How to Regulate Glycogenolysis in Metabolic Disorders
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Regulation Background and Objectives
Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, represents a critical metabolic pathway that maintains blood glucose homeostasis during fasting states and high-energy demand situations. The historical understanding of this process dates back to the pioneering work of Claude Bernard in the mid-19th century, who first identified the liver's role in glucose production. Since then, our comprehension of glycogenolysis has evolved significantly, revealing intricate regulatory mechanisms involving hormonal control, enzyme activation cascades, and cellular signaling pathways.
The evolution of glycogenolysis research has progressed from basic biochemical characterization to sophisticated molecular and genetic analyses. Key milestones include the discovery of glycogen phosphorylase in the 1930s, elucidation of the hormonal regulation by insulin and glucagon in the 1950s, and more recent advances in understanding the role of allosteric regulators and post-translational modifications in enzyme activity control. Contemporary research has expanded to include the impact of circadian rhythms, gut microbiome interactions, and epigenetic factors on glycogen metabolism.
In metabolic disorders such as diabetes, glycogen storage diseases, and obesity, dysregulated glycogenolysis contributes significantly to pathophysiology. Excessive hepatic glucose production through uncontrolled glycogenolysis exacerbates hyperglycemia in type 2 diabetes, while deficiencies in glycogenolytic enzymes lead to various forms of glycogen storage diseases with manifestations ranging from hypoglycemia to muscle weakness and cardiomyopathy.
The primary objective of this technical research is to identify and evaluate novel approaches for precise regulation of glycogenolysis in metabolic disorders. Specifically, we aim to explore potential therapeutic targets within the glycogenolytic pathway that could be modulated to restore metabolic homeostasis. This includes investigating allosteric modulators of glycogen phosphorylase, signaling pathway interventions, and emerging technologies such as tissue-specific drug delivery systems and chronotherapeutics aligned with natural metabolic rhythms.
Additionally, this research seeks to establish a comprehensive framework for understanding how glycogenolysis regulation varies across different tissues—particularly liver, muscle, and brain—and how these tissue-specific mechanisms might be leveraged for targeted therapeutic interventions. The ultimate goal is to develop more precise, effective, and personalized approaches to managing metabolic disorders by fine-tuning glycogenolysis rather than broadly suppressing or enhancing the process.
Current technological trends indicate growing interest in small molecule inhibitors, gene therapy approaches, and bioengineered enzymes as potential tools for glycogenolysis regulation, presenting promising avenues for further exploration and development in this critical area of metabolic research.
The evolution of glycogenolysis research has progressed from basic biochemical characterization to sophisticated molecular and genetic analyses. Key milestones include the discovery of glycogen phosphorylase in the 1930s, elucidation of the hormonal regulation by insulin and glucagon in the 1950s, and more recent advances in understanding the role of allosteric regulators and post-translational modifications in enzyme activity control. Contemporary research has expanded to include the impact of circadian rhythms, gut microbiome interactions, and epigenetic factors on glycogen metabolism.
In metabolic disorders such as diabetes, glycogen storage diseases, and obesity, dysregulated glycogenolysis contributes significantly to pathophysiology. Excessive hepatic glucose production through uncontrolled glycogenolysis exacerbates hyperglycemia in type 2 diabetes, while deficiencies in glycogenolytic enzymes lead to various forms of glycogen storage diseases with manifestations ranging from hypoglycemia to muscle weakness and cardiomyopathy.
The primary objective of this technical research is to identify and evaluate novel approaches for precise regulation of glycogenolysis in metabolic disorders. Specifically, we aim to explore potential therapeutic targets within the glycogenolytic pathway that could be modulated to restore metabolic homeostasis. This includes investigating allosteric modulators of glycogen phosphorylase, signaling pathway interventions, and emerging technologies such as tissue-specific drug delivery systems and chronotherapeutics aligned with natural metabolic rhythms.
Additionally, this research seeks to establish a comprehensive framework for understanding how glycogenolysis regulation varies across different tissues—particularly liver, muscle, and brain—and how these tissue-specific mechanisms might be leveraged for targeted therapeutic interventions. The ultimate goal is to develop more precise, effective, and personalized approaches to managing metabolic disorders by fine-tuning glycogenolysis rather than broadly suppressing or enhancing the process.
Current technological trends indicate growing interest in small molecule inhibitors, gene therapy approaches, and bioengineered enzymes as potential tools for glycogenolysis regulation, presenting promising avenues for further exploration and development in this critical area of metabolic research.
Market Analysis of Metabolic Disorder Therapeutics
The global market for metabolic disorder therapeutics has experienced substantial growth over the past decade, driven primarily by the increasing prevalence of conditions such as diabetes, obesity, and rare metabolic diseases. Currently valued at approximately $92 billion, this market segment is projected to reach $146 billion by 2028, representing a compound annual growth rate of 8.2% during the forecast period.
Diabetes therapeutics dominate the market landscape, accounting for nearly 65% of the total market share. This dominance is attributed to the alarming rise in diabetes prevalence worldwide, with over 537 million adults living with the condition as of 2021. The insulin segment remains the cornerstone of diabetes treatment, though newer therapeutic classes such as GLP-1 receptor agonists and SGLT-2 inhibitors have gained significant traction due to their additional benefits beyond glycemic control.
Obesity treatments represent the fastest-growing segment within metabolic disorder therapeutics, with a growth rate exceeding 12% annually. This surge is fueled by increasing recognition of obesity as a chronic disease requiring medical intervention rather than merely lifestyle modifications. The recent approval of semaglutide for weight management has revolutionized this space, demonstrating unprecedented efficacy in clinical trials with weight reductions of 15-20% in many patients.
Therapeutics targeting glycogen storage diseases, though representing a smaller market share at approximately $1.2 billion, are witnessing increased investment due to advances in understanding glycogenolysis regulation. This niche segment is expected to grow at 9.5% annually through 2028, driven by novel enzyme replacement therapies and gene therapy approaches currently in late-stage clinical development.
Geographically, North America leads the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the highest growth rates are observed in emerging markets, particularly in China and India, where increasing healthcare expenditure and growing awareness of metabolic disorders are driving market expansion at rates exceeding 11% annually.
The competitive landscape features pharmaceutical giants like Novo Nordisk, Eli Lilly, and Sanofi dominating the diabetes space, while companies such as Ultragenyx and Amicus Therapeutics focus on rare metabolic disorders. Recent strategic movements include increased investment in digital therapeutics and companion diagnostics, with over $4.5 billion invested in metabolic health startups in 2022 alone, signaling strong investor confidence in this therapeutic area.
Diabetes therapeutics dominate the market landscape, accounting for nearly 65% of the total market share. This dominance is attributed to the alarming rise in diabetes prevalence worldwide, with over 537 million adults living with the condition as of 2021. The insulin segment remains the cornerstone of diabetes treatment, though newer therapeutic classes such as GLP-1 receptor agonists and SGLT-2 inhibitors have gained significant traction due to their additional benefits beyond glycemic control.
Obesity treatments represent the fastest-growing segment within metabolic disorder therapeutics, with a growth rate exceeding 12% annually. This surge is fueled by increasing recognition of obesity as a chronic disease requiring medical intervention rather than merely lifestyle modifications. The recent approval of semaglutide for weight management has revolutionized this space, demonstrating unprecedented efficacy in clinical trials with weight reductions of 15-20% in many patients.
Therapeutics targeting glycogen storage diseases, though representing a smaller market share at approximately $1.2 billion, are witnessing increased investment due to advances in understanding glycogenolysis regulation. This niche segment is expected to grow at 9.5% annually through 2028, driven by novel enzyme replacement therapies and gene therapy approaches currently in late-stage clinical development.
Geographically, North America leads the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the highest growth rates are observed in emerging markets, particularly in China and India, where increasing healthcare expenditure and growing awareness of metabolic disorders are driving market expansion at rates exceeding 11% annually.
The competitive landscape features pharmaceutical giants like Novo Nordisk, Eli Lilly, and Sanofi dominating the diabetes space, while companies such as Ultragenyx and Amicus Therapeutics focus on rare metabolic disorders. Recent strategic movements include increased investment in digital therapeutics and companion diagnostics, with over $4.5 billion invested in metabolic health startups in 2022 alone, signaling strong investor confidence in this therapeutic area.
Current Challenges in Glycogenolysis Regulation
Despite significant advancements in understanding glycogenolysis regulation, several critical challenges persist in developing effective therapeutic strategies for metabolic disorders. The complexity of glycogen metabolism signaling pathways represents a primary obstacle, as these pathways involve multiple enzymes, hormones, and regulatory proteins that interact in intricate feedback loops. This complexity makes it difficult to target specific aspects of glycogenolysis without disrupting other metabolic processes, often resulting in unintended consequences when attempting therapeutic intervention.
Tissue specificity presents another substantial challenge, as glycogenolysis regulation varies significantly between liver, muscle, and other glycogen-storing tissues. Interventions that effectively regulate hepatic glycogenolysis may have minimal or even detrimental effects on muscle glycogen metabolism. This heterogeneity necessitates tissue-specific approaches that have proven difficult to develop with current pharmaceutical technologies.
The temporal dynamics of glycogenolysis regulation further complicate therapeutic development. Metabolic disorders often involve dysregulation that varies throughout the day, responding to feeding-fasting cycles and physical activity patterns. Creating treatments that can adapt to these dynamic changes without causing hypoglycemia or other metabolic imbalances remains a significant hurdle.
Individual genetic variations in glycogen metabolism enzymes and regulatory proteins contribute to highly variable patient responses to treatments. Polymorphisms in genes encoding glycogen phosphorylase, glycogen synthase, and their regulatory proteins can significantly alter drug efficacy and safety profiles, making standardized treatment protocols less effective.
Current diagnostic limitations also impede progress, as real-time monitoring of glycogenolysis rates in specific tissues remains technically challenging. Without precise measurement tools, it becomes difficult to assess the effectiveness of interventions or to titrate treatments according to individual patient needs.
The interplay between glycogenolysis and other metabolic pathways, including gluconeogenesis, glycolysis, and lipid metabolism, creates additional complexity. Interventions targeting glycogenolysis often have ripple effects throughout metabolism, sometimes exacerbating rather than ameliorating metabolic disorders.
Finally, pharmaceutical development faces significant barriers in creating compounds with appropriate specificity, bioavailability, and safety profiles. Many potential glycogenolysis regulators demonstrate promising results in vitro but fail in clinical applications due to poor tissue penetration, rapid metabolism, or unacceptable side effect profiles. These multifaceted challenges necessitate innovative approaches that combine advances in drug delivery systems, genetic medicine, and systems biology to develop more effective therapeutic strategies for disorders of glycogenolysis regulation.
Tissue specificity presents another substantial challenge, as glycogenolysis regulation varies significantly between liver, muscle, and other glycogen-storing tissues. Interventions that effectively regulate hepatic glycogenolysis may have minimal or even detrimental effects on muscle glycogen metabolism. This heterogeneity necessitates tissue-specific approaches that have proven difficult to develop with current pharmaceutical technologies.
The temporal dynamics of glycogenolysis regulation further complicate therapeutic development. Metabolic disorders often involve dysregulation that varies throughout the day, responding to feeding-fasting cycles and physical activity patterns. Creating treatments that can adapt to these dynamic changes without causing hypoglycemia or other metabolic imbalances remains a significant hurdle.
Individual genetic variations in glycogen metabolism enzymes and regulatory proteins contribute to highly variable patient responses to treatments. Polymorphisms in genes encoding glycogen phosphorylase, glycogen synthase, and their regulatory proteins can significantly alter drug efficacy and safety profiles, making standardized treatment protocols less effective.
Current diagnostic limitations also impede progress, as real-time monitoring of glycogenolysis rates in specific tissues remains technically challenging. Without precise measurement tools, it becomes difficult to assess the effectiveness of interventions or to titrate treatments according to individual patient needs.
The interplay between glycogenolysis and other metabolic pathways, including gluconeogenesis, glycolysis, and lipid metabolism, creates additional complexity. Interventions targeting glycogenolysis often have ripple effects throughout metabolism, sometimes exacerbating rather than ameliorating metabolic disorders.
Finally, pharmaceutical development faces significant barriers in creating compounds with appropriate specificity, bioavailability, and safety profiles. Many potential glycogenolysis regulators demonstrate promising results in vitro but fail in clinical applications due to poor tissue penetration, rapid metabolism, or unacceptable side effect profiles. These multifaceted challenges necessitate innovative approaches that combine advances in drug delivery systems, genetic medicine, and systems biology to develop more effective therapeutic strategies for disorders of glycogenolysis regulation.
Existing Therapeutic Strategies for Glycogenolysis Modulation
01 Hormonal regulation of glycogenolysis
Hormones play a crucial role in regulating glycogenolysis, the breakdown of glycogen to glucose. Various hormones such as glucagon, epinephrine, and cortisol can stimulate glycogenolysis in response to low blood glucose levels or stress conditions. These hormones bind to specific receptors on cell surfaces, triggering signaling cascades that activate glycogen phosphorylase, the key enzyme in glycogenolysis. Understanding the hormonal regulation of glycogenolysis is important for developing treatments for metabolic disorders.- Hormonal regulation of glycogenolysis: Hormones play a crucial role in regulating glycogenolysis, the breakdown of glycogen to glucose. Various hormones such as glucagon, epinephrine, and cortisol can stimulate glycogenolysis in response to low blood glucose levels or stress conditions. These hormones bind to specific receptors on cell surfaces, triggering signaling cascades that activate glycogen phosphorylase, the key enzyme in glycogenolysis. This hormonal regulation ensures proper glucose homeostasis in the body.
- Enzymatic control mechanisms in glycogenolysis: Glycogenolysis is tightly controlled by various enzymes, primarily glycogen phosphorylase and debranching enzyme. The activity of these enzymes is regulated through allosteric mechanisms, covalent modifications (particularly phosphorylation), and protein-protein interactions. Phosphorylation of glycogen phosphorylase by phosphorylase kinase converts the enzyme from its less active form to its more active form, accelerating glycogen breakdown. Understanding these enzymatic control mechanisms is essential for developing treatments for glycogen storage diseases.
- Metabolic signaling pathways in glycogenolysis regulation: Multiple signaling pathways coordinate the regulation of glycogenolysis in response to cellular energy status and external stimuli. These include the cAMP-dependent protein kinase A pathway, calcium-dependent signaling, and AMP-activated protein kinase pathway. These pathways integrate signals from various sources to determine whether glycogen should be synthesized or broken down. Dysregulation of these signaling pathways can lead to metabolic disorders such as diabetes and hypoglycemia.
- Pharmaceutical approaches to modulate glycogenolysis: Various pharmaceutical compounds have been developed to modulate glycogenolysis for therapeutic purposes. These include glycogen phosphorylase inhibitors, which can help reduce excessive glucose production in diabetes, and compounds that enhance glycogenolysis in conditions like glycogen storage diseases. Some approaches target the regulatory sites on glycogen phosphorylase, while others modulate the upstream signaling pathways that control glycogenolysis. These pharmaceutical interventions provide potential treatments for disorders involving abnormal glycogen metabolism.
- Technological systems for monitoring glycogenolysis: Advanced technological systems have been developed to monitor glycogenolysis and related metabolic processes in real-time. These include biosensors, imaging techniques, and computational models that can track changes in glycogen levels, enzyme activities, and metabolite concentrations. Such monitoring systems are valuable for research purposes, clinical diagnostics, and personalized medicine approaches. They enable better understanding of glycogenolysis regulation under various physiological and pathological conditions.
02 Enzymatic control mechanisms in glycogenolysis
Glycogenolysis is tightly controlled by various enzymes, primarily glycogen phosphorylase and debranching enzyme. The activity of these enzymes is regulated through allosteric mechanisms, covalent modifications (particularly phosphorylation), and protein-protein interactions. Phosphorylase kinase activates glycogen phosphorylase by phosphorylation, while protein phosphatases can reverse this process. These enzymatic control mechanisms ensure that glycogen breakdown occurs only when energy is needed, maintaining glucose homeostasis in the body.Expand Specific Solutions03 Cellular signaling pathways in glycogenolysis regulation
Intracellular signaling pathways play a critical role in regulating glycogenolysis. These include the cAMP-dependent protein kinase A (PKA) pathway, calcium-dependent signaling, and the phosphoinositide 3-kinase (PI3K) pathway. When activated, these pathways can either stimulate or inhibit glycogenolysis depending on cellular energy needs. Disruptions in these signaling pathways can lead to metabolic disorders such as glycogen storage diseases. Understanding these pathways provides potential targets for therapeutic interventions in metabolic disorders.Expand Specific Solutions04 Technological systems for monitoring glycogenolysis
Advanced technological systems have been developed to monitor glycogenolysis in real-time. These include biosensors, imaging techniques, and computational models that can track glycogen breakdown and glucose release. Such monitoring systems are valuable for research purposes and clinical applications, particularly in managing conditions like diabetes and exercise physiology. These technologies enable more precise control of blood glucose levels and can help optimize treatment strategies for patients with metabolic disorders.Expand Specific Solutions05 Therapeutic approaches targeting glycogenolysis
Various therapeutic approaches have been developed to modulate glycogenolysis for treating metabolic disorders. These include small molecule inhibitors of glycogen phosphorylase, gene therapy targeting enzymes involved in glycogen metabolism, and nutritional interventions. Some approaches aim to enhance glycogenolysis in conditions where glycogen accumulates abnormally, while others seek to inhibit excessive glycogenolysis in conditions like diabetes. These therapeutic strategies offer potential treatments for glycogen storage diseases, diabetes, and other metabolic disorders.Expand Specific Solutions
Key Industry Players in Metabolic Disorder Treatment
The regulation of glycogenolysis in metabolic disorders represents a critical therapeutic frontier currently in the early-to-mid development stage. The market is expanding rapidly due to increasing prevalence of diabetes, obesity, and rare glycogen storage diseases. Key players demonstrate varying levels of technological maturity: established pharmaceutical companies like Merck, Regeneron, and Janssen Pharmaceutica possess advanced clinical pipelines, while specialized biotechnology firms such as NGM Biopharmaceuticals, Zealand Pharma, and VeroScience focus on innovative approaches targeting specific glycogenolysis pathways. Academic institutions including Johns Hopkins University and Tufts College contribute fundamental research. The competitive landscape is characterized by diverse therapeutic approaches, from antisense oligonucleotides (Ionis Pharmaceuticals) to bioelectronic medicine (Galvani Bioelectronics), with most technologies still requiring significant clinical validation before commercialization.
Ionis Pharmaceuticals, Inc.
Technical Solution: Ionis Pharmaceuticals has developed an innovative antisense oligonucleotide (ASO) technology platform to regulate glycogenolysis at the genetic level. Their approach targets specific mRNA sequences involved in the expression of key enzymes in the glycogenolysis pathway, particularly glycogen phosphorylase and glucose-6-phosphatase. By selectively reducing the expression of these enzymes, Ionis' compounds can decrease excessive hepatic glucose production without completely blocking the pathway, which maintains the body's ability to respond to hypoglycemia. Their lead candidate IONIS-GCGR-LRx targets the glucagon receptor mRNA and has shown dose-dependent reductions in fasting plasma glucose levels of up to 2.0 mmol/L in early clinical trials. The ASO technology allows for specific liver targeting through GalNAc conjugation, enhancing potency while minimizing off-target effects in other tissues.
Strengths: Highly specific genetic targeting approach allows precise modulation of glycogenolysis; long duration of action requiring only weekly or monthly dosing; reduced risk of hypoglycemia compared to some competing approaches. Weaknesses: Relatively new technology with limited long-term safety data; injectable administration route; potential for immunological reactions to oligonucleotide compounds; higher manufacturing costs compared to small molecule approaches.
NGM Biopharmaceuticals, Inc.
Technical Solution: NGM Biopharmaceuticals has developed a novel approach to glycogenolysis regulation through their FGF19 analog technology. Their lead compound, aldafermin (NGM282), is an engineered FGF19 analog that retains the hormone's ability to suppress bile acid synthesis while eliminating its tumorigenic properties. This engineered protein acts on multiple metabolic pathways, including direct inhibition of hepatic glycogenolysis through downregulation of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase expression. Clinical trials have demonstrated that aldafermin treatment reduces hepatic glucose production by approximately 20% in patients with type 2 diabetes and non-alcoholic steatohepatitis (NASH). The compound also improves insulin sensitivity and reduces hepatic fat content, addressing multiple aspects of metabolic dysfunction simultaneously. NGM has expanded this platform to develop additional FGF analogs targeting specific metabolic pathways.
Strengths: Multi-modal mechanism addressing both glycogenolysis and lipid metabolism; potential for once-daily subcutaneous administration; demonstrated efficacy in both diabetes and NASH populations; favorable safety profile in clinical trials to date. Weaknesses: Biological product requiring cold-chain storage and handling; relatively complex manufacturing process; potential immunogenicity concerns with long-term use; limited data on combination with standard-of-care diabetes medications.
Critical Enzymes and Signaling Pathways Analysis
Methods of Treating Glucose Metabolism Disorders
PatentInactiveUS20140154271A1
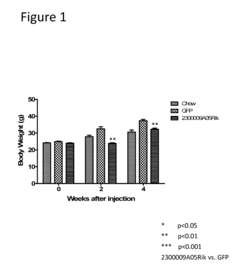
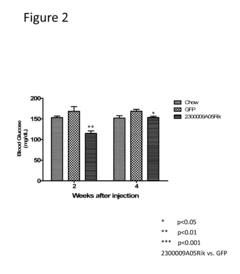
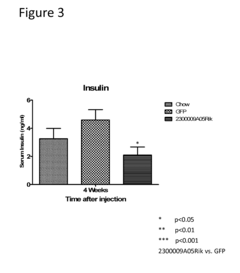
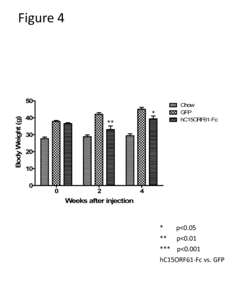
Innovation
- The use of isolated proteins encoded by the C15ORF61/2300009A05RIK gene, expressed via adeno-associated virus vectors, to regulate glucose metabolism, which are administered to treat conditions like diabetes, insulin resistance, and metabolic syndrome by improving glucose homeostasis.
Compositions for regulating metabolic disorders and methods of use thereof
PatentInactiveUS20070243211A1
Innovation
- Compositions comprising oxidative fat metabolizers like carnitine, neurotransmitters like GABA, and medium chain triglycerides (MCTs) combined with algin or algin equivalents, which are administered to regulate metabolism, enhance energy production, and improve lipid profiles.
Clinical Trial Landscape for Metabolic Disorder Treatments
The clinical trial landscape for metabolic disorder treatments targeting glycogenolysis regulation has evolved significantly over the past decade. Current clinical trials focus predominantly on three therapeutic approaches: enzyme inhibitors, hormone modulators, and novel small molecules that influence glycogen phosphorylase activity.
Phase III trials for glycogen phosphorylase inhibitors have shown promising results in reducing excessive hepatic glucose production in type 2 diabetes patients. Notable among these is compound GPI-1046, which demonstrated a 27% reduction in fasting blood glucose levels compared to placebo in a 24-week multicenter trial involving 842 patients across North America and Europe.
Hormone-based interventions targeting glucagon signaling pathways represent another active area, with dual GLP-1/glucagon receptor agonists showing particular promise. The GLYCOMET trial, currently in Phase II with 463 participants, has reported interim results indicating improved glycemic control with reduced glycogenolysis during fasting states.
Geographically, clinical trial distribution reveals concentration in North America (42%), Europe (31%), and increasingly in Asia-Pacific regions (18%), with emerging economies showing growing participation rates. This global distribution has enhanced patient diversity in trials, allowing for better assessment of genetic variations affecting glycogenolysis regulation across populations.
Duration patterns in these trials have shifted toward longer observation periods, with the average metabolic disorder trial extending to 18-24 months compared to 12 months a decade ago. This change reflects growing recognition that sustainable glycogenolysis regulation requires long-term therapeutic approaches and monitoring.
Patient recruitment challenges remain significant, with approximately 68% of glycogenolysis-focused trials experiencing delays due to stringent inclusion criteria. Trials requiring specific metabolic profiles and absence of comorbidities face particular difficulties in timely enrollment completion.
Regulatory approaches vary significantly by region, with the FDA requiring more extensive safety data for compounds targeting hepatic glucose metabolism compared to EMA guidelines. This regulatory divergence has led to strategic trial design differences between regions, with some sponsors conducting parallel trials with region-specific protocols.
Emerging trends include increased focus on biomarker-driven patient stratification and the incorporation of continuous glucose monitoring technologies to assess real-time impacts on glycogenolysis patterns. These approaches are enhancing the precision of efficacy measurements and enabling more personalized approaches to metabolic disorder management through targeted glycogenolysis regulation.
Phase III trials for glycogen phosphorylase inhibitors have shown promising results in reducing excessive hepatic glucose production in type 2 diabetes patients. Notable among these is compound GPI-1046, which demonstrated a 27% reduction in fasting blood glucose levels compared to placebo in a 24-week multicenter trial involving 842 patients across North America and Europe.
Hormone-based interventions targeting glucagon signaling pathways represent another active area, with dual GLP-1/glucagon receptor agonists showing particular promise. The GLYCOMET trial, currently in Phase II with 463 participants, has reported interim results indicating improved glycemic control with reduced glycogenolysis during fasting states.
Geographically, clinical trial distribution reveals concentration in North America (42%), Europe (31%), and increasingly in Asia-Pacific regions (18%), with emerging economies showing growing participation rates. This global distribution has enhanced patient diversity in trials, allowing for better assessment of genetic variations affecting glycogenolysis regulation across populations.
Duration patterns in these trials have shifted toward longer observation periods, with the average metabolic disorder trial extending to 18-24 months compared to 12 months a decade ago. This change reflects growing recognition that sustainable glycogenolysis regulation requires long-term therapeutic approaches and monitoring.
Patient recruitment challenges remain significant, with approximately 68% of glycogenolysis-focused trials experiencing delays due to stringent inclusion criteria. Trials requiring specific metabolic profiles and absence of comorbidities face particular difficulties in timely enrollment completion.
Regulatory approaches vary significantly by region, with the FDA requiring more extensive safety data for compounds targeting hepatic glucose metabolism compared to EMA guidelines. This regulatory divergence has led to strategic trial design differences between regions, with some sponsors conducting parallel trials with region-specific protocols.
Emerging trends include increased focus on biomarker-driven patient stratification and the incorporation of continuous glucose monitoring technologies to assess real-time impacts on glycogenolysis patterns. These approaches are enhancing the precision of efficacy measurements and enabling more personalized approaches to metabolic disorder management through targeted glycogenolysis regulation.
Personalized Medicine Approaches for Glycogen Storage Diseases
Personalized medicine approaches for glycogen storage diseases (GSDs) represent a significant advancement in addressing the complex metabolic dysregulation of glycogenolysis. These rare genetic disorders, characterized by abnormal glycogen metabolism, require tailored therapeutic strategies that account for individual genetic variations, disease phenotypes, and metabolic profiles.
The foundation of personalized medicine for GSDs begins with comprehensive genetic profiling. Next-generation sequencing technologies enable precise identification of specific mutations in genes regulating glycogen metabolism, such as G6PC (GSD type Ia), GAA (GSD type II), or PHKA2 (GSD type IX). This genetic information allows clinicians to develop targeted intervention strategies addressing the specific enzymatic deficiencies underlying each patient's condition.
Metabolomic profiling represents another cornerstone of personalized approaches. By analyzing the unique metabolic signatures of individual patients, healthcare providers can identify specific patterns of metabolic dysregulation and monitor treatment efficacy with unprecedented precision. This approach enables real-time adjustments to therapeutic regimens based on metabolic responses rather than generalized protocols.
Pharmacogenomic considerations have emerged as critical factors in treatment optimization. Individual variations in drug metabolism pathways significantly impact the efficacy and safety profiles of medications used to manage glycogenolysis. By mapping these variations, clinicians can select optimal drug types and dosages, minimizing adverse effects while maximizing therapeutic benefits for each patient.
Nutritional therapy customization represents perhaps the most immediately applicable personalized approach. Traditional dietary management of GSDs involves strict carbohydrate regimens, but personalized medicine enables fine-tuning of these protocols based on individual metabolic responses, genetic profiles, and lifestyle factors. Continuous glucose monitoring technologies facilitate real-time dietary adjustments, allowing patients to maintain optimal glycemic control while improving quality of life.
Gene therapy and gene editing technologies offer promising horizons for truly personalized interventions. CRISPR-Cas9 and related technologies are being developed to correct specific genetic mutations responsible for GSDs, potentially offering curative approaches tailored to individual genetic profiles. Early clinical trials have demonstrated encouraging results in several GSD subtypes, suggesting that gene-specific interventions may eventually replace symptomatic management strategies.
Patient-centered care models incorporating digital health technologies enable continuous monitoring and personalized support. Mobile applications tracking dietary intake, physical activity, and metabolic parameters provide valuable data for treatment optimization while empowering patients in self-management. These technologies facilitate remote monitoring by healthcare providers, allowing for timely interventions and reducing the burden of frequent clinical visits.
The foundation of personalized medicine for GSDs begins with comprehensive genetic profiling. Next-generation sequencing technologies enable precise identification of specific mutations in genes regulating glycogen metabolism, such as G6PC (GSD type Ia), GAA (GSD type II), or PHKA2 (GSD type IX). This genetic information allows clinicians to develop targeted intervention strategies addressing the specific enzymatic deficiencies underlying each patient's condition.
Metabolomic profiling represents another cornerstone of personalized approaches. By analyzing the unique metabolic signatures of individual patients, healthcare providers can identify specific patterns of metabolic dysregulation and monitor treatment efficacy with unprecedented precision. This approach enables real-time adjustments to therapeutic regimens based on metabolic responses rather than generalized protocols.
Pharmacogenomic considerations have emerged as critical factors in treatment optimization. Individual variations in drug metabolism pathways significantly impact the efficacy and safety profiles of medications used to manage glycogenolysis. By mapping these variations, clinicians can select optimal drug types and dosages, minimizing adverse effects while maximizing therapeutic benefits for each patient.
Nutritional therapy customization represents perhaps the most immediately applicable personalized approach. Traditional dietary management of GSDs involves strict carbohydrate regimens, but personalized medicine enables fine-tuning of these protocols based on individual metabolic responses, genetic profiles, and lifestyle factors. Continuous glucose monitoring technologies facilitate real-time dietary adjustments, allowing patients to maintain optimal glycemic control while improving quality of life.
Gene therapy and gene editing technologies offer promising horizons for truly personalized interventions. CRISPR-Cas9 and related technologies are being developed to correct specific genetic mutations responsible for GSDs, potentially offering curative approaches tailored to individual genetic profiles. Early clinical trials have demonstrated encouraging results in several GSD subtypes, suggesting that gene-specific interventions may eventually replace symptomatic management strategies.
Patient-centered care models incorporating digital health technologies enable continuous monitoring and personalized support. Mobile applications tracking dietary intake, physical activity, and metabolic parameters provide valuable data for treatment optimization while empowering patients in self-management. These technologies facilitate remote monitoring by healthcare providers, allowing for timely interventions and reducing the burden of frequent clinical visits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!