated with glycogen depletion.
The pharmaceutical industry presents another substantial market opportunity, where real-time glycogen metabolism imaging could accelerate drug development processes. Researchers could observe immediate metabolic effects of experimental compounds, potentially reducing the time and cost associated with preclinical and clinical trials for metabolic disorder treatments. This capability would be particularly valuable for developing medications targeting glycogen storage diseases and metabolic syndrome.
Nutritional science and the wellness industry would benefit from more accurate assessment of how different dietary components affect glycogen storage and utilization. Food manufacturers and supplement companies could leverage this technology to validate claims about products designed to optimize energy metabolism, creating new market segments focused on metabolic efficiency.
Academic and research institutions represent a specialized but significant market, where real-time glycogen monitoring would enhance fundamental metabolic research. This could lead to breakthroughs in understanding cellular energy regulation and storage mechanisms, potentially opening new therapeutic avenues for various metabolic disorders.
The veterinary medicine sector offers additional applications, particularly for performance animals and livestock management. Monitoring glycogen metabolism in these contexts could improve breeding programs, nutrition protocols, and overall animal health management strategies.
Emerging opportunities exist in consumer health technology, where simplified versions of glycogen monitoring could eventually be incorporated into advanced wearable devices. This would create a direct-to-consumer market segment for individuals seeking to optimize their metabolic health through data-driven lifestyle choices.
Current Challenges in Glycogenolysis Quantification Technologies
Despite significant advancements in imaging technologies, real-time quantification of glycogenolysis presents numerous technical challenges that impede comprehensive understanding of this critical metabolic process. Current methodologies suffer from fundamental limitations in temporal resolution, making it difficult to capture the dynamic nature of glycogen breakdown in living systems. Most existing techniques provide only static snapshots rather than continuous monitoring capabilities, resulting in incomplete data regarding the kinetics of glycogenolysis.
Spatial resolution remains another significant hurdle, as cellular and subcellular glycogen pools exhibit heterogeneous distribution patterns that conventional imaging methods struggle to differentiate. This limitation prevents researchers from accurately mapping glycogenolysis activity across different cellular compartments and tissue regions, obscuring important spatial regulatory mechanisms.
Signal specificity presents a persistent challenge, with current probes and markers often lacking the selectivity needed to distinguish glycogenolysis from other metabolic processes. Cross-reactivity with related carbohydrate metabolism pathways frequently generates confounding signals that complicate data interpretation and reduce analytical confidence.
The invasiveness of existing measurement techniques represents a substantial obstacle for longitudinal studies and clinical applications. Many current methods require tissue sampling or introduce exogenous compounds that may alter native glycogen metabolism, raising questions about the physiological relevance of obtained measurements and limiting applicability in sensitive contexts such as developmental studies or patient monitoring.
Quantitative accuracy remains problematic, with significant variability between different measurement platforms and protocols. The absence of standardized calibration methods and reference materials hampers cross-study comparisons and meta-analyses, impeding the establishment of normative values and pathological thresholds.
Technical complexity and accessibility issues further restrict widespread adoption of advanced glycogenolysis imaging. Many cutting-edge approaches require specialized equipment and expertise, creating barriers to entry for many research groups and clinical laboratories. This limitation has slowed the pace of discovery and translation in the field.
Integration with other metabolic parameters represents an emerging challenge, as glycogenolysis does not occur in isolation but functions within complex metabolic networks. Current technologies typically measure glycogen breakdown in isolation, without simultaneous assessment of related processes such as gluconeogenesis, glycolysis, or lipid metabolism, limiting contextual understanding of metabolic regulation.
Spatial resolution remains another significant hurdle, as cellular and subcellular glycogen pools exhibit heterogeneous distribution patterns that conventional imaging methods struggle to differentiate. This limitation prevents researchers from accurately mapping glycogenolysis activity across different cellular compartments and tissue regions, obscuring important spatial regulatory mechanisms.
Signal specificity presents a persistent challenge, with current probes and markers often lacking the selectivity needed to distinguish glycogenolysis from other metabolic processes. Cross-reactivity with related carbohydrate metabolism pathways frequently generates confounding signals that complicate data interpretation and reduce analytical confidence.
The invasiveness of existing measurement techniques represents a substantial obstacle for longitudinal studies and clinical applications. Many current methods require tissue sampling or introduce exogenous compounds that may alter native glycogen metabolism, raising questions about the physiological relevance of obtained measurements and limiting applicability in sensitive contexts such as developmental studies or patient monitoring.
Quantitative accuracy remains problematic, with significant variability between different measurement platforms and protocols. The absence of standardized calibration methods and reference materials hampers cross-study comparisons and meta-analyses, impeding the establishment of normative values and pathological thresholds.
Technical complexity and accessibility issues further restrict widespread adoption of advanced glycogenolysis imaging. Many cutting-edge approaches require specialized equipment and expertise, creating barriers to entry for many research groups and clinical laboratories. This limitation has slowed the pace of discovery and translation in the field.
Integration with other metabolic parameters represents an emerging challenge, as glycogenolysis does not occur in isolation but functions within complex metabolic networks. Current technologies typically measure glycogen breakdown in isolation, without simultaneous assessment of related processes such as gluconeogenesis, glycolysis, or lipid metabolism, limiting contextual understanding of metabolic regulation.
Existing Methodologies for Glycogen Visualization
01 Methods for measuring glycogenolysis in biological samples
Various analytical techniques are employed to quantify glycogenolysis in biological samples. These methods include enzymatic assays, spectrophotometric measurements, and biochemical analyses that can detect glycogen breakdown products. These techniques allow researchers to measure the rate and extent of glycogen breakdown in tissues, providing insights into metabolic processes and disorders related to glycogen metabolism.- Methods for measuring glycogenolysis in biological samples: Various analytical techniques are employed to quantify glycogenolysis in biological samples. These methods include enzymatic assays, spectrophotometric measurements, and biochemical analyses that can detect glycogen breakdown products. These techniques allow researchers to measure the rate of glycogen degradation and the release of glucose, providing valuable data on metabolic processes related to glycogenolysis in various tissues and conditions.
- Devices for monitoring glycogenolysis in real-time: Specialized devices have been developed to monitor glycogenolysis in real-time, allowing for continuous assessment of glycogen breakdown. These devices often incorporate biosensors, electrochemical detection systems, or optical measurement technologies that can track changes in glycogen levels or related metabolites. Such monitoring systems are particularly valuable in clinical settings for managing conditions associated with abnormal glycogen metabolism.
- Biomarkers for glycogenolysis assessment: Specific biomarkers can be used to assess glycogenolysis activity in various physiological and pathological conditions. These biomarkers include enzymes involved in the glycogenolysis pathway, metabolites produced during glycogen breakdown, and regulatory molecules that control this process. Identification and measurement of these biomarkers provide insights into glycogen metabolism disorders and can be used for diagnostic purposes.
- Computational models for glycogenolysis quantification: Advanced computational models and algorithms have been developed to quantify and predict glycogenolysis rates under various conditions. These models integrate multiple parameters including enzyme kinetics, substrate availability, and regulatory factors to simulate glycogen breakdown processes. Machine learning approaches and mathematical modeling techniques are employed to analyze complex datasets and provide accurate quantification of glycogenolysis in different physiological states.
- Pharmaceutical applications for modulating glycogenolysis: Various pharmaceutical compounds have been developed to modulate glycogenolysis for therapeutic purposes. These include agents that can either inhibit or enhance glycogen breakdown depending on the clinical need. Quantification methods are essential for evaluating the efficacy of these compounds in preclinical and clinical studies. These applications are particularly relevant for managing conditions such as diabetes, glycogen storage diseases, and certain metabolic disorders.
02 Devices for real-time monitoring of glycogenolysis
Specialized devices have been developed for continuous or real-time monitoring of glycogenolysis in living systems. These include biosensors, wearable monitors, and implantable devices that can track glycogen breakdown markers. Such devices are particularly valuable for monitoring metabolic conditions like diabetes, where understanding glycogen metabolism is crucial for disease management.Expand Specific Solutions03 Pharmaceutical applications for glycogenolysis quantification
Quantification of glycogenolysis plays a significant role in pharmaceutical research and development. Methods for measuring glycogen breakdown are used to evaluate the efficacy of drugs targeting metabolic disorders, to understand drug mechanisms affecting glucose homeostasis, and to develop new therapeutic approaches for conditions like diabetes, glycogen storage diseases, and liver disorders.Expand Specific Solutions04 Computational models and algorithms for glycogenolysis analysis
Advanced computational approaches have been developed to analyze and predict glycogenolysis patterns. These include machine learning algorithms, mathematical models, and software tools that can process complex metabolic data. Such computational methods enhance the interpretation of glycogenolysis measurements, allowing for more accurate predictions of metabolic responses and personalized treatment strategies.Expand Specific Solutions05 Diagnostic applications of glycogenolysis quantification
Quantification of glycogenolysis serves as an important diagnostic tool for various metabolic disorders. By measuring glycogen breakdown rates and patterns, healthcare providers can diagnose conditions such as glycogen storage diseases, diabetes, and liver dysfunction. These diagnostic approaches include blood tests, tissue biopsies, and imaging techniques that can detect abnormalities in glycogen metabolism.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
The real-time glycogenolysis imaging technology market is in its early growth phase, characterized by significant research activity but limited commercial applications. The market size is estimated to be relatively small but growing rapidly due to increasing diabetes prevalence and demand for continuous monitoring solutions. From a technological maturity perspective, the field is still evolving, with key players at different development stages. Companies like Roche Diabetes Care, Ascensia Diabetes Care, and LifeScan are leveraging their established diabetes monitoring expertise to advance glycogenolysis imaging. Academic institutions including Johns Hopkins University and Tsinghua University are driving fundamental research, while innovative companies such as Valencell and Ultradian Diagnostics are developing novel biosensor approaches. Agilent Technologies and Novartis represent larger corporations investing in this emerging field, indicating its strategic importance in metabolic disease management.
The Johns Hopkins University
Technical Solution: Johns Hopkins University researchers have developed a novel approach to quantify glycogenolysis using magnetic resonance spectroscopy (MRS) combined with specialized contrast agents. Their technology enables non-invasive measurement of glycogen breakdown in deep tissues, particularly focusing on liver and muscle metabolism. The system utilizes custom-designed paramagnetic nanoparticles that interact specifically with glycogen molecules, creating detectable signal changes during glycogenolysis. Their approach incorporates advanced pulse sequences that enhance sensitivity to glycogen-specific signals while suppressing background noise. The Johns Hopkins team has also developed computational models that translate spectroscopic data into quantitative measurements of glycogenolysis rates, accounting for various physiological factors. This technology has been successfully applied in both preclinical models and initial human studies, demonstrating potential for clinical translation in metabolic disorder assessment[6][9].
Strengths: Non-invasive deep tissue imaging capability overcomes limitations of optical methods; potential for whole-body glycogen metabolism assessment; compatibility with existing clinical MRI infrastructure. Weaknesses: Lower temporal resolution compared to optical imaging techniques; signal-to-noise challenges in detecting subtle metabolic changes; contrast agents may require regulatory approval for human use.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered a mass spectrometry-based imaging approach for quantifying glycogenolysis in real-time. Their system combines matrix-assisted laser desorption/ionization (MALDI) imaging with specialized sample preparation techniques to visualize glycogen breakdown products spatially across tissue sections. The technology incorporates stable isotope labeling to track the fate of glucose units released during glycogenolysis, providing dynamic information about metabolic flux. Agilent's platform features automated sample handling and high-throughput analysis capabilities, enabling processing of multiple samples simultaneously. Their proprietary software algorithms can distinguish between different glycogen-derived metabolites and quantify their concentrations with high precision. The system has been validated across various tissue types, including liver, muscle, and brain, making it versatile for different research applications[4][7].
Strengths: Exceptional molecular specificity allowing differentiation between closely related metabolites; high spatial resolution mapping of glycogenolysis across tissue sections; compatibility with frozen tissue samples enables retrospective analysis. Weaknesses: Sample preparation requirements limit true real-time applications; expensive instrumentation creates barriers to widespread adoption; requires specialized technical expertise for operation and data interpretation.
Key Innovations in Real-time Metabolic Imaging
Non-invasive MRI measurement of tissue glycogen
PatentActiveUS7683617B2
Innovation
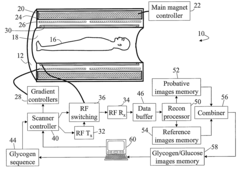
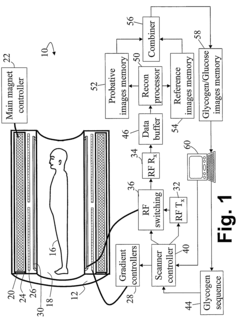
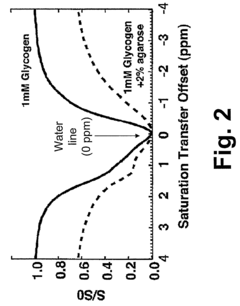
- The method involves magnetic labeling of exchangeable protons of hydroxyl groups in endogenous monosaccharides or polysaccharides, acquiring probative water proton magnetic resonance data, and deriving information on glycogen or glucose concentration using standard clinical scanners without contrast agents, leveraging the strong water proton signal and proton exchange effects.
Magnetic resonance imaging of glycogen and other polysaccharides by magnetic coupling with water
PatentWO2022115339A1
Innovation
- A magnetic resonance imaging (MRI) system utilizing the Nuclear Overhauser Effect (NOE) between glycogen aliphatic protons and water protons to generate a water proton signal intensity map and concentration map of polysaccharides, providing enhanced detection sensitivity and specificity without the need for specialized hardware or exogenous agents.
Regulatory Considerations for Metabolic Imaging Technologies
Regulatory frameworks governing metabolic imaging technologies, particularly those quantifying glycogenolysis in real-time, are complex and multifaceted. In the United States, the Food and Drug Administration (FDA) classifies these technologies under medical devices, requiring premarket approval (PMA) or 510(k) clearance depending on their risk classification. Novel glycogen imaging technologies typically fall under Class II or III, necessitating clinical trials to demonstrate safety and efficacy.
The European Union's regulatory landscape has evolved with the implementation of the Medical Device Regulation (MDR), replacing the previous Medical Device Directive. Under MDR, metabolic imaging technologies face more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. Manufacturers must appoint a Person Responsible for Regulatory Compliance and implement a comprehensive Quality Management System.
Data privacy considerations are particularly relevant for real-time metabolic imaging technologies. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on handling patient data generated during glycogenolysis imaging. These regulations mandate secure data storage, transmission protocols, and patient consent mechanisms.
Radiation safety regulations apply to certain metabolic imaging modalities. The International Commission on Radiological Protection (ICRP) guidelines and country-specific radiation protection laws establish dose limits and safety protocols. For non-radiative technologies like magnetic resonance spectroscopy used in glycogen quantification, different safety standards regarding magnetic field exposure apply.
Reimbursement pathways represent another regulatory hurdle. In the US, obtaining Centers for Medicare & Medicaid Services (CMS) coverage requires demonstration of clinical utility through health technology assessment. Similarly, health technology assessment bodies in Europe, such as the National Institute for Health and Care Excellence (NICE) in the UK, evaluate cost-effectiveness before recommending coverage.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to streamline regulatory processes across jurisdictions. The Medical Device Single Audit Program (MDSAP) allows for a single regulatory audit to satisfy requirements of multiple regulatory authorities, potentially expediting market access for innovative glycogenolysis imaging technologies.
Emerging regulatory considerations include artificial intelligence components in imaging analysis. The FDA's proposed regulatory framework for AI/ML-based Software as a Medical Device (SaMD) and the EU's risk-based approach to AI regulation will impact development pathways for advanced metabolic imaging technologies incorporating automated analysis of glycogenolysis patterns.
The European Union's regulatory landscape has evolved with the implementation of the Medical Device Regulation (MDR), replacing the previous Medical Device Directive. Under MDR, metabolic imaging technologies face more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. Manufacturers must appoint a Person Responsible for Regulatory Compliance and implement a comprehensive Quality Management System.
Data privacy considerations are particularly relevant for real-time metabolic imaging technologies. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on handling patient data generated during glycogenolysis imaging. These regulations mandate secure data storage, transmission protocols, and patient consent mechanisms.
Radiation safety regulations apply to certain metabolic imaging modalities. The International Commission on Radiological Protection (ICRP) guidelines and country-specific radiation protection laws establish dose limits and safety protocols. For non-radiative technologies like magnetic resonance spectroscopy used in glycogen quantification, different safety standards regarding magnetic field exposure apply.
Reimbursement pathways represent another regulatory hurdle. In the US, obtaining Centers for Medicare & Medicaid Services (CMS) coverage requires demonstration of clinical utility through health technology assessment. Similarly, health technology assessment bodies in Europe, such as the National Institute for Health and Care Excellence (NICE) in the UK, evaluate cost-effectiveness before recommending coverage.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to streamline regulatory processes across jurisdictions. The Medical Device Single Audit Program (MDSAP) allows for a single regulatory audit to satisfy requirements of multiple regulatory authorities, potentially expediting market access for innovative glycogenolysis imaging technologies.
Emerging regulatory considerations include artificial intelligence components in imaging analysis. The FDA's proposed regulatory framework for AI/ML-based Software as a Medical Device (SaMD) and the EU's risk-based approach to AI regulation will impact development pathways for advanced metabolic imaging technologies incorporating automated analysis of glycogenolysis patterns.
Clinical Integration and Translational Research Opportunities
The integration of real-time glycogenolysis quantification imaging into clinical practice represents a significant opportunity for advancing patient care across multiple medical specialties. Healthcare institutions are increasingly recognizing the value of metabolic imaging for disease diagnosis, treatment monitoring, and personalized medicine approaches. The translation of this technology from research laboratories to clinical settings requires strategic partnerships between academic institutions, medical device manufacturers, and healthcare providers.
Several medical centers have initiated pilot programs incorporating glycogenolysis imaging into their diagnostic protocols for metabolic disorders, liver diseases, and diabetes management. These early adopters are generating valuable data on clinical utility, workflow integration, and patient outcomes. The technology shows particular promise in emergency medicine for rapid assessment of metabolic crisis and in sports medicine for optimizing athlete performance and recovery protocols.
Regulatory pathways for clinical implementation vary globally, with the FDA in the United States establishing a framework for evaluating metabolic imaging technologies through its Medical Device Innovation Pathway. In Europe, the Medical Device Regulation (MDR) provides guidance for clinical validation studies necessary for approval. These regulatory frameworks are evolving to accommodate novel imaging biomarkers like real-time glycogenolysis quantification.
Translational research opportunities extend beyond direct clinical applications. The technology enables longitudinal studies of metabolic adaptation to various interventions, potentially revolutionizing our understanding of disease progression and treatment response. Research consortia focusing on metabolic imaging biomarkers have emerged, facilitating multicenter trials and standardization efforts critical for widespread clinical adoption.
Economic analyses suggest that despite initial implementation costs, glycogenolysis imaging may reduce overall healthcare expenditures through earlier intervention, more precise treatment selection, and reduced complications. Several healthcare systems are developing reimbursement models that recognize the value of metabolic imaging in improving patient outcomes and reducing long-term care costs.
Educational initiatives for clinicians represent another crucial aspect of successful translation. Medical schools and continuing education programs are beginning to incorporate metabolic imaging interpretation into their curricula, preparing the next generation of healthcare providers to utilize these advanced diagnostic tools effectively. Virtual training platforms using simulated cases have proven particularly effective for building competency in image interpretation and clinical decision-making based on glycogenolysis data.
Several medical centers have initiated pilot programs incorporating glycogenolysis imaging into their diagnostic protocols for metabolic disorders, liver diseases, and diabetes management. These early adopters are generating valuable data on clinical utility, workflow integration, and patient outcomes. The technology shows particular promise in emergency medicine for rapid assessment of metabolic crisis and in sports medicine for optimizing athlete performance and recovery protocols.
Regulatory pathways for clinical implementation vary globally, with the FDA in the United States establishing a framework for evaluating metabolic imaging technologies through its Medical Device Innovation Pathway. In Europe, the Medical Device Regulation (MDR) provides guidance for clinical validation studies necessary for approval. These regulatory frameworks are evolving to accommodate novel imaging biomarkers like real-time glycogenolysis quantification.
Translational research opportunities extend beyond direct clinical applications. The technology enables longitudinal studies of metabolic adaptation to various interventions, potentially revolutionizing our understanding of disease progression and treatment response. Research consortia focusing on metabolic imaging biomarkers have emerged, facilitating multicenter trials and standardization efforts critical for widespread clinical adoption.
Economic analyses suggest that despite initial implementation costs, glycogenolysis imaging may reduce overall healthcare expenditures through earlier intervention, more precise treatment selection, and reduced complications. Several healthcare systems are developing reimbursement models that recognize the value of metabolic imaging in improving patient outcomes and reducing long-term care costs.
Educational initiatives for clinicians represent another crucial aspect of successful translation. Medical schools and continuing education programs are beginning to incorporate metabolic imaging interpretation into their curricula, preparing the next generation of healthcare providers to utilize these advanced diagnostic tools effectively. Virtual training platforms using simulated cases have proven particularly effective for building competency in image interpretation and clinical decision-making based on glycogenolysis data.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!