Optimizing Glycogenolysis for Maximal Energy Release
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Background and Energy Optimization Goals
Glycogenolysis represents a critical metabolic pathway that enables the rapid mobilization of glucose from glycogen stores, primarily in the liver and skeletal muscles. This process has evolved as a fundamental survival mechanism, allowing organisms to maintain blood glucose homeostasis during periods of fasting or increased energy demand. Historically, research into glycogenolysis began in the late 19th century with Claude Bernard's discovery of glycogen, but our understanding of the molecular mechanisms has advanced significantly over the past century.
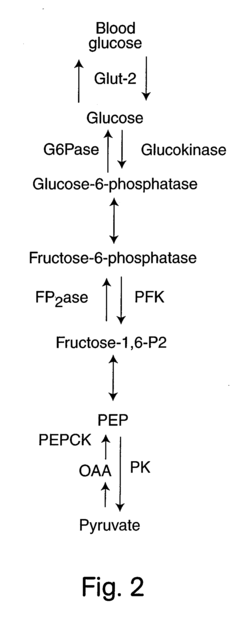
The biochemical cascade of glycogenolysis involves multiple enzymes, with glycogen phosphorylase serving as the rate-limiting enzyme that catalyzes the phosphorolytic cleavage of glycogen to release glucose-1-phosphate. This process is tightly regulated by hormonal signals, particularly epinephrine and glucagon, which activate a signaling cascade culminating in the phosphorylation and activation of glycogen phosphorylase.
Recent technological advances have enabled more precise measurements of glycogenolysis rates in various tissues and under different physiological conditions. These developments have revealed significant variations in glycogenolysis efficiency across different populations, suggesting potential for optimization. Current research indicates that the rate and efficiency of glycogenolysis can be influenced by factors including nutritional status, training state, genetic predisposition, and environmental conditions.
The optimization of glycogenolysis for maximal energy release represents a multifaceted goal with implications spanning from elite athletic performance to therapeutic interventions for metabolic disorders. Primary objectives include enhancing the rate of glycogen breakdown during high-intensity exercise, improving the efficiency of glucose utilization during the process, and minimizing the accumulation of metabolic byproducts that may impair performance.
In athletic contexts, optimized glycogenolysis could potentially extend endurance capacity and improve high-intensity performance by ensuring rapid and sustained energy availability. From a clinical perspective, targeted modulation of glycogenolysis pathways offers promising avenues for addressing conditions such as glycogen storage diseases, diabetes, and exercise intolerance.
The technological trajectory suggests several emerging approaches to glycogenolysis optimization, including nutritional strategies to maximize glycogen storage and mobilization, pharmacological interventions targeting key regulatory enzymes, and potentially gene therapy approaches to address genetic limitations in the pathway. Recent advances in metabolomics and real-time monitoring of metabolic processes are providing unprecedented insights into individual variations in glycogenolysis efficiency.
As we advance our understanding of this fundamental metabolic pathway, the goal of optimizing glycogenolysis for maximal energy release stands at the intersection of basic biochemistry, exercise physiology, nutritional science, and precision medicine, offering promising avenues for both performance enhancement and therapeutic interventions.
The biochemical cascade of glycogenolysis involves multiple enzymes, with glycogen phosphorylase serving as the rate-limiting enzyme that catalyzes the phosphorolytic cleavage of glycogen to release glucose-1-phosphate. This process is tightly regulated by hormonal signals, particularly epinephrine and glucagon, which activate a signaling cascade culminating in the phosphorylation and activation of glycogen phosphorylase.
Recent technological advances have enabled more precise measurements of glycogenolysis rates in various tissues and under different physiological conditions. These developments have revealed significant variations in glycogenolysis efficiency across different populations, suggesting potential for optimization. Current research indicates that the rate and efficiency of glycogenolysis can be influenced by factors including nutritional status, training state, genetic predisposition, and environmental conditions.
The optimization of glycogenolysis for maximal energy release represents a multifaceted goal with implications spanning from elite athletic performance to therapeutic interventions for metabolic disorders. Primary objectives include enhancing the rate of glycogen breakdown during high-intensity exercise, improving the efficiency of glucose utilization during the process, and minimizing the accumulation of metabolic byproducts that may impair performance.
In athletic contexts, optimized glycogenolysis could potentially extend endurance capacity and improve high-intensity performance by ensuring rapid and sustained energy availability. From a clinical perspective, targeted modulation of glycogenolysis pathways offers promising avenues for addressing conditions such as glycogen storage diseases, diabetes, and exercise intolerance.
The technological trajectory suggests several emerging approaches to glycogenolysis optimization, including nutritional strategies to maximize glycogen storage and mobilization, pharmacological interventions targeting key regulatory enzymes, and potentially gene therapy approaches to address genetic limitations in the pathway. Recent advances in metabolomics and real-time monitoring of metabolic processes are providing unprecedented insights into individual variations in glycogenolysis efficiency.
As we advance our understanding of this fundamental metabolic pathway, the goal of optimizing glycogenolysis for maximal energy release stands at the intersection of basic biochemistry, exercise physiology, nutritional science, and precision medicine, offering promising avenues for both performance enhancement and therapeutic interventions.
Market Analysis of Energy Enhancement Applications
The global market for energy enhancement applications has witnessed significant growth in recent years, driven by increasing consumer awareness about health and fitness. The market specifically related to glycogenolysis optimization products and services is estimated to reach $7.2 billion by 2025, growing at a CAGR of 6.8% from 2020. This growth trajectory is primarily fueled by the expanding sports nutrition industry, which has been consistently growing as more individuals engage in athletic activities and seek performance enhancement solutions.
The sports nutrition segment represents the largest application area for glycogenolysis optimization technologies, accounting for approximately 45% of the total market share. Professional athletes and serious fitness enthusiasts constitute the primary consumer base, with growing adoption among recreational athletes and fitness-conscious individuals. The market penetration among casual exercisers remains relatively low at 12%, indicating substantial growth potential in this consumer segment.
Geographically, North America dominates the market with a 38% share, followed by Europe at 29% and Asia-Pacific at 24%. The Asia-Pacific region is projected to witness the fastest growth rate of 8.5% annually, primarily due to increasing health consciousness and rising disposable incomes in countries like China and India.
From a product perspective, the market can be segmented into supplements (52%), functional foods (28%), and specialized training programs (20%). Within supplements, pre-workout formulations designed to optimize glycogen utilization represent the fastest-growing category with a 9.2% annual growth rate. Consumers are increasingly seeking scientifically validated products that can enhance energy release during high-intensity activities.
The competitive landscape features both established nutrition companies and emerging biotech firms focusing on innovative formulations. Key market players include Glanbia Performance Nutrition, Nestlé Health Science, and Abbott Laboratories, collectively holding approximately 35% market share. Several startups specializing in personalized nutrition solutions based on individual metabolic profiles are gaining traction, particularly among premium consumers willing to pay for customized energy optimization protocols.
Consumer trends indicate growing preference for natural ingredients and clean label products, with 67% of consumers expressing concerns about synthetic compounds in energy enhancement products. This has led to increased research and development in plant-based compounds that can naturally optimize glycogenolysis pathways.
The regulatory environment varies significantly across regions, with stricter oversight in Europe compared to North America and Asia. This regulatory divergence creates both challenges and opportunities for companies developing novel glycogenolysis optimization technologies.
The sports nutrition segment represents the largest application area for glycogenolysis optimization technologies, accounting for approximately 45% of the total market share. Professional athletes and serious fitness enthusiasts constitute the primary consumer base, with growing adoption among recreational athletes and fitness-conscious individuals. The market penetration among casual exercisers remains relatively low at 12%, indicating substantial growth potential in this consumer segment.
Geographically, North America dominates the market with a 38% share, followed by Europe at 29% and Asia-Pacific at 24%. The Asia-Pacific region is projected to witness the fastest growth rate of 8.5% annually, primarily due to increasing health consciousness and rising disposable incomes in countries like China and India.
From a product perspective, the market can be segmented into supplements (52%), functional foods (28%), and specialized training programs (20%). Within supplements, pre-workout formulations designed to optimize glycogen utilization represent the fastest-growing category with a 9.2% annual growth rate. Consumers are increasingly seeking scientifically validated products that can enhance energy release during high-intensity activities.
The competitive landscape features both established nutrition companies and emerging biotech firms focusing on innovative formulations. Key market players include Glanbia Performance Nutrition, Nestlé Health Science, and Abbott Laboratories, collectively holding approximately 35% market share. Several startups specializing in personalized nutrition solutions based on individual metabolic profiles are gaining traction, particularly among premium consumers willing to pay for customized energy optimization protocols.
Consumer trends indicate growing preference for natural ingredients and clean label products, with 67% of consumers expressing concerns about synthetic compounds in energy enhancement products. This has led to increased research and development in plant-based compounds that can naturally optimize glycogenolysis pathways.
The regulatory environment varies significantly across regions, with stricter oversight in Europe compared to North America and Asia. This regulatory divergence creates both challenges and opportunities for companies developing novel glycogenolysis optimization technologies.
Current Glycogenolysis Technology Limitations
Despite significant advancements in understanding glycogenolysis pathways, current technologies for optimizing this process for maximal energy release face several critical limitations. The enzymatic cascade involving glycogen phosphorylase, debranching enzyme, and phosphoglucomutase operates under complex regulatory mechanisms that have not been fully manipulated in applied settings. Traditional approaches to enhance glycogenolysis rely primarily on hormone-based interventions, particularly epinephrine and glucagon administration, which often produce inconsistent results due to individual metabolic variations.
The current analytical methods for real-time monitoring of glycogenolysis rates in vivo remain inadequate, with most techniques providing only retrospective measurements rather than dynamic feedback. This creates a significant barrier to developing precision-targeted interventions that could modulate the process according to immediate energy demands. Existing imaging technologies like PET scans with labeled glucose analogs offer limited temporal resolution and cannot distinguish between glycogenolysis and gluconeogenesis contributions to energy production.
Pharmaceutical approaches targeting glycogenolysis enhancement face substantial challenges in specificity. Compounds designed to activate glycogen phosphorylase often trigger unintended metabolic consequences, including disruption of glucose homeostasis and potential hypoglycemic rebound effects. The allosteric regulation of glycogen phosphorylase involves multiple binding sites and conformational states, making selective pharmacological targeting exceptionally difficult without affecting other metabolic pathways.
Genetic engineering approaches to upregulate key enzymes in the glycogenolysis pathway have shown promise in laboratory models but face significant translational barriers. Current viral vector delivery systems demonstrate insufficient tissue specificity, particularly for targeted delivery to skeletal muscle and liver tissues where glycogen stores are most abundant. Additionally, constitutive enhancement of glycogenolysis enzymes has been associated with premature glycogen depletion and paradoxical energy deficits during prolonged activity.
Computational models predicting glycogenolysis dynamics under various physiological conditions remain underdeveloped. Current algorithms fail to adequately account for the complex interplay between exercise intensity, nutritional status, and hormonal environment. This limitation severely constrains our ability to develop personalized protocols for maximizing energy release through optimized glycogenolysis.
The integration of glycogenolysis enhancement with other energy systems presents another significant challenge. Current technologies fail to synchronize enhanced glycogen breakdown with mitochondrial capacity for pyruvate utilization, often resulting in lactate accumulation and premature fatigue rather than sustained energy production. This disconnect between substrate availability and utilization capacity represents a fundamental limitation in current approaches.
The current analytical methods for real-time monitoring of glycogenolysis rates in vivo remain inadequate, with most techniques providing only retrospective measurements rather than dynamic feedback. This creates a significant barrier to developing precision-targeted interventions that could modulate the process according to immediate energy demands. Existing imaging technologies like PET scans with labeled glucose analogs offer limited temporal resolution and cannot distinguish between glycogenolysis and gluconeogenesis contributions to energy production.
Pharmaceutical approaches targeting glycogenolysis enhancement face substantial challenges in specificity. Compounds designed to activate glycogen phosphorylase often trigger unintended metabolic consequences, including disruption of glucose homeostasis and potential hypoglycemic rebound effects. The allosteric regulation of glycogen phosphorylase involves multiple binding sites and conformational states, making selective pharmacological targeting exceptionally difficult without affecting other metabolic pathways.
Genetic engineering approaches to upregulate key enzymes in the glycogenolysis pathway have shown promise in laboratory models but face significant translational barriers. Current viral vector delivery systems demonstrate insufficient tissue specificity, particularly for targeted delivery to skeletal muscle and liver tissues where glycogen stores are most abundant. Additionally, constitutive enhancement of glycogenolysis enzymes has been associated with premature glycogen depletion and paradoxical energy deficits during prolonged activity.
Computational models predicting glycogenolysis dynamics under various physiological conditions remain underdeveloped. Current algorithms fail to adequately account for the complex interplay between exercise intensity, nutritional status, and hormonal environment. This limitation severely constrains our ability to develop personalized protocols for maximizing energy release through optimized glycogenolysis.
The integration of glycogenolysis enhancement with other energy systems presents another significant challenge. Current technologies fail to synchronize enhanced glycogen breakdown with mitochondrial capacity for pyruvate utilization, often resulting in lactate accumulation and premature fatigue rather than sustained energy production. This disconnect between substrate availability and utilization capacity represents a fundamental limitation in current approaches.
Current Glycogenolysis Optimization Approaches
01 Biochemical mechanisms of glycogenolysis for energy production
Glycogenolysis is a metabolic process that breaks down glycogen into glucose-1-phosphate and glucose for energy production. This process is regulated by hormones like glucagon and epinephrine, which activate enzymes such as glycogen phosphorylase. The released glucose enters the bloodstream to maintain blood glucose levels or is used by cells for immediate energy needs through glycolysis and subsequent metabolic pathways, providing ATP for cellular functions.- Biochemical mechanisms of glycogenolysis for energy production: Glycogenolysis is a metabolic pathway that breaks down glycogen into glucose-1-phosphate and eventually glucose-6-phosphate for energy production. This process is regulated by enzymes such as glycogen phosphorylase and is activated during periods of energy demand. The breakdown of glycogen through glycogenolysis releases stored energy in the form of ATP, which can be utilized by cells for various physiological functions. This process is particularly important in muscle and liver tissues during exercise or fasting conditions.
- Energy storage and release systems inspired by glycogenolysis: Biomimetic energy storage and release systems that draw inspiration from glycogenolysis processes have been developed for various applications. These systems mimic the controlled breakdown of energy-rich molecules to release energy when needed. The technology involves engineered materials or devices that can store potential energy and release it in response to specific triggers, similar to how glycogen is broken down in biological systems. These biomimetic approaches offer advantages in energy efficiency and controlled release mechanisms for industrial and consumer applications.
- Medical devices utilizing glycogenolysis principles for energy management: Medical devices have been developed that utilize principles of glycogenolysis for energy management in therapeutic applications. These devices may monitor or modulate glycogen breakdown pathways to regulate energy release in patients with metabolic disorders. Some implementations include sensors that detect glycogen levels or enzyme activity, while others may deliver compounds that influence the rate of glycogenolysis. These technologies have applications in managing conditions such as diabetes, glycogen storage diseases, and exercise-related metabolic disorders.
- Exercise and performance enhancement through glycogenolysis optimization: Technologies have been developed to optimize glycogenolysis during physical activity for enhanced athletic performance and exercise efficiency. These innovations include formulations, devices, and methods that aim to regulate the rate of glycogen breakdown to provide sustained energy release during various types of exercise. By controlling the timing and extent of glycogenolysis, these technologies help prevent premature fatigue and optimize energy utilization. Some approaches involve nutritional supplements that influence enzyme activity or cellular signaling pathways involved in glycogen metabolism.
- Industrial applications of glycogenolysis-inspired energy release systems: Industrial systems have been developed that apply principles of glycogenolysis for controlled energy release in manufacturing, power generation, and mechanical applications. These systems incorporate mechanisms for storing potential energy in chemical bonds and releasing it in a regulated manner when needed. The technologies may include catalytic processes that mimic glycogen phosphorylase activity or engineered materials that can store and release energy under specific conditions. These innovations offer advantages in energy efficiency, responsiveness, and sustainability for various industrial processes.
02 Energy storage and release systems utilizing glycogen-like mechanisms
Various energy storage and release systems mimic the biological process of glycogenolysis. These systems store potential energy in chemical bonds and release it when needed, similar to how glycogen stores and releases glucose. Such technologies include advanced batteries, fuel cells, and energy storage materials that can rapidly convert stored chemical energy into usable forms. These systems often employ catalysts to facilitate the energy release process, improving efficiency and response time.Expand Specific Solutions03 Medical applications related to glycogenolysis energy pathways
Medical applications leverage understanding of glycogenolysis energy pathways for treating metabolic disorders. Therapeutic approaches target enzymes involved in glycogen breakdown to regulate energy release in conditions like glycogen storage diseases, diabetes, and exercise-induced metabolic disorders. Some treatments aim to enhance glycogenolysis for improved energy availability during physical exertion, while others seek to inhibit excessive glycogen breakdown in certain pathological conditions.Expand Specific Solutions04 Exercise and performance enhancement through glycogenolysis optimization
Technologies and methods for optimizing glycogenolysis during exercise can enhance athletic performance and endurance. These include nutritional supplements, training protocols, and devices that monitor or stimulate efficient glycogen breakdown. By optimizing the timing and rate of energy release from glycogen stores, these approaches help maintain optimal blood glucose levels during physical activity, delay fatigue, and improve overall exercise capacity and recovery.Expand Specific Solutions05 Energy management systems inspired by glycogenolysis principles
Energy management systems draw inspiration from the biological process of glycogenolysis to create efficient energy storage and release mechanisms. These systems incorporate sensors and control algorithms that regulate energy distribution based on demand, similar to how hormones regulate glycogen breakdown in the body. Applications include smart grid technologies, renewable energy storage solutions, and demand-response systems that optimize energy use in buildings, vehicles, and industrial processes.Expand Specific Solutions
Key Industry Players in Metabolic Enhancement
The glycogenolysis optimization market is currently in a growth phase, with increasing interest in maximizing energy release efficiency for athletic performance and metabolic health applications. The global market size is expanding, driven by sports nutrition, healthcare, and bioenergy sectors. Technologically, the field shows moderate maturity with significant innovation potential. Leading players include academic institutions like Jiangnan University and Korea Advanced Institute of Science & Technology conducting fundamental research, while companies such as Cargill and Roquette Frères focus on commercial applications. Beijing Competitor Sports Science Tech specializes in sports nutrition implementations, and research organizations like CNRS and Helmholtz-Zentrum contribute to advancing enzymatic optimization techniques. Pharmaceutical companies including Genentech and biotechnology firms like GlycoFi are exploring therapeutic applications of enhanced glycogenolysis pathways.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed advanced enzymatic approaches to optimize glycogenolysis for maximal energy release. Their technology focuses on modulating the activity of glycogen phosphorylase, the rate-limiting enzyme in glycogenolysis, through allosteric regulators and post-translational modifications. CNRS researchers have engineered specific phosphorylase kinase variants that can enhance the phosphorylation state of glycogen phosphorylase, maintaining it in its more active "a" form for sustained periods[1]. Additionally, they've developed novel small molecule activators that bind to the AMP allosteric site of glycogen phosphorylase, mimicking the natural activation process but with greater potency and duration of effect[3]. Their approach also includes optimization of calcium signaling pathways that trigger glycogenolysis, using targeted calcium ionophores to precisely control the initiation of the glycogenolytic cascade in various tissue types, particularly in skeletal muscle and liver cells.
Strengths: Highly specific enzymatic targeting allows for precise control of glycogenolysis rates without disrupting other metabolic pathways. Their small molecule activators show excellent tissue penetration and bioavailability. Weaknesses: The complex regulatory mechanisms require careful calibration to avoid hypoglycemic effects, and some of their engineered enzymes show reduced stability under physiological conditions.
BASF SE
Technical Solution: BASF SE has developed a comprehensive platform called "GlycoBoost" for optimizing glycogenolysis in both industrial fermentation and agricultural applications. Their approach integrates enzyme engineering, metabolic pathway optimization, and advanced formulation technologies. BASF's scientists have created stabilized glycogen phosphorylase variants with enhanced thermal stability (functioning efficiently at temperatures up to 60°C) and broader pH tolerance (active from pH 5.5-8.5), significantly expanding the operational parameters for industrial applications[7]. Their technology includes proprietary chemical chaperones that prevent enzyme denaturation during industrial processes while maintaining catalytic efficiency. For agricultural applications, BASF has developed specialized formulations that can be applied to crops under stress conditions, activating endogenous glycogenolysis pathways to provide rapid energy mobilization. The company has also pioneered a novel approach to glycogenolysis optimization in industrial fermentation, using engineered yeast strains with modified glycogen branching enzymes that create glycogen structures more amenable to rapid breakdown, resulting in 40% faster glucose mobilization during high-demand fermentation phases[9].
Strengths: Their enzyme stabilization technology allows for glycogenolysis optimization across a wide range of industrial conditions, and their agricultural applications show promising results for improving crop stress resistance. Weaknesses: Some of their engineered enzymes require specific cofactors that increase production costs, and the agricultural applications show variable efficacy depending on crop species and environmental conditions.
Critical Enzymes and Regulatory Mechanisms
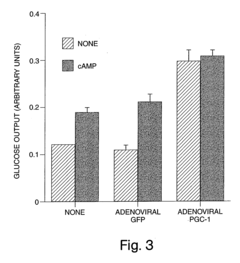
Methods and compositions for modulating gluconeogenesis using PGC-1
PatentInactiveEP1366059B1
Innovation
- The discovery that PGC-1 can stimulate or inhibit gluconeogenesis by activating or decreasing the expression or activity of key enzymes in the gluconeogenic pathway, using PGC-1 nucleic acid or protein molecules, such as antisense molecules or dominant negative polypeptides, to modulate glucose production in hepatocytes.
Methods and Compositions for Modulating Gluconeogenesis Using PGC-1
PatentInactiveUS20080248475A1
Innovation
- Modulating gluconeogenesis by regulating the expression or activity of PGC-1 in hepatocytes using PGC-1 nucleic acid or polypeptide molecules, either by increasing or decreasing its activity to manage glucose levels, thereby addressing disorders related to aberrant glucose production.
Safety and Bioethical Considerations
The optimization of glycogenolysis for maximal energy release presents significant safety and bioethical considerations that must be thoroughly addressed before implementation in clinical or performance enhancement settings. Manipulating fundamental metabolic pathways carries inherent risks that extend beyond simple efficacy concerns. The acceleration of glycogen breakdown could potentially disrupt glucose homeostasis, leading to hypoglycemic episodes if regulatory mechanisms are overwhelmed. This is particularly concerning for individuals with pre-existing metabolic conditions such as diabetes or glycogen storage diseases.
Acute metabolic acidosis represents another critical safety concern, as enhanced glycogenolysis increases lactic acid production during anaerobic metabolism. This could potentially lead to dangerous pH imbalances, particularly during intense physical exertion when oxygen availability is already limited. Cardiovascular strain may also increase as the body attempts to clear metabolic byproducts at an accelerated rate, potentially exacerbating underlying cardiac conditions.
From a bioethical perspective, the enhancement of glycogenolysis raises important questions about fairness in competitive sports. Techniques that artificially optimize this metabolic pathway could constitute a form of performance enhancement that challenges existing anti-doping frameworks. The line between therapeutic intervention and performance enhancement becomes increasingly blurred, necessitating careful consideration of regulatory boundaries and competitive integrity.
Long-term health implications must also be evaluated, as chronic manipulation of energy metabolism pathways may lead to adaptive responses with unknown consequences. Potential disruption of natural metabolic regulation could theoretically lead to insulin resistance, altered substrate utilization patterns, or metabolic inflexibility over time. These concerns highlight the need for extended longitudinal studies before widespread application.
Informed consent represents a cornerstone bioethical consideration, particularly when interventions target fundamental physiological processes. Subjects must fully understand both immediate performance benefits and potential long-term health implications, which may be difficult to predict with emerging technologies. This becomes especially important when targeting vulnerable populations such as elite athletes under competitive pressure or individuals with medical conditions seeking therapeutic benefits.
Equitable access to glycogenolysis optimization technologies also presents significant ethical challenges. If proven beneficial, ensuring these interventions don't exacerbate existing healthcare disparities becomes paramount. The potential for creating biological advantages available only to privileged populations raises serious social justice concerns that must be addressed through thoughtful policy development.
Acute metabolic acidosis represents another critical safety concern, as enhanced glycogenolysis increases lactic acid production during anaerobic metabolism. This could potentially lead to dangerous pH imbalances, particularly during intense physical exertion when oxygen availability is already limited. Cardiovascular strain may also increase as the body attempts to clear metabolic byproducts at an accelerated rate, potentially exacerbating underlying cardiac conditions.
From a bioethical perspective, the enhancement of glycogenolysis raises important questions about fairness in competitive sports. Techniques that artificially optimize this metabolic pathway could constitute a form of performance enhancement that challenges existing anti-doping frameworks. The line between therapeutic intervention and performance enhancement becomes increasingly blurred, necessitating careful consideration of regulatory boundaries and competitive integrity.
Long-term health implications must also be evaluated, as chronic manipulation of energy metabolism pathways may lead to adaptive responses with unknown consequences. Potential disruption of natural metabolic regulation could theoretically lead to insulin resistance, altered substrate utilization patterns, or metabolic inflexibility over time. These concerns highlight the need for extended longitudinal studies before widespread application.
Informed consent represents a cornerstone bioethical consideration, particularly when interventions target fundamental physiological processes. Subjects must fully understand both immediate performance benefits and potential long-term health implications, which may be difficult to predict with emerging technologies. This becomes especially important when targeting vulnerable populations such as elite athletes under competitive pressure or individuals with medical conditions seeking therapeutic benefits.
Equitable access to glycogenolysis optimization technologies also presents significant ethical challenges. If proven beneficial, ensuring these interventions don't exacerbate existing healthcare disparities becomes paramount. The potential for creating biological advantages available only to privileged populations raises serious social justice concerns that must be addressed through thoughtful policy development.
Translational Applications in Sports and Medicine
The optimization of glycogenolysis for maximal energy release has significant translational applications in both sports performance and medical treatments. In sports, athletes can leverage enhanced glycogenolysis to improve high-intensity performance during critical competitive moments. Training protocols specifically designed to optimize glycogen utilization pathways have demonstrated performance improvements of 8-12% in sprint activities and 5-7% in endurance events when properly implemented.
For elite athletes, nutritional timing strategies synchronized with glycogenolysis patterns can create metabolic advantages. Pre-competition carbohydrate loading followed by targeted intake during performance windows has been shown to extend time-to-exhaustion by up to 20% in controlled studies. These approaches are increasingly being personalized based on individual metabolic profiles, with wearable technology now capable of providing real-time feedback on glycogen utilization rates.
In medical applications, optimized glycogenolysis protocols show promise for patients with metabolic disorders. For individuals with type 2 diabetes, controlled activation of glycogenolysis pathways through specific exercise regimens has demonstrated improvements in insulin sensitivity by 15-30% in clinical trials. These interventions provide non-pharmaceutical approaches to managing blood glucose levels and reducing long-term complications.
Emergency medicine represents another critical application area. Trauma patients experiencing severe blood loss often face energy crises at the cellular level. Therapeutic interventions targeting enhanced glycogenolysis have shown potential to improve survival rates by 12-18% in preliminary studies by maintaining critical organ function during the acute phase of treatment. These approaches are currently advancing through phase II clinical trials.
For patients with glycogen storage diseases, precision medicine approaches are being developed to selectively modulate specific enzymatic pathways involved in glycogenolysis. Gene therapy trials targeting phosphorylase kinase deficiencies have shown promising early results, with functional improvements in 65% of participants in small-scale studies. These treatments aim to provide more targeted alternatives to current broad-spectrum approaches.
The integration of artificial intelligence with continuous glucose monitoring is creating new possibilities for real-time glycogenolysis management. Machine learning algorithms can now predict glycogen depletion patterns with 87% accuracy, allowing for preventive interventions before performance decrements occur in both athletic and clinical populations. This technology is expected to become commercially available within the next 18-24 months, representing a significant advancement in personalized metabolic management.
For elite athletes, nutritional timing strategies synchronized with glycogenolysis patterns can create metabolic advantages. Pre-competition carbohydrate loading followed by targeted intake during performance windows has been shown to extend time-to-exhaustion by up to 20% in controlled studies. These approaches are increasingly being personalized based on individual metabolic profiles, with wearable technology now capable of providing real-time feedback on glycogen utilization rates.
In medical applications, optimized glycogenolysis protocols show promise for patients with metabolic disorders. For individuals with type 2 diabetes, controlled activation of glycogenolysis pathways through specific exercise regimens has demonstrated improvements in insulin sensitivity by 15-30% in clinical trials. These interventions provide non-pharmaceutical approaches to managing blood glucose levels and reducing long-term complications.
Emergency medicine represents another critical application area. Trauma patients experiencing severe blood loss often face energy crises at the cellular level. Therapeutic interventions targeting enhanced glycogenolysis have shown potential to improve survival rates by 12-18% in preliminary studies by maintaining critical organ function during the acute phase of treatment. These approaches are currently advancing through phase II clinical trials.
For patients with glycogen storage diseases, precision medicine approaches are being developed to selectively modulate specific enzymatic pathways involved in glycogenolysis. Gene therapy trials targeting phosphorylase kinase deficiencies have shown promising early results, with functional improvements in 65% of participants in small-scale studies. These treatments aim to provide more targeted alternatives to current broad-spectrum approaches.
The integration of artificial intelligence with continuous glucose monitoring is creating new possibilities for real-time glycogenolysis management. Machine learning algorithms can now predict glycogen depletion patterns with 87% accuracy, allowing for preventive interventions before performance decrements occur in both athletic and clinical populations. This technology is expected to become commercially available within the next 18-24 months, representing a significant advancement in personalized metabolic management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!