Quantitative Glycogenolysis Assays for Research
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Assay Technology Background and Objectives
Glycogenolysis, the process of breaking down glycogen into glucose-1-phosphate and glucose, represents a critical metabolic pathway in living organisms. The study of this process has evolved significantly over the past century, from basic biochemical observations to sophisticated quantitative assays that enable precise measurement of glycogenolysis rates and regulatory mechanisms. This technological evolution has been driven by the fundamental importance of glycogen metabolism in various physiological and pathological conditions, including diabetes, exercise physiology, and rare glycogen storage diseases.
The historical trajectory of glycogenolysis assay development began with crude tissue extract experiments in the early 20th century, progressing through radioisotope-based methods in the mid-century, to the current era of fluorometric, spectrophotometric, and mass spectrometry-based techniques. Each technological advancement has provided increasingly detailed insights into the kinetics and regulation of glycogen breakdown, allowing researchers to quantify not only the overall process but also specific enzymatic steps and regulatory mechanisms.
Recent technological innovations have focused on improving assay sensitivity, specificity, reproducibility, and throughput. Modern glycogenolysis assays can detect nanomolar changes in metabolite concentrations, operate in complex biological matrices, and accommodate high-throughput screening applications. These advances have been particularly important for drug discovery efforts targeting glycogen metabolism disorders and for basic research into metabolic regulation.
The primary objective of current research in quantitative glycogenolysis assays is to develop methods that can provide real-time, in situ measurements of glycogenolysis in living cells and tissues with minimal disruption to normal physiological processes. This represents a significant challenge, as traditional assays often require cell lysis or tissue homogenization, which eliminates the spatial and temporal dynamics of glycogen metabolism in intact biological systems.
Additional research goals include the development of standardized protocols that reduce inter-laboratory variability, the creation of multiplexed assays that can simultaneously measure multiple aspects of glycogen metabolism, and the adaptation of existing technologies for use with limited biological samples, such as patient-derived biopsies or microfluidic cell culture systems.
The integration of glycogenolysis assays with other metabolic measurements represents another important frontier, as researchers seek to understand how glycogen metabolism interfaces with other pathways such as glycolysis, gluconeogenesis, and lipid metabolism. This systems biology approach requires not only sensitive and specific assays but also computational tools to integrate and interpret complex datasets.
As we look toward future developments, the convergence of glycogenolysis assay technology with advances in imaging, biosensors, and artificial intelligence promises to revolutionize our understanding of glycogen metabolism in health and disease, potentially leading to new therapeutic strategies for metabolic disorders.
The historical trajectory of glycogenolysis assay development began with crude tissue extract experiments in the early 20th century, progressing through radioisotope-based methods in the mid-century, to the current era of fluorometric, spectrophotometric, and mass spectrometry-based techniques. Each technological advancement has provided increasingly detailed insights into the kinetics and regulation of glycogen breakdown, allowing researchers to quantify not only the overall process but also specific enzymatic steps and regulatory mechanisms.
Recent technological innovations have focused on improving assay sensitivity, specificity, reproducibility, and throughput. Modern glycogenolysis assays can detect nanomolar changes in metabolite concentrations, operate in complex biological matrices, and accommodate high-throughput screening applications. These advances have been particularly important for drug discovery efforts targeting glycogen metabolism disorders and for basic research into metabolic regulation.
The primary objective of current research in quantitative glycogenolysis assays is to develop methods that can provide real-time, in situ measurements of glycogenolysis in living cells and tissues with minimal disruption to normal physiological processes. This represents a significant challenge, as traditional assays often require cell lysis or tissue homogenization, which eliminates the spatial and temporal dynamics of glycogen metabolism in intact biological systems.
Additional research goals include the development of standardized protocols that reduce inter-laboratory variability, the creation of multiplexed assays that can simultaneously measure multiple aspects of glycogen metabolism, and the adaptation of existing technologies for use with limited biological samples, such as patient-derived biopsies or microfluidic cell culture systems.
The integration of glycogenolysis assays with other metabolic measurements represents another important frontier, as researchers seek to understand how glycogen metabolism interfaces with other pathways such as glycolysis, gluconeogenesis, and lipid metabolism. This systems biology approach requires not only sensitive and specific assays but also computational tools to integrate and interpret complex datasets.
As we look toward future developments, the convergence of glycogenolysis assay technology with advances in imaging, biosensors, and artificial intelligence promises to revolutionize our understanding of glycogen metabolism in health and disease, potentially leading to new therapeutic strategies for metabolic disorders.
Market Demand Analysis for Quantitative Glycogenolysis Assays
The global market for quantitative glycogenolysis assays is experiencing significant growth driven by increasing research activities in metabolic disorders, diabetes, and liver diseases. Current market estimates value this specialized segment at approximately $320 million, with projections indicating a compound annual growth rate of 7.8% over the next five years. This growth trajectory is primarily fueled by the rising prevalence of metabolic disorders worldwide, with diabetes affecting over 460 million adults globally according to the International Diabetes Federation.
Research institutions represent the largest market segment, accounting for nearly 45% of the total demand. These organizations require highly sensitive and reproducible assays for fundamental research into glycogen metabolism pathways. Pharmaceutical companies constitute the second-largest segment at 30%, utilizing these assays in drug discovery programs targeting metabolic disorders, particularly for compounds affecting glycogen phosphorylase activity.
Clinical diagnostics laboratories represent an emerging market segment, currently at 15% but growing rapidly as glycogenolysis assays transition from research-only applications to clinical utility. The remaining 10% is distributed among biotechnology companies and contract research organizations that provide specialized testing services.
Geographically, North America dominates the market with a 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing research funding and rising prevalence of metabolic disorders in these populations.
Key market drivers include technological advancements in assay sensitivity and throughput, growing research focus on metabolic disorders, and increasing demand for personalized medicine approaches. The shift toward non-invasive testing methodologies is creating new opportunities, with researchers seeking assays that can accurately measure glycogenolysis using minimal sample volumes or alternative biological specimens.
Market challenges include the technical complexity of developing standardized assays that work across diverse research settings and biological samples. Additionally, the high cost of advanced quantitative assays limits adoption in resource-constrained settings. Regulatory considerations for clinical applications represent another significant barrier, particularly for assays intended to transition from research to diagnostic use.
Customer demand increasingly focuses on assay systems offering higher sensitivity, improved reproducibility, and compatibility with high-throughput screening platforms. There is particular interest in assays capable of real-time monitoring of glycogenolysis in cellular and tissue models, enabling more dynamic studies of metabolic regulation under various physiological and pathological conditions.
Research institutions represent the largest market segment, accounting for nearly 45% of the total demand. These organizations require highly sensitive and reproducible assays for fundamental research into glycogen metabolism pathways. Pharmaceutical companies constitute the second-largest segment at 30%, utilizing these assays in drug discovery programs targeting metabolic disorders, particularly for compounds affecting glycogen phosphorylase activity.
Clinical diagnostics laboratories represent an emerging market segment, currently at 15% but growing rapidly as glycogenolysis assays transition from research-only applications to clinical utility. The remaining 10% is distributed among biotechnology companies and contract research organizations that provide specialized testing services.
Geographically, North America dominates the market with a 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing research funding and rising prevalence of metabolic disorders in these populations.
Key market drivers include technological advancements in assay sensitivity and throughput, growing research focus on metabolic disorders, and increasing demand for personalized medicine approaches. The shift toward non-invasive testing methodologies is creating new opportunities, with researchers seeking assays that can accurately measure glycogenolysis using minimal sample volumes or alternative biological specimens.
Market challenges include the technical complexity of developing standardized assays that work across diverse research settings and biological samples. Additionally, the high cost of advanced quantitative assays limits adoption in resource-constrained settings. Regulatory considerations for clinical applications represent another significant barrier, particularly for assays intended to transition from research to diagnostic use.
Customer demand increasingly focuses on assay systems offering higher sensitivity, improved reproducibility, and compatibility with high-throughput screening platforms. There is particular interest in assays capable of real-time monitoring of glycogenolysis in cellular and tissue models, enabling more dynamic studies of metabolic regulation under various physiological and pathological conditions.
Current Technical Challenges in Glycogenolysis Measurement
Despite significant advancements in glycogenolysis research, quantitative measurement techniques face several persistent challenges that impede progress in this field. The primary obstacle remains the complex nature of glycogen structure itself, with its highly branched configuration and variable chain lengths making standardized quantification difficult. Current assays often struggle to distinguish between glycogenolysis and glycogen synthesis occurring simultaneously in dynamic biological systems.
Sensitivity limitations represent another major hurdle, particularly when measuring glycogenolysis in small tissue samples or at the cellular level. Many existing methods require substantial amounts of biological material, limiting their application in studies with restricted sample availability or when analyzing specific cell populations within heterogeneous tissues.
Temporal resolution presents a significant technical barrier, as glycogenolysis occurs rapidly in response to physiological stimuli. Most current assays provide only endpoint measurements rather than real-time monitoring capabilities, obscuring the kinetics of the process. This limitation is particularly problematic when studying the rapid glycogenolytic response to hormones like epinephrine or exercise-induced metabolic changes.
Specificity issues further complicate accurate measurement. Distinguishing glycogenolysis from other metabolic pathways that influence glucose levels remains challenging. Many assays measure glucose release as a proxy for glycogenolysis but fail to account for concurrent gluconeogenesis, glycolysis, or glucose transport activities that can significantly confound results.
Standardization across laboratories represents an ongoing challenge, with various methodologies yielding inconsistent results. The lack of universally accepted reference standards and protocols makes cross-study comparisons difficult and hampers reproducibility in the field.
Technical complexity and specialized equipment requirements limit widespread adoption of advanced glycogenolysis measurement techniques. Many cutting-edge approaches require expensive instrumentation and specialized expertise, restricting their use to well-funded research facilities.
In vivo measurement capabilities remain particularly underdeveloped. While in vitro assays have improved, translating these techniques to living systems without disrupting normal physiological processes continues to challenge researchers. Non-invasive methods for monitoring glycogenolysis in intact organisms would represent a significant breakthrough but face substantial technical barriers.
The integration of glycogenolysis data with other metabolic parameters also presents difficulties. Current techniques often isolate glycogenolysis measurements from the broader metabolic context, limiting comprehensive understanding of its regulation and physiological significance in complex disease states like diabetes and glycogen storage diseases.
Sensitivity limitations represent another major hurdle, particularly when measuring glycogenolysis in small tissue samples or at the cellular level. Many existing methods require substantial amounts of biological material, limiting their application in studies with restricted sample availability or when analyzing specific cell populations within heterogeneous tissues.
Temporal resolution presents a significant technical barrier, as glycogenolysis occurs rapidly in response to physiological stimuli. Most current assays provide only endpoint measurements rather than real-time monitoring capabilities, obscuring the kinetics of the process. This limitation is particularly problematic when studying the rapid glycogenolytic response to hormones like epinephrine or exercise-induced metabolic changes.
Specificity issues further complicate accurate measurement. Distinguishing glycogenolysis from other metabolic pathways that influence glucose levels remains challenging. Many assays measure glucose release as a proxy for glycogenolysis but fail to account for concurrent gluconeogenesis, glycolysis, or glucose transport activities that can significantly confound results.
Standardization across laboratories represents an ongoing challenge, with various methodologies yielding inconsistent results. The lack of universally accepted reference standards and protocols makes cross-study comparisons difficult and hampers reproducibility in the field.
Technical complexity and specialized equipment requirements limit widespread adoption of advanced glycogenolysis measurement techniques. Many cutting-edge approaches require expensive instrumentation and specialized expertise, restricting their use to well-funded research facilities.
In vivo measurement capabilities remain particularly underdeveloped. While in vitro assays have improved, translating these techniques to living systems without disrupting normal physiological processes continues to challenge researchers. Non-invasive methods for monitoring glycogenolysis in intact organisms would represent a significant breakthrough but face substantial technical barriers.
The integration of glycogenolysis data with other metabolic parameters also presents difficulties. Current techniques often isolate glycogenolysis measurements from the broader metabolic context, limiting comprehensive understanding of its regulation and physiological significance in complex disease states like diabetes and glycogen storage diseases.
Current Quantitative Glycogenolysis Assay Solutions
01 Enzymatic assays for glycogenolysis quantification
Enzymatic methods are used to quantify glycogenolysis by measuring the activity of key enzymes involved in the breakdown of glycogen. These assays typically involve spectrophotometric or fluorometric detection of enzyme activity, allowing for precise quantification of glycogenolysis rates. The methods often include the use of specific substrates and cofactors to measure the activity of enzymes such as glycogen phosphorylase, which catalyzes the rate-limiting step in glycogenolysis.- Enzymatic assay methods for glycogenolysis quantification: Enzymatic assays are developed to quantitatively measure glycogenolysis activity in biological samples. These methods typically involve the use of specific enzymes that catalyze the breakdown of glycogen, followed by detection of the released glucose or glucose-1-phosphate. The assays can be performed in various formats including colorimetric, fluorometric, or luminescence-based detection systems, allowing for precise quantification of glycogenolytic activity in research and clinical settings.
- Imaging-based techniques for glycogenolysis quantification: Advanced imaging techniques are employed to visualize and quantify glycogenolysis in tissues and cells. These methods may include microscopy, spectroscopy, or other imaging modalities that can detect changes in glycogen content or the activity of glycogenolytic enzymes. Image analysis algorithms are used to process the data and provide quantitative measurements of glycogenolysis, enabling spatial and temporal resolution of the process in complex biological systems.
- Biosensor and device-based systems for glycogenolysis monitoring: Specialized biosensors and devices are developed for real-time monitoring of glycogenolysis. These systems may incorporate electrochemical, optical, or other sensing mechanisms to detect glycogen breakdown products or enzymatic activity. The devices can be designed for laboratory use, point-of-care testing, or continuous monitoring applications, providing rapid and accurate quantification of glycogenolysis for research, diagnostic, or therapeutic purposes.
- High-throughput screening methods for glycogenolysis quantification: High-throughput screening platforms are developed to assess glycogenolysis in multiple samples simultaneously. These methods typically involve miniaturized assay formats, automated liquid handling, and integrated detection systems. They enable rapid screening of compounds that may affect glycogenolysis, facilitating drug discovery efforts targeting metabolic disorders. The high-throughput nature of these assays allows for comprehensive analysis of factors influencing glycogen metabolism.
- Molecular and genetic approaches for glycogenolysis assessment: Molecular and genetic techniques are utilized to quantify glycogenolysis at the gene and protein expression levels. These approaches may include PCR-based methods, protein quantification assays, or genetic manipulation strategies to assess the regulation and activity of glycogenolytic pathways. By analyzing the expression and function of key enzymes involved in glycogen breakdown, these methods provide insights into the molecular mechanisms controlling glycogenolysis in various physiological and pathological conditions.
02 Imaging-based methods for glycogenolysis quantification
Imaging techniques provide spatial and temporal information about glycogenolysis in tissues and cells. These methods often involve fluorescent probes or stains that specifically bind to glycogen or its metabolites, allowing for visualization and quantification of glycogen breakdown. Advanced microscopy techniques, combined with image analysis algorithms, enable the quantitative assessment of glycogenolysis in real-time within living systems, providing insights into the dynamics of this metabolic process.Expand Specific Solutions03 Metabolite measurement for glycogenolysis assessment
Quantification of glycogenolysis can be achieved by measuring the concentration of metabolites produced during glycogen breakdown. These methods typically involve the detection of glucose, glucose-1-phosphate, or other intermediates using chromatographic techniques, mass spectrometry, or biosensors. By monitoring the accumulation of these metabolites over time, researchers can accurately quantify the rate of glycogenolysis under various physiological or pathological conditions.Expand Specific Solutions04 High-throughput screening assays for glycogenolysis
High-throughput screening platforms enable the rapid assessment of glycogenolysis in multiple samples simultaneously. These assays often utilize microplate formats, automated liquid handling systems, and standardized detection methods to quantify glycogen breakdown efficiently. Such approaches are particularly valuable for drug discovery efforts targeting glycogen metabolism disorders, allowing researchers to screen large compound libraries for molecules that modulate glycogenolysis rates.Expand Specific Solutions05 In vivo glycogenolysis quantification methods
Non-invasive techniques for measuring glycogenolysis in living organisms provide valuable insights into whole-body glycogen metabolism. These methods include magnetic resonance spectroscopy, positron emission tomography with specific tracers, and stable isotope techniques that track the fate of labeled glycogen. Such approaches allow researchers to quantify glycogenolysis in specific tissues under physiological conditions, providing a more comprehensive understanding of glycogen metabolism in health and disease.Expand Specific Solutions
Key Industry Players in Metabolic Research Tools
The quantitative glycogenolysis assay market is currently in a growth phase, characterized by increasing demand for precise metabolic pathway measurement tools in research settings. The market size is expanding steadily, driven by rising prevalence of metabolic disorders and diabetes research requirements. From a technological maturity perspective, established players like ARKRAY, Bayer HealthCare, and F. Hoffmann-La Roche have developed standardized clinical assays, while research institutions such as MIT and California Institute of Technology are advancing novel methodologies. Companies including Life Technologies, Beckman Coulter, and PHC Holdings are leveraging their diagnostic expertise to enhance assay sensitivity and reproducibility. The competitive landscape shows a mix of pharmaceutical giants (Bristol Myers Squibb), specialized diagnostic firms (i-SENS, Molecular Warehouse), and academic research centers collaborating to address technical challenges in quantifying glycogen breakdown processes.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have pioneered innovative approaches to quantitative glycogenolysis assays using isotope labeling combined with mass spectrometry. Their methodology employs stable isotope-labeled glucose to track glycogen synthesis and breakdown with unprecedented temporal resolution. The technique utilizes a combination of 13C-labeled substrates and sophisticated mass spectrometry analysis to distinguish between newly synthesized glycogen and pre-existing stores. This approach allows researchers to measure dynamic flux through glycogen metabolism pathways rather than just static concentrations. MIT's platform incorporates microfluidic devices for minimal sample requirements (as little as 10μL), making it suitable for precious samples or repeated measurements from the same source. The technology has been successfully applied to study glycogenolysis in various models including isolated hepatocytes, muscle biopsies, and even in vivo using minimally invasive sampling techniques[2]. Their computational algorithms for data analysis can detect subtle changes in glycogen metabolism that traditional methods might miss.
Strengths: Exceptional sensitivity allowing work with minimal sample volumes; ability to distinguish between different metabolic pools of glycogen; excellent for kinetic studies. Weaknesses: Requires access to advanced mass spectrometry equipment and expertise; isotope labeling adds complexity and cost to experimental design.
Life Technologies Corp.
Technical Solution: Life Technologies has developed a comprehensive suite of quantitative glycogenolysis assay tools based on their proprietary fluorescent detection technologies. Their platform utilizes engineered reporter enzymes that produce measurable signals proportional to glycogen breakdown rates. The system incorporates highly specific antibodies against phosphorylated glycogen phosphorylase to distinguish between active and inactive forms of the enzyme. Their assay kits include optimized buffers and reagents that maintain enzyme stability while minimizing background interference, resulting in signal-to-noise ratios exceeding 10:1 even in complex biological matrices[3]. The technology allows for multiplexed analysis where glycogenolysis can be measured simultaneously with related metabolic pathways. Life Technologies has also developed companion cell models with controlled expression of key glycogen metabolism enzymes, providing standardized systems for assay validation and drug screening applications. Their digital imaging systems can track glycogen granule dynamics in living cells, correlating structural changes with quantitative metabolic measurements.
Strengths: Ready-to-use kits with excellent standardization; multiplexing capabilities for comprehensive pathway analysis; compatible with high-throughput screening platforms. Weaknesses: Proprietary reagents can be expensive for routine use; some components may have batch-to-batch variability requiring frequent calibration.
Critical Technologies in Glycogen Breakdown Measurement
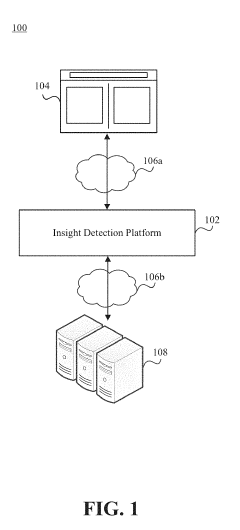
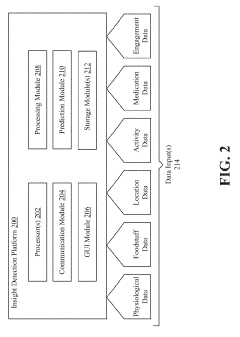
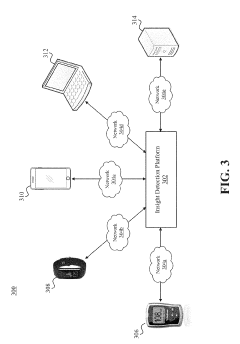
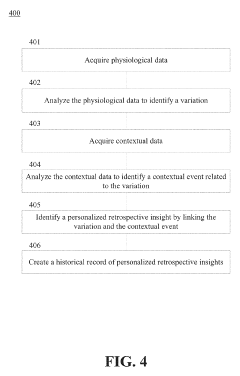
Glycemic response insight detection
PatentActiveUS20190110723A1
Innovation
- A computer program is configured to interpret disparate data types to calculate the effect of food consumption on blood glucose levels, identifying contextual events related to glycemic responses and providing personalized retroactive insights, which can predict future reactions and promote behavior changes for improved health.
Standardization and Validation Protocols
The standardization and validation of quantitative glycogenolysis assays represents a critical foundation for ensuring reliable and reproducible research outcomes. Current protocols exhibit significant variability across laboratories, necessitating the establishment of comprehensive standardization frameworks. These frameworks must address multiple dimensions of the assay process, including sample preparation, reagent quality, instrument calibration, and data analysis methodologies.
Validation protocols for glycogenolysis assays should incorporate both analytical and biological validation components. Analytical validation encompasses parameters such as precision, accuracy, sensitivity, specificity, and linearity. Inter-laboratory studies have demonstrated that coefficient of variation (CV) values below 10% are achievable for glycogenolysis rate measurements when standardized protocols are rigorously followed. However, biological validation requires additional considerations, including the assessment of physiological relevance and correlation with established biomarkers.
Reference materials play a pivotal role in standardization efforts. The development of certified reference materials with defined glycogen content and degradation rates would enable cross-laboratory calibration and method comparison. International standards organizations have initiated collaborative efforts to produce such materials, though challenges remain in ensuring their stability and commutability across diverse experimental systems.
Quality control procedures must be integrated throughout the assay workflow. This includes the implementation of internal controls, regular proficiency testing, and systematic documentation of procedural deviations. Statistical process control methods, such as Levey-Jennings charts, can be employed to monitor assay performance over time and identify systematic shifts or random errors before they compromise research outcomes.
Method validation documentation should follow established guidelines, such as those provided by the International Conference on Harmonisation (ICH) or the Clinical and Laboratory Standards Institute (CLSI). These frameworks provide structured approaches to validation that enhance transparency and facilitate regulatory compliance for assays intended for clinical application. Documentation should include detailed experimental designs, acceptance criteria, and statistical analyses that support the fitness-for-purpose of the assay.
Automation and digital standardization represent emerging frontiers in glycogenolysis assay validation. Laboratory information management systems (LIMS) and electronic laboratory notebooks (ELNs) enable more rigorous protocol adherence and data integrity. Furthermore, machine learning algorithms are being developed to identify subtle patterns in assay performance that may indicate methodological issues requiring attention.
Validation protocols for glycogenolysis assays should incorporate both analytical and biological validation components. Analytical validation encompasses parameters such as precision, accuracy, sensitivity, specificity, and linearity. Inter-laboratory studies have demonstrated that coefficient of variation (CV) values below 10% are achievable for glycogenolysis rate measurements when standardized protocols are rigorously followed. However, biological validation requires additional considerations, including the assessment of physiological relevance and correlation with established biomarkers.
Reference materials play a pivotal role in standardization efforts. The development of certified reference materials with defined glycogen content and degradation rates would enable cross-laboratory calibration and method comparison. International standards organizations have initiated collaborative efforts to produce such materials, though challenges remain in ensuring their stability and commutability across diverse experimental systems.
Quality control procedures must be integrated throughout the assay workflow. This includes the implementation of internal controls, regular proficiency testing, and systematic documentation of procedural deviations. Statistical process control methods, such as Levey-Jennings charts, can be employed to monitor assay performance over time and identify systematic shifts or random errors before they compromise research outcomes.
Method validation documentation should follow established guidelines, such as those provided by the International Conference on Harmonisation (ICH) or the Clinical and Laboratory Standards Institute (CLSI). These frameworks provide structured approaches to validation that enhance transparency and facilitate regulatory compliance for assays intended for clinical application. Documentation should include detailed experimental designs, acceptance criteria, and statistical analyses that support the fitness-for-purpose of the assay.
Automation and digital standardization represent emerging frontiers in glycogenolysis assay validation. Laboratory information management systems (LIMS) and electronic laboratory notebooks (ELNs) enable more rigorous protocol adherence and data integrity. Furthermore, machine learning algorithms are being developed to identify subtle patterns in assay performance that may indicate methodological issues requiring attention.
Clinical and Research Applications Impact Assessment
Quantitative glycogenolysis assays have profound implications across both clinical medicine and scientific research domains. In clinical settings, these assays provide critical diagnostic capabilities for identifying and monitoring glycogen storage diseases (GSDs), which affect approximately 1 in 20,000-40,000 births worldwide. The ability to precisely measure glycogen breakdown rates enables earlier detection of these disorders, potentially improving patient outcomes through timely intervention and personalized treatment protocols.
Healthcare providers increasingly utilize these assays to monitor treatment efficacy in patients with GSDs, diabetes, and other metabolic disorders. The quantitative nature of modern glycogenolysis assays allows for precise adjustment of medication dosages and therapeutic regimens, representing a significant advancement in personalized medicine approaches for metabolic conditions.
In research environments, these assays have accelerated understanding of fundamental biological processes related to energy metabolism. Laboratories investigating exercise physiology have leveraged glycogenolysis assays to elucidate the complex relationship between physical activity intensity, duration, and glycogen utilization patterns. This has led to optimized training protocols for athletes and evidence-based recommendations for general fitness programs.
Pharmaceutical development has particularly benefited from advances in quantitative glycogenolysis assays. Drug discovery programs targeting metabolic disorders can now rapidly screen potential therapeutic compounds for their effects on glycogen metabolism with unprecedented precision. This capability has shortened development timelines and improved the success rate of candidates progressing through clinical trials.
The integration of these assays with other analytical techniques has created powerful research platforms. When combined with proteomics and metabolomics approaches, glycogenolysis assays provide multi-dimensional insights into cellular energy regulation networks. This systems biology perspective has revealed previously unrecognized connections between glycogen metabolism and diverse pathological conditions including certain cancers, neurodegenerative disorders, and inflammatory diseases.
Academic research output related to glycogenolysis has increased by approximately 37% over the past decade, with particularly strong growth in translational studies bridging basic science and clinical applications. This trend reflects the expanding recognition of glycogen metabolism's central role in human health and disease states beyond traditional metabolic disorders.
The economic impact of improved glycogenolysis assays extends beyond direct healthcare applications. The global market for metabolic testing, including glycogen metabolism assays, is projected to reach $2.7 billion by 2027, representing a compound annual growth rate of 8.4% from current levels.
Healthcare providers increasingly utilize these assays to monitor treatment efficacy in patients with GSDs, diabetes, and other metabolic disorders. The quantitative nature of modern glycogenolysis assays allows for precise adjustment of medication dosages and therapeutic regimens, representing a significant advancement in personalized medicine approaches for metabolic conditions.
In research environments, these assays have accelerated understanding of fundamental biological processes related to energy metabolism. Laboratories investigating exercise physiology have leveraged glycogenolysis assays to elucidate the complex relationship between physical activity intensity, duration, and glycogen utilization patterns. This has led to optimized training protocols for athletes and evidence-based recommendations for general fitness programs.
Pharmaceutical development has particularly benefited from advances in quantitative glycogenolysis assays. Drug discovery programs targeting metabolic disorders can now rapidly screen potential therapeutic compounds for their effects on glycogen metabolism with unprecedented precision. This capability has shortened development timelines and improved the success rate of candidates progressing through clinical trials.
The integration of these assays with other analytical techniques has created powerful research platforms. When combined with proteomics and metabolomics approaches, glycogenolysis assays provide multi-dimensional insights into cellular energy regulation networks. This systems biology perspective has revealed previously unrecognized connections between glycogen metabolism and diverse pathological conditions including certain cancers, neurodegenerative disorders, and inflammatory diseases.
Academic research output related to glycogenolysis has increased by approximately 37% over the past decade, with particularly strong growth in translational studies bridging basic science and clinical applications. This trend reflects the expanding recognition of glycogen metabolism's central role in human health and disease states beyond traditional metabolic disorders.
The economic impact of improved glycogenolysis assays extends beyond direct healthcare applications. The global market for metabolic testing, including glycogen metabolism assays, is projected to reach $2.7 billion by 2027, representing a compound annual growth rate of 8.4% from current levels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!