Comparative Study of Glycogenolysis vs Lipolysis
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metabolic Pathways Background and Research Objectives
Metabolic energy production is a fundamental process that has evolved over billions of years, enabling organisms to convert stored energy into usable forms. Glycogenolysis and lipolysis represent two distinct yet complementary pathways that organisms utilize to maintain energy homeostasis under varying physiological conditions. These processes have been extensively studied since the early 20th century, with significant breakthroughs occurring in the 1960s through the work of researchers like Christian de Duve and Earl Sutherland.
The evolutionary development of these pathways demonstrates remarkable conservation across species while exhibiting specialized adaptations in different organisms. From single-celled eukaryotes to complex mammals, the core enzymatic machinery has remained largely intact, highlighting the essential nature of these metabolic processes for survival.
Recent technological advances in metabolomics, proteomics, and computational biology have dramatically enhanced our understanding of these pathways at molecular and systems levels. The integration of multi-omics approaches has revealed previously unknown regulatory mechanisms and interconnections between glycogenolysis and lipolysis, suggesting more complex coordination than previously recognized.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of glycogenolysis and lipolysis pathways, examining their molecular mechanisms, regulatory networks, energetic efficiency, and physiological significance across different biological contexts. We aim to identify key differences in pathway activation, substrate utilization efficiency, energy yield, and regulatory control mechanisms.
Secondary objectives include mapping the cross-talk between these pathways under various metabolic states, identifying potential therapeutic targets for metabolic disorders, and exploring biotechnological applications that could leverage these natural energy conversion systems. The research will particularly focus on how these pathways respond to different stressors, including exercise, fasting, and pathological conditions.
The technological landscape surrounding metabolic research has evolved significantly, with tools like CRISPR-Cas9 gene editing, real-time metabolic flux analysis, and advanced imaging techniques providing unprecedented insights into pathway dynamics. These technologies enable more precise manipulation and observation of metabolic processes than ever before, creating opportunities for novel discoveries and applications.
By establishing a comprehensive understanding of the comparative aspects of glycogenolysis and lipolysis, this research aims to contribute to the development of targeted interventions for metabolic disorders, optimize energy utilization strategies for athletic performance, and potentially inform bioenergy production systems inspired by natural metabolic processes.
The evolutionary development of these pathways demonstrates remarkable conservation across species while exhibiting specialized adaptations in different organisms. From single-celled eukaryotes to complex mammals, the core enzymatic machinery has remained largely intact, highlighting the essential nature of these metabolic processes for survival.
Recent technological advances in metabolomics, proteomics, and computational biology have dramatically enhanced our understanding of these pathways at molecular and systems levels. The integration of multi-omics approaches has revealed previously unknown regulatory mechanisms and interconnections between glycogenolysis and lipolysis, suggesting more complex coordination than previously recognized.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of glycogenolysis and lipolysis pathways, examining their molecular mechanisms, regulatory networks, energetic efficiency, and physiological significance across different biological contexts. We aim to identify key differences in pathway activation, substrate utilization efficiency, energy yield, and regulatory control mechanisms.
Secondary objectives include mapping the cross-talk between these pathways under various metabolic states, identifying potential therapeutic targets for metabolic disorders, and exploring biotechnological applications that could leverage these natural energy conversion systems. The research will particularly focus on how these pathways respond to different stressors, including exercise, fasting, and pathological conditions.
The technological landscape surrounding metabolic research has evolved significantly, with tools like CRISPR-Cas9 gene editing, real-time metabolic flux analysis, and advanced imaging techniques providing unprecedented insights into pathway dynamics. These technologies enable more precise manipulation and observation of metabolic processes than ever before, creating opportunities for novel discoveries and applications.
By establishing a comprehensive understanding of the comparative aspects of glycogenolysis and lipolysis, this research aims to contribute to the development of targeted interventions for metabolic disorders, optimize energy utilization strategies for athletic performance, and potentially inform bioenergy production systems inspired by natural metabolic processes.
Market Analysis of Metabolic Research Applications
The metabolic research market has witnessed substantial growth in recent years, driven by increasing prevalence of metabolic disorders and growing interest in understanding fundamental metabolic processes. The global market for metabolic research tools and technologies was valued at approximately $12.3 billion in 2022 and is projected to reach $18.7 billion by 2027, representing a compound annual growth rate of 8.7%.
Research focusing on energy metabolism pathways, particularly glycogenolysis and lipolysis, constitutes a significant segment of this market. These fundamental metabolic processes have applications across multiple industries including pharmaceuticals, nutrition, sports science, and personalized medicine. The pharmaceutical sector remains the largest end-user, accounting for roughly 42% of the market share, followed by academic and research institutions at 28%.
North America dominates the metabolic research market with approximately 38% market share, attributed to substantial R&D investments and presence of major research institutions. Europe follows closely at 30%, while Asia-Pacific represents the fastest-growing region with a projected CAGR of 10.5% through 2027, primarily driven by increasing research activities in China, Japan, and India.
The comparative study of glycogenolysis versus lipolysis has particular commercial relevance in several high-growth application areas. The sports nutrition segment, valued at $3.8 billion in 2022, has seen increasing demand for products that optimize energy utilization during different exercise intensities. Understanding the balance between these metabolic pathways has led to the development of targeted nutritional supplements with specific timing protocols to enhance athletic performance.
In the diabetes management sector, technologies for monitoring glycogenolysis pathways have created a specialized market niche worth approximately $2.1 billion. Companies developing continuous glucose monitoring systems are increasingly incorporating metabolic pathway analytics to provide more comprehensive patient insights.
The obesity treatment market, currently valued at $4.2 billion, has witnessed significant investment in research comparing fat mobilization (lipolysis) versus carbohydrate utilization (glycogenolysis) under various dietary and pharmaceutical interventions. This has spurred development of targeted therapeutics designed to modulate specific metabolic pathways.
Key market drivers include increasing prevalence of metabolic disorders, growing adoption of personalized medicine approaches, and technological advancements in metabolic research tools. However, market challenges persist, including high research costs, complex regulatory requirements for metabolic biomarkers, and difficulties in translating basic metabolic research into commercial applications.
Research focusing on energy metabolism pathways, particularly glycogenolysis and lipolysis, constitutes a significant segment of this market. These fundamental metabolic processes have applications across multiple industries including pharmaceuticals, nutrition, sports science, and personalized medicine. The pharmaceutical sector remains the largest end-user, accounting for roughly 42% of the market share, followed by academic and research institutions at 28%.
North America dominates the metabolic research market with approximately 38% market share, attributed to substantial R&D investments and presence of major research institutions. Europe follows closely at 30%, while Asia-Pacific represents the fastest-growing region with a projected CAGR of 10.5% through 2027, primarily driven by increasing research activities in China, Japan, and India.
The comparative study of glycogenolysis versus lipolysis has particular commercial relevance in several high-growth application areas. The sports nutrition segment, valued at $3.8 billion in 2022, has seen increasing demand for products that optimize energy utilization during different exercise intensities. Understanding the balance between these metabolic pathways has led to the development of targeted nutritional supplements with specific timing protocols to enhance athletic performance.
In the diabetes management sector, technologies for monitoring glycogenolysis pathways have created a specialized market niche worth approximately $2.1 billion. Companies developing continuous glucose monitoring systems are increasingly incorporating metabolic pathway analytics to provide more comprehensive patient insights.
The obesity treatment market, currently valued at $4.2 billion, has witnessed significant investment in research comparing fat mobilization (lipolysis) versus carbohydrate utilization (glycogenolysis) under various dietary and pharmaceutical interventions. This has spurred development of targeted therapeutics designed to modulate specific metabolic pathways.
Key market drivers include increasing prevalence of metabolic disorders, growing adoption of personalized medicine approaches, and technological advancements in metabolic research tools. However, market challenges persist, including high research costs, complex regulatory requirements for metabolic biomarkers, and difficulties in translating basic metabolic research into commercial applications.
Current Understanding and Technical Challenges in Energy Metabolism
The field of energy metabolism has witnessed significant advancements in understanding the biochemical pathways of glycogenolysis and lipolysis. Current research has established that glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and ultimately glucose-6-phosphate, serves as a rapid energy mobilization mechanism primarily in the liver and skeletal muscles. This process is triggered by hormones like glucagon and epinephrine, particularly during short-term, high-intensity exercise or fasting states.
Conversely, lipolysis involves the hydrolysis of triglycerides into glycerol and free fatty acids, occurring predominantly in adipose tissue. This process provides a more sustained energy source during prolonged exercise or extended fasting periods. Recent metabolomic studies have revealed intricate regulatory networks governing these pathways, including the identification of novel signaling molecules and feedback mechanisms.
Despite these advances, several technical challenges persist in fully elucidating the comparative dynamics of these processes. Real-time monitoring of pathway activation remains difficult, particularly in human subjects under physiological conditions. Current technologies like stable isotope tracers provide valuable insights but often lack temporal resolution to capture rapid metabolic shifts between pathways.
Another significant challenge lies in understanding the tissue-specific variations in these processes. While liver glycogenolysis and adipose tissue lipolysis are well-characterized, the regulatory mechanisms in other tissues such as cardiac muscle, brain, and kidney remain incompletely understood. Recent single-cell metabolomic approaches show promise but face limitations in sample preparation and metabolite stability.
The integration of these pathways with broader metabolic networks presents additional complexity. Cross-talk between glycogenolysis and lipolysis pathways involves numerous regulatory proteins and post-translational modifications that respond dynamically to nutritional status and energy demands. Current computational models struggle to incorporate this complexity while maintaining predictive accuracy.
Emerging evidence suggests that disruptions in the balance between these pathways contribute to metabolic disorders including diabetes, obesity, and non-alcoholic fatty liver disease. However, therapeutic interventions targeting specific aspects of these pathways have shown limited success, highlighting gaps in our understanding of their integrated regulation in pathological states.
Technical limitations in distinguishing between different sources of circulating glucose (glycogenolysis vs. gluconeogenesis) and fatty acids (dietary vs. lipolysis-derived) in vivo remain significant obstacles. Advanced tracer methodologies and imaging techniques are being developed but require further refinement to achieve the necessary specificity and sensitivity for clinical applications.
Conversely, lipolysis involves the hydrolysis of triglycerides into glycerol and free fatty acids, occurring predominantly in adipose tissue. This process provides a more sustained energy source during prolonged exercise or extended fasting periods. Recent metabolomic studies have revealed intricate regulatory networks governing these pathways, including the identification of novel signaling molecules and feedback mechanisms.
Despite these advances, several technical challenges persist in fully elucidating the comparative dynamics of these processes. Real-time monitoring of pathway activation remains difficult, particularly in human subjects under physiological conditions. Current technologies like stable isotope tracers provide valuable insights but often lack temporal resolution to capture rapid metabolic shifts between pathways.
Another significant challenge lies in understanding the tissue-specific variations in these processes. While liver glycogenolysis and adipose tissue lipolysis are well-characterized, the regulatory mechanisms in other tissues such as cardiac muscle, brain, and kidney remain incompletely understood. Recent single-cell metabolomic approaches show promise but face limitations in sample preparation and metabolite stability.
The integration of these pathways with broader metabolic networks presents additional complexity. Cross-talk between glycogenolysis and lipolysis pathways involves numerous regulatory proteins and post-translational modifications that respond dynamically to nutritional status and energy demands. Current computational models struggle to incorporate this complexity while maintaining predictive accuracy.
Emerging evidence suggests that disruptions in the balance between these pathways contribute to metabolic disorders including diabetes, obesity, and non-alcoholic fatty liver disease. However, therapeutic interventions targeting specific aspects of these pathways have shown limited success, highlighting gaps in our understanding of their integrated regulation in pathological states.
Technical limitations in distinguishing between different sources of circulating glucose (glycogenolysis vs. gluconeogenesis) and fatty acids (dietary vs. lipolysis-derived) in vivo remain significant obstacles. Advanced tracer methodologies and imaging techniques are being developed but require further refinement to achieve the necessary specificity and sensitivity for clinical applications.
Established Methodologies for Studying Energy Substrate Utilization
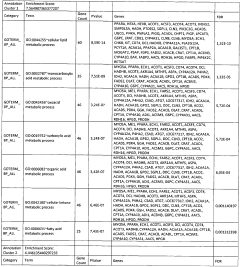
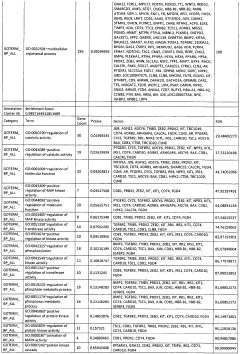
01 Mechanisms of glycogenolysis and lipolysis in metabolic processes
Glycogenolysis and lipolysis are fundamental metabolic processes that break down glycogen and fat stores respectively to provide energy for the body. These processes are regulated by various hormones and enzymes that respond to energy demands. Understanding these mechanisms is crucial for developing treatments for metabolic disorders such as obesity and diabetes. Research in this area focuses on the molecular pathways involved in these processes and how they can be modulated for therapeutic purposes.- Mechanisms of glycogenolysis and lipolysis in metabolic processes: Glycogenolysis and lipolysis are fundamental metabolic processes that break down glycogen and lipids respectively to provide energy for the body. These processes are regulated by various hormones and enzymes that control the rate of breakdown. Understanding these mechanisms is crucial for developing treatments for metabolic disorders such as obesity and diabetes. The breakdown of glycogen into glucose and the hydrolysis of triglycerides into fatty acids and glycerol are essential for maintaining energy homeostasis during fasting or exercise.
- Therapeutic applications targeting glycogenolysis and lipolysis pathways: Pharmaceutical compounds and therapeutic approaches that target glycogenolysis and lipolysis pathways have been developed to treat various metabolic conditions. These include medications that can either stimulate or inhibit these processes depending on the desired therapeutic outcome. For instance, stimulating lipolysis can be beneficial for weight management, while controlling glycogenolysis may help manage blood glucose levels in diabetic patients. These therapeutic approaches often involve targeting specific enzymes or receptors involved in these metabolic pathways.
- Devices and methods for enhancing glycogenolysis and lipolysis for weight management: Various devices and methods have been developed to enhance glycogenolysis and lipolysis for weight management and body contouring purposes. These include electrical stimulation devices, ultrasound technology, and other non-invasive approaches that aim to increase the breakdown of stored glycogen and fat. By targeting specific areas of the body, these technologies can help reduce localized fat deposits and improve body composition. Some approaches combine multiple modalities to achieve synergistic effects on metabolic processes.
- Cosmetic and dermatological applications of glycogenolysis and lipolysis: Cosmetic and dermatological formulations have been developed that utilize principles of glycogenolysis and lipolysis to improve skin appearance and texture. These formulations often contain active ingredients that can stimulate these metabolic processes in skin cells or subcutaneous fat tissue. Applications include anti-aging products, cellulite treatments, and skin firming solutions. By enhancing local metabolic activity, these products aim to improve skin elasticity, reduce the appearance of cellulite, and promote a more youthful appearance.
- Novel compounds and compositions affecting glycogenolysis and lipolysis: Research has led to the discovery of novel compounds and compositions that can modulate glycogenolysis and lipolysis processes. These include natural extracts, synthetic molecules, and bioactive compounds that can either stimulate or inhibit these metabolic pathways. Some compositions combine multiple active ingredients to achieve synergistic effects on metabolism. These compounds often work by interacting with specific enzymes, receptors, or signaling pathways involved in the regulation of glycogen and lipid metabolism, offering potential applications in both pharmaceutical and nutraceutical products.
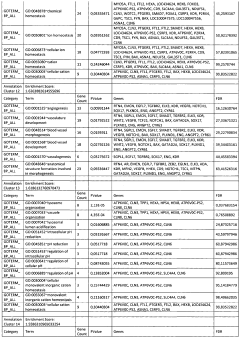
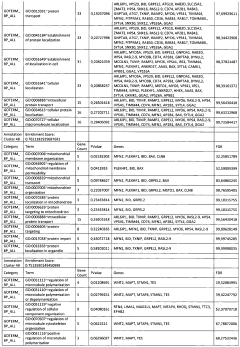
02 Therapeutic applications targeting glycogenolysis and lipolysis for weight management
Compounds and compositions that modulate glycogenolysis and lipolysis pathways have been developed for weight management applications. These therapeutics aim to enhance fat breakdown and reduce fat storage by targeting specific enzymes or receptors involved in these metabolic processes. Such approaches can help in reducing body fat, improving body composition, and addressing obesity-related health issues. The formulations may include natural extracts, synthetic compounds, or combinations that effectively stimulate lipolysis while maintaining metabolic balance.Expand Specific Solutions03 Electrical stimulation devices for enhancing glycogenolysis and lipolysis
Various electrical stimulation devices have been developed to enhance glycogenolysis and lipolysis in targeted body areas. These devices apply electrical impulses to specific muscle groups or fat deposits to stimulate metabolic processes, increase energy expenditure, and promote fat breakdown. The technology includes wearable devices, implantable systems, and clinical equipment that can be used for both therapeutic and aesthetic purposes. The stimulation parameters are carefully designed to optimize metabolic effects while ensuring safety and comfort for users.Expand Specific Solutions04 Exercise and physical therapy methods to promote glycogenolysis and lipolysis
Specific exercise protocols and physical therapy methods have been developed to optimize glycogenolysis and lipolysis during physical activity. These methods focus on exercise intensity, duration, and timing to maximize fat utilization as an energy source. The approaches may include interval training, resistance exercises, or specialized movement patterns that effectively engage metabolic pathways. Some innovations combine physical activity with supplementary techniques such as heat application, cooling, or respiratory control to further enhance metabolic effects.Expand Specific Solutions05 Pharmaceutical compositions targeting glycogenolysis and lipolysis pathways
Advanced pharmaceutical compositions have been formulated to specifically target enzymes and receptors involved in glycogenolysis and lipolysis pathways. These formulations may include beta-adrenergic agonists, phosphodiesterase inhibitors, or compounds that modulate hormone-sensitive lipase activity. The pharmaceutical approaches aim to address metabolic disorders, enhance athletic performance, or support medical weight management programs. Some compositions incorporate delivery systems that ensure targeted action in specific tissues while minimizing systemic effects.Expand Specific Solutions
Key Research Institutions and Industry Players
The glycogenolysis vs. lipolysis market is currently in a growth phase, with increasing research focus on metabolic disorders and energy regulation. The global market size for related therapeutics and diagnostics is expanding, driven by rising obesity and diabetes prevalence. Technologically, this field shows moderate maturity with established understanding of basic mechanisms, but significant innovation potential remains. Leading players include pharmaceutical giants like Pfizer, GlaxoSmithKline, and Hoffmann-La Roche, who leverage their R&D capabilities for drug development targeting metabolic pathways. Research institutions such as MIT, Duke University, and Tsinghua University contribute fundamental discoveries, while specialized biotechnology firms like Novozymes and Valerion Therapeutics focus on enzyme-based approaches and targeted therapies, respectively. The competitive landscape features increasing collaboration between academia and industry to accelerate translation of research into clinical applications.
Hoffmann-La Roche, Inc.
Technical Solution: Hoffmann-La Roche has developed comprehensive metabolic pathway analysis platforms focusing on the comparative regulation of glycogenolysis and lipolysis. Their approach utilizes proprietary biomarkers to monitor the relative activation of these pathways under various physiological conditions. Their research has identified specific molecular triggers that can selectively activate glycogenolysis over lipolysis, particularly in liver and muscle tissues. The company has developed small molecule modulators that can influence the PKA-dependent phosphorylation cascade central to both pathways, allowing for selective intervention. Their technology includes advanced imaging techniques that can visualize glycogen depletion versus lipid mobilization in real-time within living tissues, providing unprecedented insights into the temporal dynamics of energy substrate utilization.
Strengths: Robust pharmaceutical development pipeline allowing rapid translation of metabolic research into therapeutic candidates; extensive clinical trial infrastructure for testing metabolic interventions. Weaknesses: Primarily focused on disease states rather than normal physiology; research heavily oriented toward pharmaceutical applications rather than fundamental metabolic understanding.
Duke University
Technical Solution: Duke University has established a multidisciplinary research program examining the comparative regulation and physiological significance of glycogenolysis versus lipolysis. Their approach integrates molecular biology, exercise physiology, and clinical research to understand how these pathways are coordinated during various metabolic challenges. Duke researchers have developed novel in vivo imaging techniques using positron emission tomography with specialized tracers to visualize the activation of these pathways in human subjects during exercise and fasting. Their work has particularly focused on the role of AMPK as a master regulator that differentially affects glycogenolysis and lipolysis depending on tissue type and energy status. Duke's research has identified specific exercise protocols that preferentially activate one pathway over the other, with implications for training strategies in athletes and therapeutic interventions in metabolic disorders. Their studies have demonstrated that the relative contribution of these pathways varies significantly with age, training status, and metabolic health, providing insights into personalized approaches to exercise and nutrition.
Strengths: Strong integration of basic science with clinical and translational research; sophisticated in vivo imaging capabilities for human metabolic studies. Weaknesses: Research spread across multiple departments with varying priorities; more limited resources for drug development compared to pharmaceutical companies.
Critical Molecular Mechanisms and Regulatory Pathways
TFEB for use in treating obesity or metabolic syndrome
PatentWO2013186398A1
Innovation
- A vector comprising a TFEB coding sequence, under the control of a liver-specific promoter, is used to enhance lipid breakdown and energy expenditure through gene therapy, specifically targeting key genes involved in lipid catabolism and ketogenesis.
Compositions and methods for induced brown fat differentiation
PatentActiveUS20200206317A1
Innovation
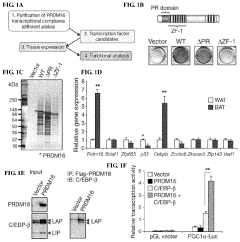
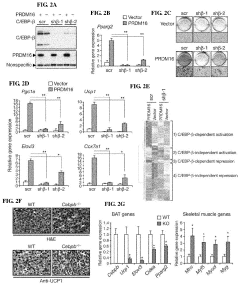
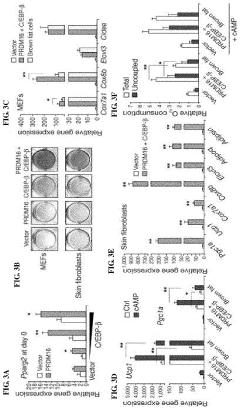
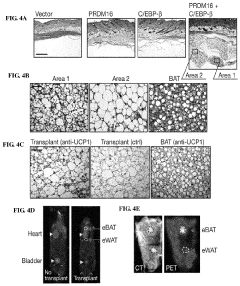
- The discovery that Prdm16 and CCAAT/enhancer binding protein beta (C/EBPβ) can cooperatively induce brown fat differentiation in non-adipocyte mammalian cells, increasing energy expenditure through enhanced mitochondrial gene expression and cellular respiration, which can be used to treat or prevent obesity-related disorders.
Clinical and Therapeutic Applications of Metabolic Pathway Research
The clinical applications of metabolic pathway research, particularly focusing on glycogenolysis and lipolysis, have significant implications for therapeutic interventions across multiple disease states. Understanding these fundamental energy-mobilizing processes has enabled the development of targeted treatments for metabolic disorders, with distinct approaches based on pathway-specific mechanisms.
In diabetes management, the differential regulation of glycogenolysis has become central to pharmaceutical development. Glucagon receptor antagonists specifically target the hyperactive glycogenolytic response observed in diabetic patients, helping to reduce excessive hepatic glucose output. Similarly, glycogen phosphorylase inhibitors have emerged as promising therapeutic agents for type 2 diabetes, offering pathway-specific intervention that avoids the broader metabolic disruptions associated with earlier generation treatments.
Cardiovascular disease treatment strategies increasingly incorporate knowledge of lipolytic pathways. The selective modulation of hormone-sensitive lipase (HSL) activity presents opportunities for reducing circulating free fatty acid levels, thereby potentially mitigating atherosclerotic progression. Clinical trials investigating partial fatty acid oxidation inhibitors have demonstrated improved myocardial efficiency in heart failure patients by shifting metabolic substrate utilization away from fatty acids derived from lipolysis toward glucose oxidation.
Obesity therapeutics have evolved substantially through deeper understanding of adipose tissue lipolysis regulation. Beta-adrenergic agonists targeting specific receptor subtypes can enhance lipolytic rates in adipocytes, while simultaneously minimizing unwanted cardiovascular effects. The clinical application of adipose triglyceride lipase (ATGL) modulators represents a newer therapeutic avenue with promising early-phase clinical results for weight management.
Exercise physiology and sports medicine have benefited from pathway-specific interventions based on the temporal dynamics of glycogenolysis versus lipolysis during different exercise intensities. Nutritional strategies and supplementation protocols now commonly incorporate timing considerations to optimize substrate availability based on the predominant energy pathway utilized during specific training modalities.
Rare metabolic disorders, including glycogen storage diseases and lipodystrophies, represent areas where pathway-specific therapeutic approaches have yielded remarkable clinical advances. Enzyme replacement therapies targeting specific deficiencies in glycogenolytic pathways have transformed management of conditions like Pompe disease, while gene therapy approaches for familial lipolysis disorders are advancing through clinical development pipelines.
In diabetes management, the differential regulation of glycogenolysis has become central to pharmaceutical development. Glucagon receptor antagonists specifically target the hyperactive glycogenolytic response observed in diabetic patients, helping to reduce excessive hepatic glucose output. Similarly, glycogen phosphorylase inhibitors have emerged as promising therapeutic agents for type 2 diabetes, offering pathway-specific intervention that avoids the broader metabolic disruptions associated with earlier generation treatments.
Cardiovascular disease treatment strategies increasingly incorporate knowledge of lipolytic pathways. The selective modulation of hormone-sensitive lipase (HSL) activity presents opportunities for reducing circulating free fatty acid levels, thereby potentially mitigating atherosclerotic progression. Clinical trials investigating partial fatty acid oxidation inhibitors have demonstrated improved myocardial efficiency in heart failure patients by shifting metabolic substrate utilization away from fatty acids derived from lipolysis toward glucose oxidation.
Obesity therapeutics have evolved substantially through deeper understanding of adipose tissue lipolysis regulation. Beta-adrenergic agonists targeting specific receptor subtypes can enhance lipolytic rates in adipocytes, while simultaneously minimizing unwanted cardiovascular effects. The clinical application of adipose triglyceride lipase (ATGL) modulators represents a newer therapeutic avenue with promising early-phase clinical results for weight management.
Exercise physiology and sports medicine have benefited from pathway-specific interventions based on the temporal dynamics of glycogenolysis versus lipolysis during different exercise intensities. Nutritional strategies and supplementation protocols now commonly incorporate timing considerations to optimize substrate availability based on the predominant energy pathway utilized during specific training modalities.
Rare metabolic disorders, including glycogen storage diseases and lipodystrophies, represent areas where pathway-specific therapeutic approaches have yielded remarkable clinical advances. Enzyme replacement therapies targeting specific deficiencies in glycogenolytic pathways have transformed management of conditions like Pompe disease, while gene therapy approaches for familial lipolysis disorders are advancing through clinical development pipelines.
Technological Innovations in Metabolic Pathway Monitoring
Recent technological innovations in metabolic pathway monitoring have revolutionized our understanding of glycogenolysis and lipolysis processes. Advanced biosensors utilizing fluorescence resonance energy transfer (FRET) technology now enable real-time tracking of metabolic pathway activation with unprecedented precision. These sensors can detect minute changes in enzyme activity and substrate concentrations, providing valuable insights into the comparative dynamics of glycogenolysis and lipolysis under various physiological conditions.
Continuous glucose monitoring (CGM) systems have evolved to incorporate machine learning algorithms that can differentiate between glucose derived from glycogenolysis versus other metabolic sources. This technological advancement allows researchers to quantify the relative contributions of different energy pathways during various metabolic states, such as exercise, fasting, and feeding cycles.
Mass spectrometry techniques have undergone significant refinements, particularly in the area of metabolomics. High-resolution liquid chromatography-mass spectrometry (LC-MS) platforms can now simultaneously track hundreds of metabolites involved in both glycogenolysis and lipolysis pathways. This comprehensive metabolic profiling enables researchers to construct detailed pathway flux maps, revealing previously unrecognized regulatory interactions between these two critical energy-mobilizing processes.
Wearable technology has emerged as a promising frontier for non-invasive metabolic monitoring. Multi-modal sensors incorporating impedance measurements, temperature sensing, and sweat analysis can now provide indirect measurements of metabolic pathway activity. These devices are being calibrated to distinguish between predominant glycogenolysis versus lipolysis states based on biomarker patterns detected through the skin.
Imaging technologies have also advanced considerably, with positron emission tomography (PET) tracers specifically designed to bind to key enzymes in the glycogenolysis and lipolysis pathways. These molecular imaging approaches allow visualization of tissue-specific metabolic activity in living subjects, revealing spatial and temporal differences in how various organs utilize these pathways during different physiological challenges.
Microfluidic "organ-on-chip" platforms represent another innovative approach, where miniaturized tissue constructs can be monitored for metabolic activity under precisely controlled conditions. These systems incorporate integrated sensors that can measure oxygen consumption, pH changes, and specific metabolite production, providing a controlled environment to compare glycogenolysis and lipolysis regulation across different tissue types and disease states.
Continuous glucose monitoring (CGM) systems have evolved to incorporate machine learning algorithms that can differentiate between glucose derived from glycogenolysis versus other metabolic sources. This technological advancement allows researchers to quantify the relative contributions of different energy pathways during various metabolic states, such as exercise, fasting, and feeding cycles.
Mass spectrometry techniques have undergone significant refinements, particularly in the area of metabolomics. High-resolution liquid chromatography-mass spectrometry (LC-MS) platforms can now simultaneously track hundreds of metabolites involved in both glycogenolysis and lipolysis pathways. This comprehensive metabolic profiling enables researchers to construct detailed pathway flux maps, revealing previously unrecognized regulatory interactions between these two critical energy-mobilizing processes.
Wearable technology has emerged as a promising frontier for non-invasive metabolic monitoring. Multi-modal sensors incorporating impedance measurements, temperature sensing, and sweat analysis can now provide indirect measurements of metabolic pathway activity. These devices are being calibrated to distinguish between predominant glycogenolysis versus lipolysis states based on biomarker patterns detected through the skin.
Imaging technologies have also advanced considerably, with positron emission tomography (PET) tracers specifically designed to bind to key enzymes in the glycogenolysis and lipolysis pathways. These molecular imaging approaches allow visualization of tissue-specific metabolic activity in living subjects, revealing spatial and temporal differences in how various organs utilize these pathways during different physiological challenges.
Microfluidic "organ-on-chip" platforms represent another innovative approach, where miniaturized tissue constructs can be monitored for metabolic activity under precisely controlled conditions. These systems incorporate integrated sensors that can measure oxygen consumption, pH changes, and specific metabolite production, providing a controlled environment to compare glycogenolysis and lipolysis regulation across different tissue types and disease states.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!